Abstract
Myoepithelial cells (MECs) are considered to be a key participant in most salivary gland diseases, particularly tumors. MECs structurally resemble both epithelial cells and smooth muscles. Diagnostic dilemmas caused are due to inadequacy of characterizing the wide spectrum of morphologic and immunologic features which are different for both normal and neoplastic MECs. This article discusses the development, functions and structure of both normal and neoplastic MECs, their staining properties and differences in the morphologic and immunophenotypic properties of the MEC in detail. It also describes the role of MEC in pathogenesis and morphogenesis of various nonneoplastic and neoplastic salivary gland lesions and thereby are responsible for the myriad histopathology of salivary gland tumors.
Keywords: Myoepithelial cell, neoplasm, salivary gland tumor
INTRODUCTION
In salivary glands and other exocrine glands, there are star-shaped cells lying between the basal lamina and the acinar and ductal cells. These cells structurally resemble epithelial cells and smooth muscles and, thus, are referred to as myoepithelial cells (MECs). Because of their shape and interwoven processes, they were commonly referred to as “star-shaped cells” or “basket cells.” Tamarin described these cells as being “like an octopus sitting on a rock” [Figure 1].[1,2]
Figure 1.
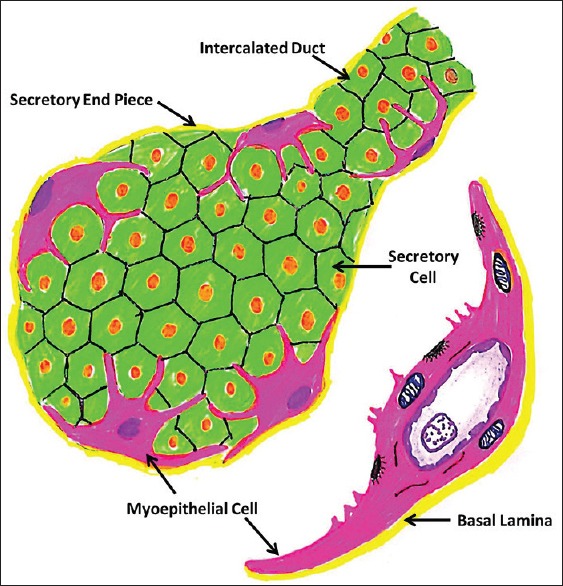
Diagrammatic representation of Myoepithelial Cells (Image modified from: Antonio Nanci: TenCate's Oral Histology: Development, Structure, and Function 8th edition. Elsevier Mosby
HISTORICAL INTRODUCTION
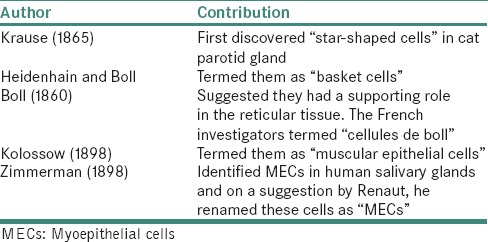
A brief history about the various terminologies used for these cells is shown in Table 1.[3,4]
Table 1.
History of myoepithelial cells
ORIGIN OF SALIVARY GLAND MYOEPITHELIAL CELLS
The salivary duct system is entirely epithelial in origin. The stem cells in the primordium differentiate into various components of the ducto-acinar unit including the secretory end pieces [Figure 2].[5]
Figure 2.
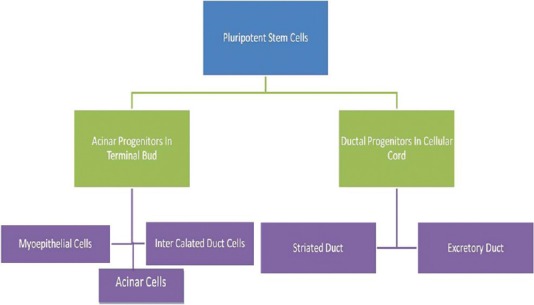
Origin of ducto-acinar components of salivary glands from pluripotent stem cells
DEVELOPMENT OF SALIVARY GLAND MYOEPITHELIAL CELLS
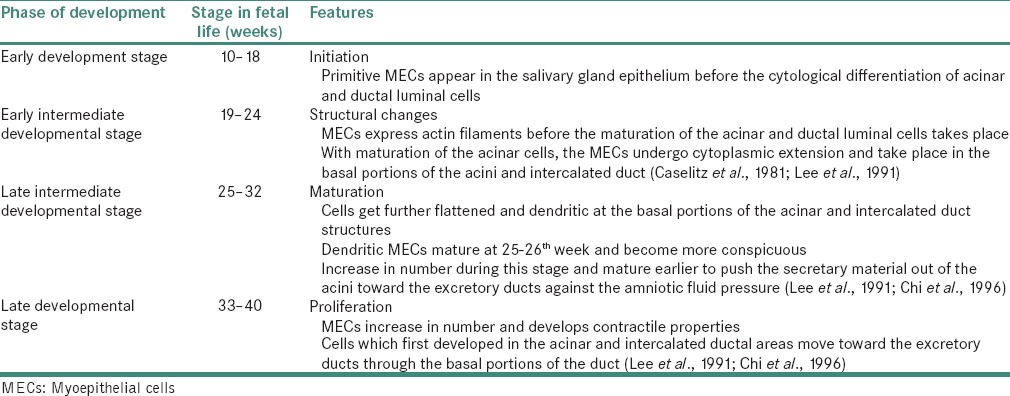
The various stages in the development of MECs are given in Table 2.[6]
Table 2.
Stages in development of myoepithelial cell
DISTRIBUTION
MECs are located beneath the basement membrane of the terminal portion of most exocrine glands including salivary, lacrimal, mammary and sweat glands. They have variable distribution among the glands and species and also occasionally within the same gland during the development.[2]
In salivary glands, they are typically arranged to form arcs around the acini and orient along the long axes of ducts. In the major salivary glands, MECs are seen in relation to acini, intercalated ducts and striated ducts. Whereas, in the minor salivary glands, MECs invest the acini with processes continuing onto intercalated ducts. The excretory ducts and rather rudimentary striated ducts are devoid of MECs [Figure 3].
Figure 3.
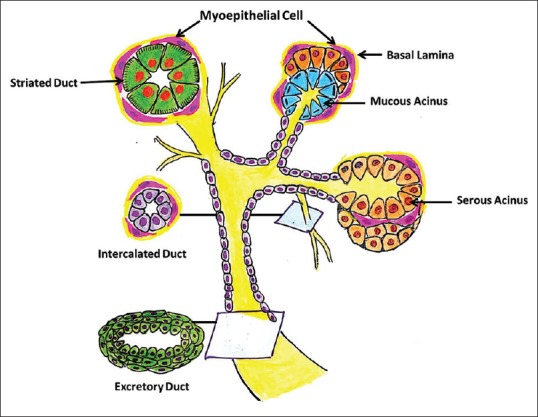
Diagrammatic representation of the distribution of MECs along the ducto-acinar units of salivary gland
FUNCTIONS OF MYOEPITHELIAL CELLS
They serve diverse functions as follows.
