Abstract
Ameloblastoma is the second most common benign epithelial odontogenic tumor and though it is of a benign nature, it is locally invasive, has a high recurrence rate and could potentially become malignant. Many theories have been proposed to explain the pathogenesis of ameloblastoma. Proper understanding of the pathogenic mechanism involved in ameloblastoma and its proliferation aids in constituting proper treatment of choice at an early stage, preventing morbidity associated with extensive therapy. An attempt has been made to discuss the current concepts related to molecular and genetic changes that occur in ameloblastoma as these could affect treatment plan and prognosis.
Keywords: Ameloblastoma, odontogenic tumor, molecular pathogenesis
INTRODUCTION
Ameloblastoma is a benign but a locally invasive odontogenic neoplasm arising from the odontogenic epithelium. They account for about 1% of all oral tumors and about 18% of odontogenic tumors. It is primarily seen in adults in the third to fifth decades of life, with almost equal sex predilection.[1] The tumor is more common in the mandible than in the maxilla and shows a predilection for various parts of the mandible. Clinically, it often presents as a slow growing, painless swelling causing expansion of the cortical bone.[2] Radiographically, it appears as an expansile radiolucency with thinned and perforated cortices, frequently causing root resorption. Large tumors can break through the cortex and involve the soft tissues.[1] There are many histological variants of ameloblastoma, but the most common ones are follicular (32.5%), plexiform (28.2%), acanthomatous (12.1%), granular cell (5%), basal cell (2.02%) and desmoplastic (4–13%) type. Based on the site and type of presentation, the ameloblastomas can be classified as solid/multicystic type, extraosseous/peripheral type and unicystic type.[3] The most common and aggressive is the solid/multicystic type whereas less common and less aggressive are the unicystic and peripheral ameloblastomas.[4]
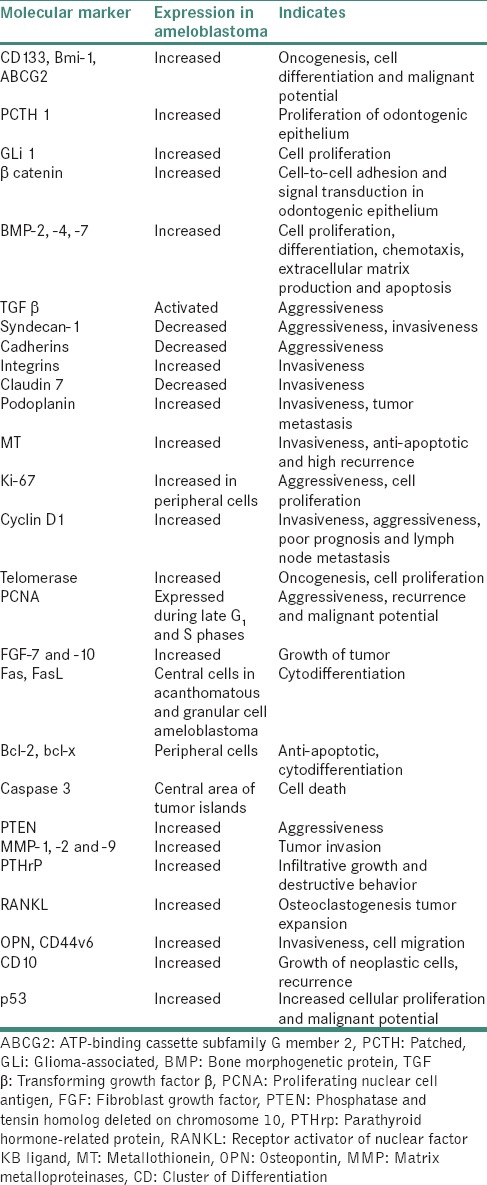
These tumors have high chances of local recurrence and due to its slow growing nature, often there is a delay in diagnosis by the dentists. Numerous studies have been found to explain the molecular pathogenesis of ameloblastoma. Every cellular change, including proliferation, differentiation and tumorigenesis occurs through the activation or inactivation of the related molecular signaling pathways. The important signaling molecules are either overexpressed or underexpressed during tumorigenesis of ameloblastomas.[5] Ameloblastoma histologically resembles the epithelial odontogenic apparatus, such as enamel organ and dental lamina; however, the detailed mechanism of oncogenesis, cytodifferentiation and tumor progression remains unknown. The molecular and genetic alterations that occur in ameloblastoma would be discussed in this review which is helpful for better treatment and prognosis. Table 1 illustrates molecular markers expressed in ameloblastoma.
Table 1.