Epithelial cell differentiation
During embryonic development, MECs are involved in the branching morphogenesis of the developing salivary glands and the promotion of epithelial cell differentiation by secreting growth factors and cytokines (basic fibroblast growth factor, transforming growth factor α and interleukin-6).[7]
Contractile function
Contraction of MEC facilitates expulsion of secretion by rupturing “ripe” mucous cells, reducing luminal volume and preventing distention of acini.
Contraction of elongated MECs along the intercalated ducts helps overcome peripheral resistance.[8,9,10,11,12,13]
Sensory
Myoepithelial cilia projecting into the invaginations in adjacent secretory cells may act as chemoreceptors.[14]
Maintenance of gland patency
Extension of MEC processes onto proximal regions of allied acini facilitates rigidity and patency in glands which may become distorted by masticatory movements.[10]
Transportation of metabolites
The role of MECs in this is questionable. However, the presence of pinocytic vesicles, a positive staining for iron-binding protein ferritin and high levels of alkaline phosphatase and magnesium-dependent ATPase activity suggests that MECs are involved in transportation of metabolites in the secretory process.[9,14,15,16,17,18,19,20,21]
Formation and maintenance of basement membrane
MECs play an important role by producing fibronectin, laminin and elastin, which are major components of basement membrane and extracellular matrix (ECM).
Interestingly, with neoplastic transformation, MECs usually augment and modify this matrix-synthesizing ability resulting in production of large amounts of both basement membrane and nonbasement membrane elements, the latter often predominating. The most dominant component of the nonbasement membrane matrix is chondroitin sulfate proteoglycan. The other forms of matrix produced by the MECs is eosinophilic hyalinized material, which represents the basement membrane-related proteins (type IV collagen and laminin) and interstitial matrix proteins (fibronectin, type I and II collagens).[18,21]
Tumor suppression
There is evidence that MECs also produce a number of proteins that have tumor-suppressor activity, such as proteinase inhibitors and anti-angiogenesis factors, which act as barriers against invasive epithelial neoplasms.
The MECs exert a paracrine anti-invasive effect by promoting epithelial differentiation, synthesis of basement membrane, secreting proteinase inhibitors and inhibiting angiogenesis.[22,23] The other properties of MEC signifying a tumor-suppressor role include secretion of high levels of maspin (an inhibitor of tumor growth and invasion) and tissue inhibitor of metalloproteinase-1, protease nexin II and α1-antitrypsin.[7,22,23]
STRUCTURE OF MYOEPITHELIAL CELLS
MECs appear considerably similar in structure, irrespective of the organ or species. MECs on acini have 4–8 primary cytoplasmic processes each showing 2 or more secondary branching processes. SEM also revealed that MEC processes ramify into numerous secondary and tertiary divisions. There can be up to thirty terminal processes extending from each MEC. The average number of processes extending from MECs is more in submandibular gland as compared to sublingual gland. It is also noted that the thicker MEC processes occur on the glands with more viscous secretions [Figure 4].[2,24]
Figure 4.
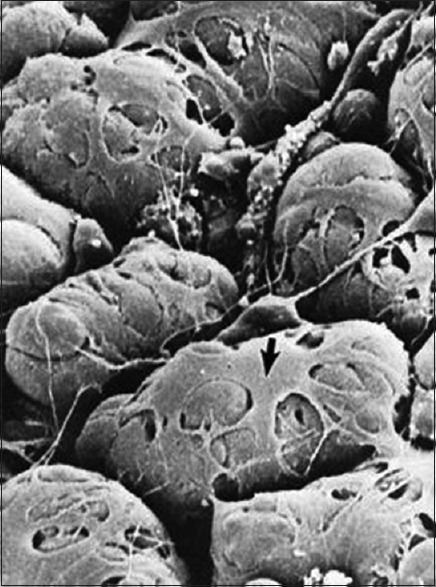
Structure of myoepithelial cells showing cell body (arrow) and numerous cytoplasmic processes surrounding the acini (Coutesy: Antonio Nanci: TenCate's Oral Histology: Development, Structure, and Function 8th edition. Elsevier Mosby)
The outer surface of MECs contains abundant caveolar invaginations in areas where nerve fibers abut. The smooth visceral surface is attached to secretory cells by desmosomes and may show isolated cilia invaginating the basal cytoplasmic membrane and protruding deep into the cytoplasm of the secretory epithelial cells.[25]
The MECs and their processes were filled with parallel streams of myofilaments which displayed focal densities. The myofilaments formed attachment plaques on the inner face of the plasmalemma. The cytoplasm contained ribosomes, polyribosomes, pinocytotic vesicles, vacuoles and lysosomes but contained few mitochondria and little rough endoplasmic reticulum. The nucleus is usually elongated, dense and irregular.[26]
The acinar MECs lie entirely on the epithelial side of the basal lamina. The intervening spaces between the MECs and the acinar cells display interdigitations and infoldings of the lateral plasma membrane. On the intercalated ducts, they are located between the basal lamina and ductal epithelium. Squamous metaplasia of MECs in this region is not uncommon and is characterized by the presence of numerous small and large tufts of cytoplasmic tonofilaments. MECs are also seen in association with the intralobular striated ducts and lie between the basal lamina and the ductal cells. Here, the MECs may be spindle-shaped, stellate, with their long axes are oriented parallel to the basal lamina. Occasionally, they may be oriented vertically, extending between the ductal cells or on top of another. Irrespective of their orientation, the MECs displayed similar ultrastructural features.[26]
Comparison of myoepithelial cells with smooth muscle cells
MECs share many features with smooth muscle cells. These include parallel arrays of filaments which are gathered in “dense bodies” and are anchored to the basal plasmalemma in attachment plaques; fusiform nuclei parallel to the long axis of the muscle cell or its longest processes; numerous caveolae, mostly in the basal plasmalemma; occasional collections of glycogen particles; sparse rough endoplasmic reticulum and Golgi zones and a basal lamina.[2]
MEC can be distinguished from smooth muscle cells in being attached to other parenchymal (acinar and ductal) cells and to each other by desmosomes and gap junctions and to the basal lamina by hemidesmosomes. In addition, smooth muscle cells are spindle shaped and bipolar while MECs have multiple processes[2,27,28] [Figure 5a and b].
Figure 5.