Molecular markers expressed in ameloblastoma
IMMUNOEXPRESSION OF CANCER STEM CELLS IN AMELOBLASTOMA
It has been hypothesized that tumors are more likely to be initiated in normal stem cells or their immediate descendants and then are perpetuated by a minority of these cells known as cancer stem cells (CSCs).[6,7] Kumamoto et al. investigated the role of the expression of stem cell-related molecules in oncogenesis and cytodifferentiation of odontogenic tumors, expression of CD 133, Bmi-1 and ATP-binding cassette subfamily G member 2 (ABCG2) in ameloblastic tumors and tooth germs. CD 133, prominin-1, a product of single copy gene on chromosome 4 (4p 15.33) in humans has been found to have an important role in cell growth, development and tumor biology. It is expressed in the differentiated epithelia of various organs, and lack of expression of this marker could initiate tumors, mainly brain tumors. Another marker known as polycomb complex protein Bmi-1 or polycomb group RING finger protein 4 is a protein in humans encoded by Bmi-1 gene. This complex is an epigenetic repressor of multiple regulatory genes involved in self-renewal in somatic stem cells. It interacts with several signaling pathways such as Wnt, notch, hedgehog (Hh) and Akt and has an important role in DNA damage repair. Aberrant expression of this complex leads to many cancers. Positive expression of CD 133 and Bmi-1 was observed in odontogenic epithelial cells, ameloblastomas and metastasizing ameloblastomas. Ameloblastic carcinomas also showed reactivity for CD 133 and Bmi-1. When compared to tooth germs and ameloblastomas, malignant ameloblastic tumors. showed increased expression of CD 133. The study proved that stem cell-related molecules have a role in oncogenesis, cell differentiation and malignant potential of odontogenic epithelium.[8]
In a study by Sathi et al., two distinct cell types were observed such as peripheral columnar epithelium and central stellate reticulum-like cells. Peripheral cells were situated at invasive front, and they were positive for cancer stem cells (CSC) markers and cell proliferation marker Ki-67. Therefore, in these cells, it is possible that CSC markers only maintain cellular proliferation and tumor progression. In contrast, few CSC marker-positive central stellate reticulum cells situated at the close vicinity of the peripheral cells were devoid of Ki-67 expression. These cells may have the potential to be cancer stem-like cells. In accordance with this hypothesis, stellate reticula-like cells could change their morphology and differentiate into different cellular patterns such as granular, squamous and acanthomatous types.[7]
SIGNALING PATHWAYS
Various signaling pathways have been identified to explain the pathogenesis of ameloblastoma. Sonic Hedgehog (SHH), a mammalian homolog of drosophila segment polarity gene Hedgehog, encodes a secreted protein that activates a membrane receptor complex formed by patched 1 (PCTH 1) and smoothened (SMO).[9,10,11,12] In the absence of SHH, PCTH inhibits SMO. When PCTH-SHH binding occurs, SMO is released, glioma-associated (GLi 1) family transcription factor gene is activated and SHH signaling is mediated from cytoplasm to nucleas. High expression of SHH, SMO and GLi 1 was reported in ameloblastoma.[9,10] The benign and metastasizing types of ameloblastoma showed stronger PCTH 1 expression in neoplastic cells than stromal cells. Studies suggested that SHH signaling molecules may play a role in epithelial-mesenchymal interaction and cell proliferation in ameloblastoma.[13]
WNT genes encode a family of 38–43 kDa glycoproteins, first identified in mammals as proto-oncogenic integration site for mouse mammary tumor virus. These proteins activate a number of signaling pathways that are divided into two categories: Canonical β-catenin pathway and noncanonical β-catenin independent pathway. WNT 5a signaling was found to have a crucial role in modulating tumorigenesis and behaviors of enamel epithelial cells in ameloblastoma. Overexpression of WNT 5a increases enamel epithelial cell migration while suppression impairs their migration and fails to form actin re-organization. Canonical WNT pathway is associated with the accumulation and translocation of adherens junction-associated protein β catenin into nucleus. Therefore, the main action is stabilization of β catenin and its translocation into nucleus, where it exerts its effect on gene transcription. This mechanism was demonstrated in ameloblastomas.[14,15,16]
Bone morphogenetic protein (BMP) is a mesenchymal cell differentiation factor and a morphogen. It plays a crucial role in cell proliferation, differentiation, chemotaxis, extracellular matrix (ECM) production and apoptosis during the development process.[17,18] Kumamoto examined tooth germs, ameloblastomas, malignant ameloblastomas and adenomatoid odontogenic tumors by reverse transcription polymerase chain reaction and immunohistochemistry for BMP-2, -4, -7, BMP receptors I and II (BMPR I and II), core-binding factor alpha 1 (CBFA 1) and osterix. They found that mRNA expression of BMPs, BMPRs, CBFA 1 and osterix was detected in all odontogenic tissues. BMPs and BMPRs was evidently expressed in odontogenic epithelial cells in tooth germs and epithelial odontogenic tumors. Acanthomatous ameloblastomas showed an increased BMP-7 reactivity in keratinizing cells. Ameloblastic carcinomas showed low reactivity for BMPs, BMPRs and CBFA1.[18]