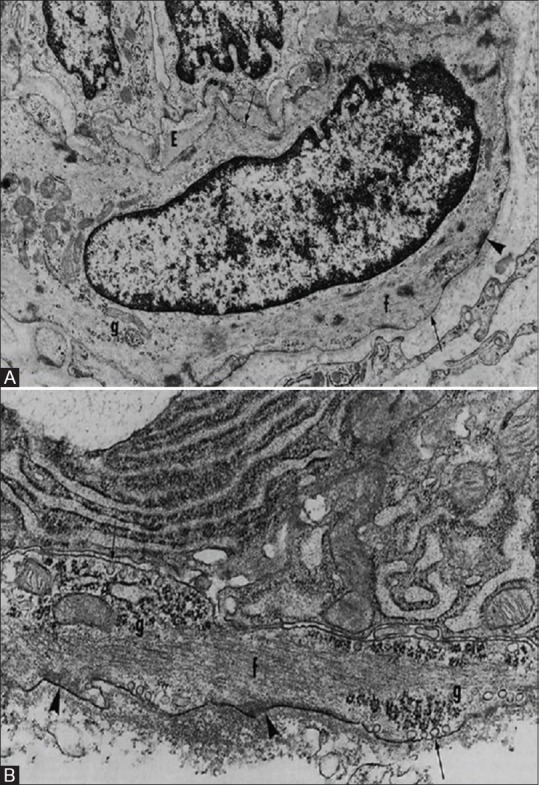
Comparison between myoepithelial cells and smooth muscle cells. (A) Smooth Muscle cell (B) Myoepithelial cell. There are groups of filaments (f); dense bodies; attachment plaques (arrowheads); caveolae (arrows) in the plasmalemma, that are more numerous basally; basal laminae; and focal collections of glycogen particles (g) (Redman R.S. Myoepithelium of Salivary Glands. Microscopy research and technique 2725-45 [1994])
NEOPLASTIC MYOEPITHELIAL CELLS
Basal and/or MECs form a continuum along the acini and ducts in the normal salivary gland. In salivary gland tumors largely composed of neoplastic basal and/or MECs, this relationship may persist. However, in some tumors, hybrid tumor cell forms are present along with typical basal and/or MECs or both.[29]
Morphologic spectrum of neoplastic myoepithelial cell
Histologic diversity is the hallmark of neoplastic MECs. These cells have the potential to undergo divergent differentiation giving rise to different morphological cell types. The complex morphological patterns of neoplastic MEC results from a complex morphologic interplay of 3 characteristics:[30,31,32,33]
Cytological differentiation
Extracellular matrix production
Architectural patterns.
Cytological differentiation
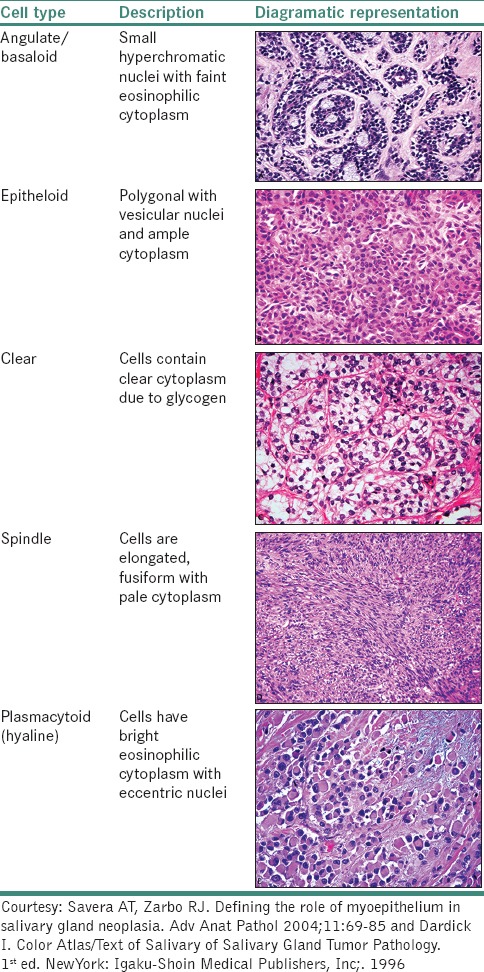
MECs potentially differentiate into various morphological cell types [Table 3]. Most tumors with MEC differentiation show more than one cell type.[7,34,35,36]
Table 3.
Various cell types of myoepithelial cells due to cytological differentiation
Extracellular matrix production
In the normal state, myoepithelium contributes to the synthesis of basement membrane components and ECM. This matrix-synthesizing ability is modified and augmented with their neoplastic transformation leading to production of abundance of both basement membrane and non-basement membrane elements.[22,23]
The most dominant component of the nonbasement matrix is chondroitin sulfate proteoglycan, which histologically appears as the bluish-gray myxochondroid material and is alcian blue positive. The other form of matrix produced by neoplastic myoepithelium is the eosinophilic hyalinized material, which represents the basement membrane-related (type IV collagen and laminin) and interstitial matrix (fibronectin and type I and type II collagens) components.[22]
Determining the true nature of ECM particularly the myxoid material is important in salivary gland tumor identification since it forms a component in various salivary gland tumors (SGTs) including pleomorphic adenoma, carcinoma ex pleomorphic adenoma and myoepithelioma.[22]
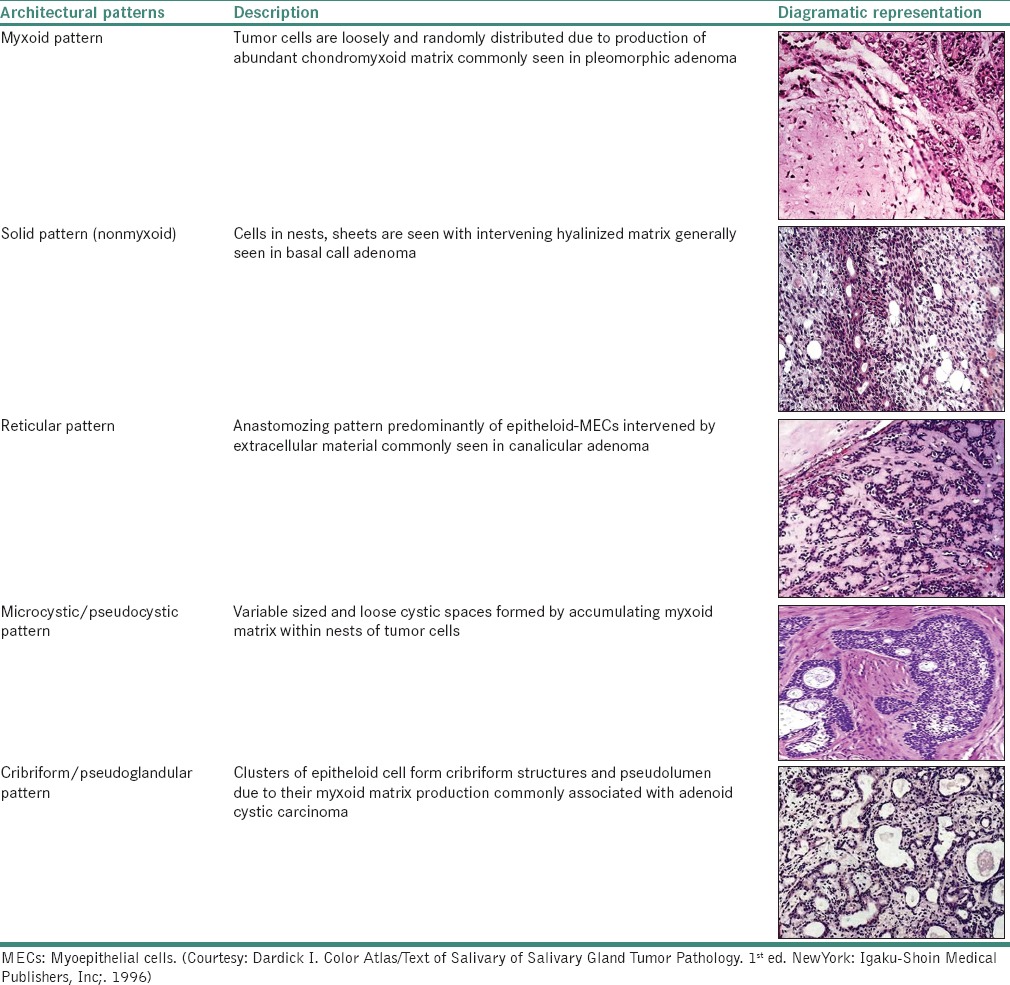
Architectural patterns
These cells can undergo metaplastic changes such as chondroid, squamous and oncocytic metaplasia. Dardick described various architectural differentiation patterns of MEC [Table 4].[7,29]
Table 4.
Various architectural patterns of myoepithelial cell
IDENTIFICATION OF MYOEPITHELIAL CELLS
Staining
Hematoxylin and eosin staining
In their normal environment, they are eosinophilic, birefringent structures which lie deep to the basement membranes of the secretory acini and smaller ducts and may appear fusiform or spider-like, depending on the plane of section. They stain intensely with phosphotungstic acid hematoxylin and iron hematoxylin [Figure 6].[24]
Figure 6.
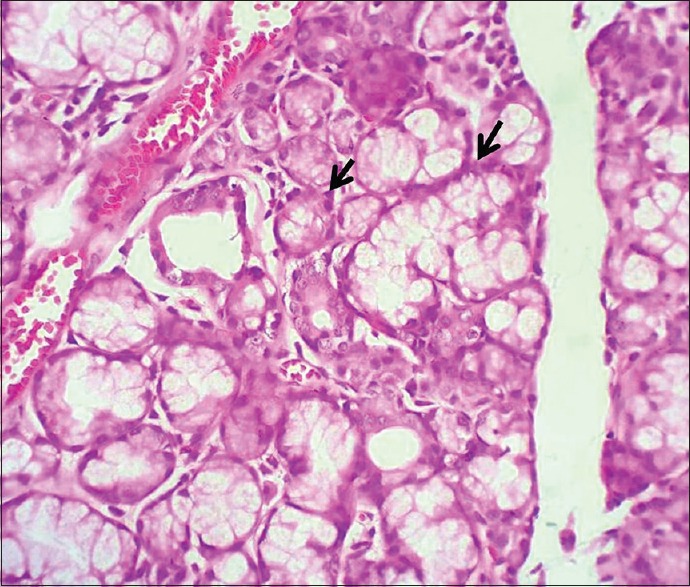
Demonstration of myoepithelial cells (H&E stain, ×400)
Special stains
A special staining procedure, the tannic acid-phosphomolybdic acid amido black technique of Puchtler and Leblond, has been found to be particularly suitable for staining myofibrils, thus demonstrating MECs.[37,38,39]
Enzyme histochemistry
ATPase was found to be the enzyme marker of MEC in human glands. However, although ATPase histochemistry can be helpful in identifying neoplastic MECs, some MECs in normal glands lack activity of this enzyme and persistence of enzyme activity in routinely processed specimens is too spotty to be reliable. A similar problem was encountered on examination of glycogen phosphorylase activity as a marker for neoplastic MEC.[40,41,42,43,44,45]
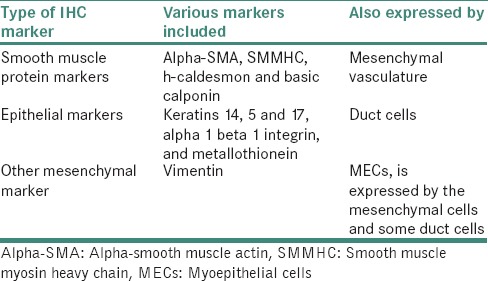
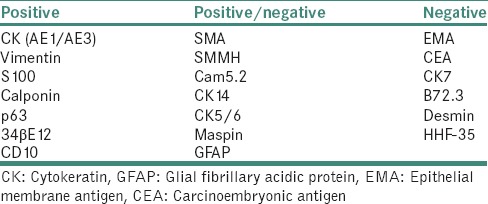
Immunocytochemistry
MECs can be best observed by immunocytochemistry. There are three types of immunocytochemical markers of MECs in salivary glands [Table 5].[46]
Table 5.
Types of immunohistochemical markers for myoepithelial cells
Due to the morphologic diversity of these cells, the immunohistochemical profile of neoplastic MECs has also been a perplexing issue. The reason for this is probably two-fold and interrelated as follows: First, immunophenotypic modifications are associated with neoplastic transformation which leads to variability/loss of immunoreactivity of some of the markers invariably present in normal myoepithelium (CK14, muscle markers [Figure 7]); second, there is expression of certain markers by neoplastic myoepithelium that are usually absent in nonneoplastic counterpart (S100 protein, vimentin and glial fibrillary acidic protein).[47,48]
Figure 7.
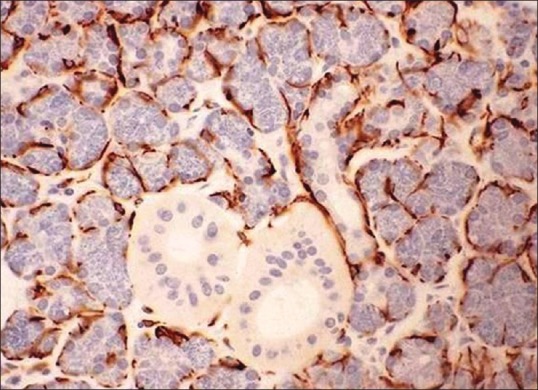
Demonst ration of myoepithelial cells using immunohistochemistry marker cytokeratin AE1/AE3 (IHC stain, ×400) (Courtesy: http://www.pathologyoutlines.com/topic/salivaryglandssuperpage.html [last viewed on 10/04/2015])
The various markers seen during the development of salivary glands are shown in Table 6, and the various markers used for demonstrating neoplastic MECs are given in Table 7.[46,47,48,49,50,51,52,53,54,55,56,57]
Table 6.
Myoepithelial markers in developing and fully developed human salivary glands
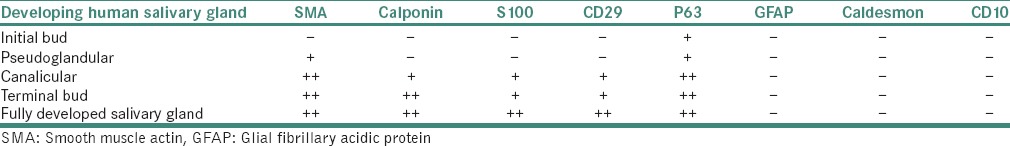
Table 7.
Immunohistochemical profile of neoplastic myoepithelium
Electron microscopy
Transmission electron microscopy remains the best method for the accurate identification and characterization of normal and neoplastic MEC. In human submandibular glands, two types of MECs can be distinguished in serial ultrathin sections. The dark MEC type was stellate in shape and exhibited a pronounced electron density due to numerous myofilaments with focal densities and accounted for around 76% of MEC and, furthermore, showed adenosine triphosphatase activity. The light MEC type was large and ellipsoid with a few short-thick processes and was characterized by an electron lucent cytoplasm which included scant and unevenly distributed myofilaments. They showed positive ATPase activity and accounted for only 17% of the MEC number. Transitional forms between these two types were also observed. The light MEC type may mature into the dark MEC type by means of the transitional form. In addition, clear cells were sometimes encountered between the MEC and the acinar or intercalated duct cells.[58]
ROLE OF MYOEPITHELIAL CELLS IN PATHOLOGIC CONDITIONS
MECs have been implicated in various neoplastic as well as nonneoplastic disorders of the salivary glands.
Nonneoplastic disorders associated with myoepithelial cells
Lymphoreticular cell proliferation associated with atrophy of the glandular parenchyma and ductal changes ending in the so-called “epimyoepithelial islands” are characteristics of chronic recurrent (punctate) sialadenitis, sicca syndrome, Sjogren's syndrome and benign lymphoepithelial lesion. The epimyoepithelial islands are formed by metaplastic transformation of ductal epithelial and MECs. Some believe that MECs are few in number and located only around the periphery of the islands whereas majority of the workers agree that myoepithelium forms an integral part of epimyoepithelial islands.[25,59]
HISTOMORPHOGENESIS OF SALIVARY GLAND NEOPLASMS
Rationale
In pathology, histogenesis is synonymous with the “cell of origin” for a neoplasm rather than the developmental process underlying the tumor. On the other hand, morphogenesis represents the process of differentiation inherent in neoplasms and the resulting histopathology characteristic for that particular tumor.[60,61,62]
Histogenetic concept
A variety of histogenetic concepts for salivary gland tumors have evolved. However, the semipluripotential bicellular reserve cell hypothesis given by Eversole in 1971 is considered to be the main concept wherein it is observed that specific reserve or basal cells of the excretory and intercalated ducts or both are responsible for the placement of all types of cells in the normal gland and, hence, are the sole source for neoplastic transformation. However, this theory excluded the role of cells of the striated duct.[63]
Morphogenetic concept
It is now apparent that acini and ducts including excretory, striated and intercalated ducts are associated with some form of basal/MECs.
Based on the pattern of tumor cell differentiation and tumor cell organization, it has been suggested that three basic types of tumors can develop.
Composed entirely or primarily of acinar or luminal epithelium
Composed of both luminal and basal/MECs
Composed only of basal/MECs.
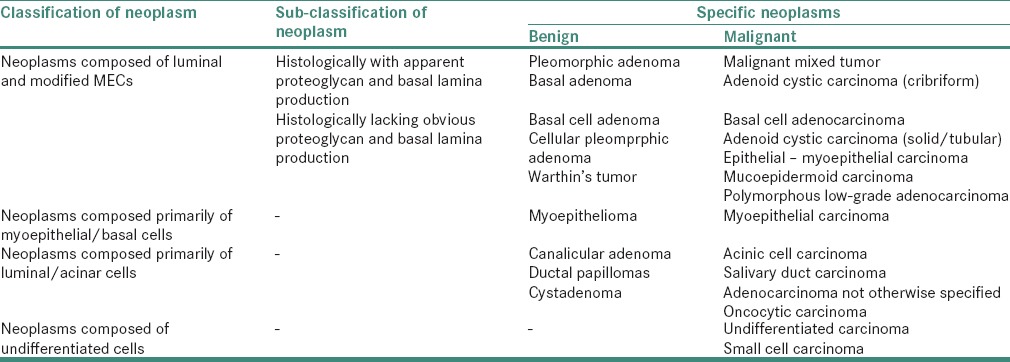
However, this concept was modified and it was suggested that the main differentiation process underlying the histomorphology of many salivary gland tumors can be divided into five main categories as shown in Table 8.[64]
Table 8.
Classification of salivary gland neoplasms
Neoplastic myoepithelial cells: Are they host friendly?
Malignant neoplasms have a near universal ability to degrade extracellular matrices, which otherwise play roles in containment of the neoplasm. Sternlicht and Barsky are credited for adding objective evidence to the subjective presumption of the tumor suppressive, or tumor ameliorative behavior of MECs. In a series of publications, these investigators have answered their hypotheses that MECs are natural tumor suppressors and resist malignant transformation and progression of the neoplasm.[23]
The MECs appear to have a modifying effect on biologic behavior of the salivary gland tumors. MECs surround benign epithelial proliferations and in situ carcinomas but are absent in invasive cancers. Thus, the mere presence of MECs can distinguish benign or in situ disease from malignant disease. Salivary gland carcinomas in which there is histopathological evidence of an active participation of MECs are generally those taking origin from the intercalated duct/secretory end piece part of the salivary duct unit. From the clinicobiological point of view, it is also those carcinomas which are regarded as low grade as judged by their relatively low ability to metastasize or a long-term progression, when compared to carcinomas without MEC participation.[23]
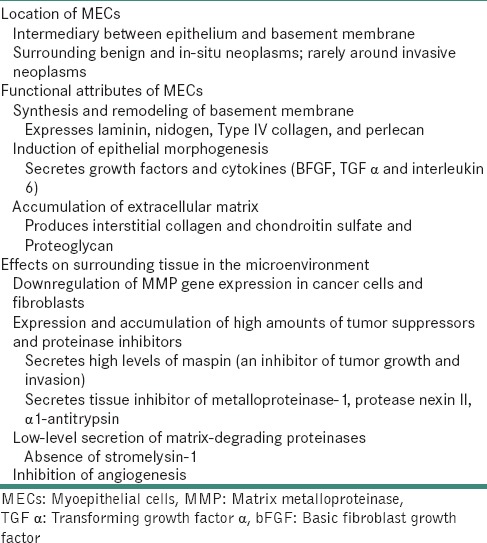
In the neoplastic state, myoepithelium presents with lower proliferation rates than basal type epithelial cells and secretes excess substances that inhibit tissue invasion and metastasis. These accumulated myxoid ground substances and basement membrane components contribute to an anti-invasive matrix for myoepithelial-rich SGTs as evidenced by lobulated and pushing rather than infiltrative tissue growth patterns in tumors showing MEC proliferation and prolonged survivals despite distant metastasis.[65] The various properties of MEC signifying a tumor-suppressor role are summarized in Table 9.[7,23,66]
Table 9.
Properties of myoepithelial cell signifying a tumor-suppressor role
Neoplastic disorders associated with myoepithelial cell proliferation
The wide spectrum of morphologic presentation of salivary gland neoplasms is greatly a result of two-sided expression of myoepithelium. Table 10 summarizes the expression of MEC differentiation in the various salivary gland neoplasms.[67]
Table 10.
Expression of myoepithelial cells in various salivary gland neoplasms
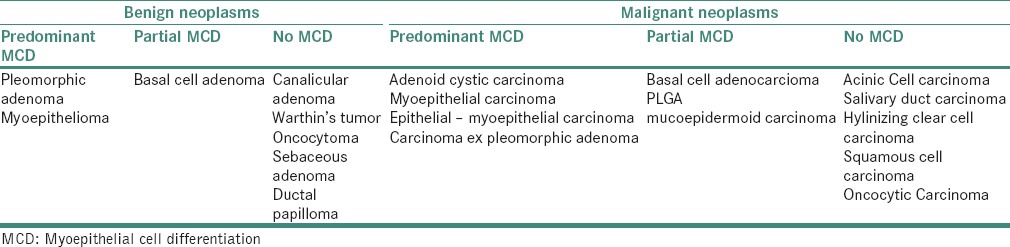
PLEOMORPHIC ADENOMA/CARCINOMA EX PLEOMORPHIC ADENOMA
It is the most common benign salivary gland tumor in which MECs form the principal cell type. These cells can assume a variety of cytological forms such as hyaline, myxoid or epithelial cells, but they frequently occur as angular, slightly separated cells surrounding ducts or forming variably sized clusters or sheet-like regions and do not present the classical features of normal MECs. Initially, these cells are related to duct luminal cells. However, with proliferation, they are gradually separated by increasing amounts of matrix material, resulting in the development of the myxoid and chondroid areas.[29,68]
In carcinoma ex pleomorphic adenoma, the earliest changes typically consist of tumor cells replacing the normal inner duct epithelial layer leaving the normal peripherally located myoepithelial layer intact. Atypical changes within these tumors range from focal to diffuse often with multifocal areas containing carcinoma, which frequently overgrows and replaces many of the benign elements.[29]
MYOEPITHELIOMA/MYOEPITHELIAL CARCINOMA
Myoepitheliomas are a type of pleomorphic adenoma in which neoplastic MECs exclusively or predominantly proliferate. Cytologically, tumor cells can be either spindle-shaped, epithelioid or plasmacytoid or clear cells. Occasionally, however, myxoid and chondroid matrix may also form a significant part of the histology. Predomination of any one cell type, not necessarily a homogenous population of tumor cells, underlies a particular histomorphologic form.[35,69]
The cells in myoepithelial carcinoma resemble tumor cells in benign myoepithelioma and the MECs in pleomorphic adenoma; however, they usually demonstrate increased mitotic activity and cytologic pleomorphism, large and more vesicular nuclei and more prominent nucleoli.[70]
BASAL CELL ADENOMA/BASAL CELL ADENOCARCINOMA
It has been found that although basal cell adenoma subtypes appear microscopically basaloid and monomorphic in architectural patterns compared with pleomorphic adenoma, periductal, epithelioid and spindled (stromal-like) MECs contribute to the proliferation of these tumors. The MECs in these tumors are truly neoplastic rather than entrapped normal cells for the following reasons. First, the morphologic heterogeneity of stained cells (periductal, spindled and epithelioid) departs from that of normal myoepithelium. Second, the MECs highlighted by the monoclonal antibodies form an integral part of the tumors and are not merely situated at the periphery.[71]
Other than invasion, basal cell adenocarcinomas are morphologically very similar to the benign counterpart.[7]
ADENOID CYSTIC CARCINOMA
Adenoid cystic carcinoma is a basaloid tumor consisting of epithelial and MECs in variable morphologic configurations. The complexity underlying the histologic features of adenoid cystic carcinoma is based on the frequency and prominence of ductal structures, basal/myoepithelial cells and intercellular matrix materials resulting in the formation of three histologic variants such as cribriform, solid and tubular.[29]
EPITHELIAL - MYOEPITHELIAL CARCINOMA
A malignant tumor that comprises variable proportions of two cell types, which typically form duct-like structures is epithelial-myoepithelial carcinoma. The two cell types are arranged as an inner layer of darker cells that represents the intercalated duct epithelial component and an outer layer of cells with clear, glycogen-rich cytoplasm that represents the myoepithelial component, the proportions of which may vary from one neoplasm and in different fields within the same tumor.[29]
POLYMORPHOUS LOW GRADE ADENOCARCINOMA (PLGA)
A malignant epithelial tumor is characterized by cytologic uniformity, morphologic diversity, an infiltrative growth pattern and low metastatic potential. There is a significant, if not predominant, myoepithelial differentiation in these tumors denoted by the presence of analogous histologic patterns (architectural arrangements, myxoid and hyalinized matrices) as seen in myoepitheliomatous zones of pleomorphic adenoma and adenoid cystic carcinoma.[67]
MUCOEPIDERMOID CARCINOMA
Current classifications of SGTs separate mucoepidermoid carcinoma from other neoplasms on the basis of a number of histological features, in particular, the lack of participation of neoplastic MECs. However, ultrastructural examination of low- and intermediate-grade mucoepidermoid carcinomas and pleomorphic adenomas reveals many common organizational and cellular features. The relationship of intermediate cells to the luminal cells in mucoepidermoid carcinomas is of prime importance, which is remarkably similar to that seen between modified MECs and luminal cells in pleomorphic adenomas. These results suggest that intermediate cells of mucoepidermoid carcinoma are the counterpart of the modified MECs of pleomorphic adenoma.[33]
SUMMARY AND CONCLUSION
In this article, we reviewed the various aspects of MECs in normal conditions as well the morphologic diversity of these cells in various pathologic conditions. It can be observed that the neoplastic counterpart of these cells shows certain morphological and architectural alterations which are responsible for variations in the histopathologic patterns of the various salivary gland neoplasms. Thus, an evaluation of SGTs based on an accurate recognition of myoepithelial differentiation (as described), their various morphologic and immunophenotypic expressions is essential in establishing a definitive role of myoepithelium in salivary gland neoplasm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nanci A. Ten Cate's Oral Histology: Development, Structure, and Function. 8th ed. Pheladelphia: Elsevier Mosby; 2012. [Google Scholar]
- 2.Redman RS. Myoepithelium of Salivary Glands. Microscopy Research and Technique. 1994;1(27):25–45. doi: 10.1002/jemt.1070270103. [DOI] [PubMed] [Google Scholar]
- 3.Travill AA, Hill MF. Histochemical demonstration of myoepithelial cell activity. Q J Exp Physiol Cogn Med Sci. 1963;48:423–6. doi: 10.1113/expphysiol.1963.sp001684. [DOI] [PubMed] [Google Scholar]
- 4.Garrett JR, Ekström J, Anderson LC, editors. Glandular mechanisms of salivary secretion. 1st ed. Basel: Karger publications; 1998. [Google Scholar]
- 5.Batsakis JG, Kraemer B, Sciubba JJ. The pathology of head and neck tumors: The myoepithelial cell and its participation in salivary gland neoplasia, Part 17. Head Neck Surg. 1983;5:222–33. doi: 10.1002/hed.2890050307. [DOI] [PubMed] [Google Scholar]
- 6.Nirmalkumar H, Jagannathan N. Myoepithelial cells: Revisited – Review. Ann RSCB. 2013;XVIII:9–15. [Google Scholar]
- 7.Savera AT, Zarbo RJ. Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol. 2004;11:69–85. doi: 10.1097/00125480-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Raubenheimer EJ. The myoepithelial cell: Embryology, function, and proliferative aspects. Crit Rev Clin Lab Sci. 1987;25:161–93. doi: 10.3109/10408368709105881. [DOI] [PubMed] [Google Scholar]
- 9.Garrett JR, Emmelin N. Activities of salivary myoepithelial cells: A review. Med Biol. 1979;57:1–28. [PubMed] [Google Scholar]
- 10.Garrett JR, Parsons PA. Alkaline phosphatase and myoepithelial cells in the parotid gland of the rat. Histochem J. 1973;5:463–71. doi: 10.1007/BF01012003. [DOI] [PubMed] [Google Scholar]
- 11.Cope GH, Pratten MK, Williams MA. Correlative morphological and biochemical study of the effects of isoprenaline on the organelle and membrane content of the rabbit parotid gland. Histochem J. 1976;8:403–18. doi: 10.1007/BF01003829. [DOI] [PubMed] [Google Scholar]
- 12.Linzell JL. Some observations on the contractile tissue of the mammary glands. J Physiol. 1955;130:257–67. doi: 10.1113/jphysiol.1955.sp005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young JA, Van Lennep EW. Morphology and physiology of salivary myoepithelial cells. Int Rev Physiol. 1977;12:105–25. [PubMed] [Google Scholar]
- 14.Tandler B, Denning CR, Mandel ID, Kutscher AH. Ultrastructure of human labial salivary glands 3. Myoepithelium and ducts. J Morphol. 1970;130:227–45. doi: 10.1002/jmor.1051300208. [DOI] [PubMed] [Google Scholar]
- 15.Tamarin A. Myoepithelium of the rat submaxillary gland. J Ultrastruct Res. 1966;16:320–38. doi: 10.1016/s0022-5320(66)80066-7. [DOI] [PubMed] [Google Scholar]
- 16.Nagai T, Nagai M. Scanning electron microscopy of the human submandibular gland. Arch Otorhinolaryngol. 1985;241:265–66. doi: 10.1007/BF00453698. [DOI] [PubMed] [Google Scholar]
- 17.Ruby JR. Ultrastructure of the parotid gland of the nine-banded armadillo. Anat Rec. 1978;192:389–405. doi: 10.1002/ar.1091920306. [DOI] [PubMed] [Google Scholar]
- 18.Toto PD, Hsu DJ. Product definition of pleomorphic adenoma of minor salivary glands. J Oral Pathol. 1985;14:818–32. doi: 10.1111/j.1600-0714.1985.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 19.Bogart BI. The fine structural localization of alkaline and acid phosphatase activity in the rat submandibular gland. J Histochem Cytochem. 1968;16:572–81. doi: 10.1177/16.9.572. [DOI] [PubMed] [Google Scholar]
- 20.Yoshihara T, Kanda T, Kaneko T. A cytochemical study on the salivary gland pleomorphic adenoma (mixed tumor) and the fetal and adult salivary gland. Arch Otorhinolaryngol. 1984;240:231–8. doi: 10.1007/BF00453376. [DOI] [PubMed] [Google Scholar]
- 21.David R, Buchner A. Elastosis in benign and malignant salivary gland tumors. A histochemical and ultrastructural study. Cancer. 1980;45:2301–10. doi: 10.1002/1097-0142(19800501)45:9<2301::aid-cncr2820450913>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Sternlicht MD, Safarians S, Rivera SP, Barsky SH. Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest. 1996;74:781–96. [PubMed] [Google Scholar]
- 23.Sternlicht MD, Barsky SH. The myoepithelial defense: A host defense against cancer. Med Hypotheses. 1997;48:37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 24.Shear M. The structure and function of myoepithelial cells in salivary glands. Arch Oral Biol. 1966;11:769–80. doi: 10.1016/0003-9969(66)90003-3. [DOI] [PubMed] [Google Scholar]
- 25.Raubenheimer EJ, van Nickerk JP, Hauman CH. Salivary myoepithelium: Distribution, structure, functions and pathologic proliferations. J Dent Assoc S Afr. 1987;42:631–7. [PubMed] [Google Scholar]
- 26.Chaudhry AP, Cutler LS, Yamane GM, Labay GR, Sunderraj M, Manak JR., Jr Ultrastructure of normal human parotid gland with special emphasis on myoepithelial distribution. J Anat. 1987;152:1–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Tandler B. In: Structure of the human parotid and submandibular glands. The Salivary System. 1st ed. Sreebny LM, editor. Boca Raton FL: CRC Press; 1987. pp. 21–41. [Google Scholar]
- 28.Bois RM. The organization of the contractile apparatus of vertebrate smooth muscle. Anat Rec. 1973;177:61–77. doi: 10.1002/ar.1091770107. [DOI] [PubMed] [Google Scholar]
- 29.Dardick I. Color Atlas/Text of Salivary of Salivary Gland Tumor Pathology. 1st ed. New York: Igaku-Shoin Medical Publishers, Inc; 1996. [Google Scholar]
- 30.Dardick I, Ostrynski VL, Ekem JK, Leung R, Burford-Mason AP. Immunohistochemical and ultrastructural correlates of muscle-actin expression in pleomorphic adenomas and myoepitheliomas based on comparison of formalin and methanol fixation. Virchows Arch A Pathol Anat Histopathol. 1992;421:95–104. doi: 10.1007/BF01607041. [DOI] [PubMed] [Google Scholar]
- 31.Erlandson RA, Cardon-Cardo C, Higgins PJ. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1984;8:803–20. doi: 10.1097/00000478-198411000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Dardick I. Myoepithelioma: Definitions and diagnostic criteria. Ultrastruct Pathol. 1995;19:335–45. doi: 10.3109/01913129509021906. [DOI] [PubMed] [Google Scholar]
- 33.Dardick I, Gliniecki MR, Heathcote JG, Burford-Mason A. Comparative histogenesis and morphogenesis of mucoepidermoid carcinoma and pleomorphic adenoma. An ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1990;417:405–17. doi: 10.1007/BF01606029. [DOI] [PubMed] [Google Scholar]
- 34.Dardick I, van Nostrand AW. Myoepithelial cells in salivary gland tumors – Revisited. Head Neck Surg. 1985;7:395–408. doi: 10.1002/hed.2890070509. [DOI] [PubMed] [Google Scholar]
- 35.Dardick I, Thomas MJ, van Nostrand AW. Myoepithelioma – New concepts of histology and classification: A light and electron microscopic study. Ultrastruct Pathol. 1989;13:187–224. doi: 10.3109/01913128909057442. [DOI] [PubMed] [Google Scholar]
- 36.Dardick I, Cavell S, Boivin M, Hoppe D, Parks WR, Stinson J, et al. Salivary gland myoepithelioma variants. Histological, ultrastructural, and immunocytological features. Virchows Arch A Pathol Anat Histopathol. 1989;416:25–42. doi: 10.1007/BF01606467. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar K, Kallenbach E. Myoepithelial cells in carcinoma of human breast. Am J Pathol. 1966;49:301–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Puchtler H, Leblond CP. Histochemical analysis of cell membranes and associated structures as seen in the intestinal epithelium. Am J Anat. 1958;102:1–31. doi: 10.1002/aja.1001020102. [DOI] [PubMed] [Google Scholar]
- 39.Leblond CP, Puchtler H, Clermont Y. Structures corresponding to terminal bars and terminal web in many types of cells. Nature. 1960;186:784–8. doi: 10.1038/186784a0. [DOI] [PubMed] [Google Scholar]
- 40.Auger DW, Harrison JD. Ultrastructural phosphatase cytochemistry of the intercalary ducts of the parotid and submandibular salivary glands of man. Arch Oral Biol. 1982;27:79–81. doi: 10.1016/0003-9969(82)90181-9. [DOI] [PubMed] [Google Scholar]
- 41.Cutler LS, Chaudhry A, Innes DJ., Jr Ultrastructure of the parotid duct. Cytochemical studies of the striated duct and papillary cystadenoma lymphomatosum of the human parotid gland. Arch Pathol Lab Med. 1977;101:420–4. [PubMed] [Google Scholar]
- 42.Garrett JR, Harrison JD. Alkaline-phosphatase and adenosine-triphosphatase histochemical reactions in the salivary glands of cat, dog and man, with particular reference to the myoepithelial cells. Histochemie. 1971;24:214–29. doi: 10.1007/BF00304191. [DOI] [PubMed] [Google Scholar]
- 43.Harrison JD. Cystic adenoma of a minor salivary gland: A histochemical study. J Pathol. 1973;114:29–38. doi: 10.1002/path.1711140107. [DOI] [PubMed] [Google Scholar]
- 44.Harrison JD. Minor salivary glands of man: Enzyme and mucosubstance histochemical studies. Histochem J. 1974;6:633–47. doi: 10.1007/BF01011504. [DOI] [PubMed] [Google Scholar]
- 45.Innes DJ, Jr, Cutler LS. Phosphatase enzymes. Cytochemical study of pleomorphic adenoma and normal human salivary glands. Arch Pathol Lab Med. 1978;102:90–4. [PubMed] [Google Scholar]
- 46.Ogawa Y. Immunocytochemistry of myoepithelial cells in the salivary glands. Prog Histochem Cytochem. 2003;38:343–426. doi: 10.1016/s0079-6336(03)80001-3. [DOI] [PubMed] [Google Scholar]
- 47.Leoncini P, Cintorino M, Vindigni C, Leoncini L, Armellini D, Bugnoli M, et al. Distribution of cytoskeletal and contractile proteins in normal and tumour bearing salivary and lacrimal glands. Virchows Arch A Pathol Anat Histopathol. 1988;412:329–37. doi: 10.1007/BF00750259. [DOI] [PubMed] [Google Scholar]
- 48.Longtine JA, Pinkus GS, Fujiwara K, Corson JM. Immunohistochemical localization of smooth muscle myosin in normal human tissues. J Histochem Cytochem. 1985;33:179–84. doi: 10.1177/33.3.3882826. [DOI] [PubMed] [Google Scholar]
- 49.Savera AT, Gown AM, Zarbo RJ. Immunolocalization of three novel smooth muscle-specific proteins in salivary gland pleomorphic adenoma: Assessment of the morphogenetic role of myoepithelium. Mod Pathol. 1997;10:1093–100. [PubMed] [Google Scholar]
- 50.Bilal H, Handra-Luca A, Bertrand JC, Fouret PJ. P63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem. 2003;51:133–9. doi: 10.1177/002215540305100201. [DOI] [PubMed] [Google Scholar]
- 51.Moritani S, Kushima R, Sugihara H, Bamba M, Kobayashi TK, Hattori T. Availability of CD10 immunohistochemistry as a marker of breast myoepithelial cells on paraffin sections. Mod Pathol. 2002;15:397–405. doi: 10.1038/modpathol.3880536. [DOI] [PubMed] [Google Scholar]
- 52.Savera A, Huvos A, Klimstra D. Comparative analysis of the purported immunohistochemical “myoepithelial markers”. Mod Pathol. 1999;12:130A. [Google Scholar]
- 53.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: A clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–74. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Dairkee SH, Blayney C, Smith HS, Hackett AJ. Monoclonal antibody that defines human myoepithelium. Proc Natl Acad Sci U S A. 1985;82:7409–13. doi: 10.1073/pnas.82.21.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prasad AR, Savera AT, Gown AM, Zarbo RJ. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801–6. doi: 10.5858/1999-123-0801-TMIIBA. [DOI] [PubMed] [Google Scholar]
- 56.Carmichael JD, Winder SJ, Walsh MP, Kargacin GJ. Calponin and smooth muscle regulation. Can J Physiol Pharmacol. 1994;72:1415–9. doi: 10.1139/y94-204. [DOI] [PubMed] [Google Scholar]
- 57.Reis-Filho JS, Milanezi F, Silva P, Schmitt FC. Maspin expression in myoepithelial tumors of the breast. Pathol Res Pract. 2001;197:817–21. doi: 10.1078/0344-0338-00165. [DOI] [PubMed] [Google Scholar]
- 58.Nagashima Y, Ono K. Myoepithelial cell ultrastructure in the submandibular gland of man. Anat Embryol (Berl) 1985;171:259–65. doi: 10.1007/BF00347014. [DOI] [PubMed] [Google Scholar]
- 59.Takeda Y. Histopathological studies of the labial salivary glands in patients with Sjogrens syndrome. Part II. Electron microscopic study. Bull Tokyo Med Dent Univ. 1980;27:27–42. [PubMed] [Google Scholar]
- 60.Dardick I, van Nostrand AW. Morphogenesis of salivary gland tumors. A prerequisite to improving classification. Pathol Annu. 1987;22(Pt 1):1–53. [PubMed] [Google Scholar]
- 61.Dardick I. In: Histogenesis and morphogenesis of salivary gland neoplasms. Surgical Pathology of the Salivary Glands. Ellis GL, Auclair PL, Gnepp DR, editors. Philadelphia: W.B. Saunders; 1991. p. 108. [Google Scholar]
- 62.Dardick I, Burford-Mason AP. Current status of histogenetic and morphogenetic concepts of salivary gland tumorigenesis. Crit Rev Oral Biol Med. 1993;4:639–77. doi: 10.1177/10454411930040050201. [DOI] [PubMed] [Google Scholar]
- 63.Eversole LR. Histogenic classification of salivary tumors. Arch Pathol. 1971;92:433–43. [PubMed] [Google Scholar]
- 64.Ellis GL, Auclair PL, Gnepp DR, editors. Surgical Pathology of the Salivary Glands. Major Problems in Pathology in the Series. 1 ed. Vol. 25. Philadelphia: W.B. Saunders Co; 1991. [Google Scholar]
- 65.Zarbo RJ. Salivary gland neoplasia: A review for the practicing pathologist. Mod Pathol. 2002;15:298–323. doi: 10.1038/modpathol.3880525. [DOI] [PubMed] [Google Scholar]
- 66.Batsakis JG, el-Naggar AK. Myoepithelium in salivary and mammary neoplasms is host-friendly. Adv Anat Pathol. 1999;6:218–26. [PubMed] [Google Scholar]
- 67.Redder CP, Kandagal VS, Vibhute N, Ingaleshwar PS, Shetty SJ, Ahamad S. Myoepithelial cells: Current perspectives insalivary gland tumors. Clin Cancer Invest J. 2013;2:101–5. [Google Scholar]
- 68.Ponce Bravo S, Ledesma Montes C, López Becerril U, Morales Sánchez I. Myoepithelial cells are the main component in pleomorphic adenomas? Med Oral Patol Oral Cir Bucal. 2007;12:E110–5. [PubMed] [Google Scholar]
- 69.Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: Report of 23 cases. Cancer. 1982;49:562–72. doi: 10.1002/1097-0142(19820201)49:3<562::aid-cncr2820490328>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 70.El-Mofty SK, O’Leary TR, Swanson PE. Malignant myoepithelioma od Salivary glands: Clinicopathologic and immunophenotypic features. Review of literature and report of two cases. Int J Surg Pathol. 1994;2:133–40. [Google Scholar]
- 71.Zarbo RJ, Prasad AR, Regezi JA, Gown AM, Savera AT. Salivary gland basal cell and canalicular adenomas: Immunohistochemical demonstration of myoepithelial cell participation and morphogenetic considerations. Arch Pathol Lab Med. 2000;124:401–5. doi: 10.5858/2000-124-0401-SGBCAC. [DOI] [PubMed] [Google Scholar]