SMAD proteins are classified into different groups based on their function to regulate transforming growth factor β (TGF β) pathway or BMP pathway. SMAD 2/3 mainly mediates TGF β pathway. It has been investigated that SMAD 2 and 4 function as tumor suppressors and their deletions or mutations lead to tumor progression. In contrast, SMAD 3 plays a role in TGF β-induced inflammation and its mutation or deletion is infrequent. The TGF β/SMAD signaling pathway has an important role in the invasiveness of ameloblastoma, especially in later stages. In early stages, TGF β inhibits not only the growth, but also the invasion of the tumor, but in later stages, it promotes invasion of the tumor. Various mechanisms have been proposed such as loss of inhibitory response to TGF β, activation of ligand and reduced apoptosis of tumor, but the exact mechanism is still unclear, for which further research is mandatory.[19,20]
Expression of enamel matrix proteins
Expression of ameloblastin, anamelin and sheathlin protein was not found in ameloblastoma, suggesting that ameloblasts have not attained functional maturation in tumor cells.[21] In contrast, Snead et al. found that amelogenin is transcribed only by differentiated ameloblasts, which was expressed by amelostastic epithelial cells.[22] It was found that presecretory ameloblasts in inner enamel epithelium express calretinin during odontogenesis. This protein plays a role in the transition of dental lamina remnants to ameloblastoma.[23]
Molecular markers involved in cell adhesion and migration
Syndecan-1 also known as transmembrane heparan sulfate proteoglycan, CD 138, regulates many biological processes: Cytoskeletal organization, growth factor signaling, cell-to-cell signaling and ECM attachment. It is expressed by tumor epithelial cells, and loss of Syndecan-1 indicates unfavorable prognosis in epithelial tumors. The decreased expression of Syndecan-1 was seen in ameloblastomas which could attribute to its aggressive behavior.[24] Bologna-Molina et al. found reduced expression of syndecan-1 in solid ameloblastoma when compared to unicystic ameloblastoma.[25]
Cadherins, keratin 7 (KRT 7) and Notch are cell adhesion molecules that are expressed on cell membranes in adherens junctions and have the ability to communicate with different intercellular controls.[26] As E-cadherin is an important regulator of cell adhesion with KRT 7 and Notch, loss of these genes could be associated with tumor progression in ameloblastomas. It was found that poorly differentiated tumors showed reduced expression of E-cadherin.[27]
Integrins are transmembrane receptor proteins that bind to cell surfaces and ECM ligands, where they participate in anchorage to ECM proteins and in the modulation of multiple molecules involved in growth, adhesion, migration, proliferation, apoptosis and cell proliferation.[27] Integrin α5 β 1 is the classic receptor for fibronectin, a protein that plays an important role in epithelial mesenchymal interactions in odontogenic tumors. High amount of α5 β 1 integrin was detected in ameloblastoma, thus contributing to its invasiveness by binding to fibronectin, increasing the secretion of metalloproteinases. Modolo et al. observed strong expression of the α1, α2, α3, α5, αv, β1, β3 and β 4 integrins in follicular, acanthomatous and plexiform form solid/multicystic ameloblastoma, as well as in luminal unicystic ameloblastoma. It was observed that a decrease in integrin expression is related to tumor growth and invasion of neighboring structures.[28]
Claudins are a family of proteins that participate in tight junctions; they are present in epithelial and endothelial cells and also participate in embryogenesis and organogenesis, mainly in epithelial-mesenchymal interaction. Bello et al. found in ameloblastoma an intense immunoreactivity of claudin 1, 4 and 7, principally in stellate reticulum-like cells. This increased expression indicated efforts of this protein to maintain cell-to-cell adhesion. Claudin 1 expression is seen in the regions of squamous differentiation of ameloblastoma. Decreased expression of claudin 7 is associated with invasive behavior of carcinomas.[29]
González-Alva et al. suggested the role of podoplanin, a type 1 transmembrane sialo mucin-like glycoprotein composed of 162 amino acids, which is a lymphocyte-specific marker, reported to be associated with tumor-induced platelet aggregation, tumor metastasis and tumor invasiveness.[30] Metallothionein (MT) comprises a group of low molecular weight cysteine-rich intracellular proteins which can bind to both zinc and copper. It is a negative regulator of apoptosis, its overexpression promotes cell growth in some tumors. Ribeiro et al. studied the expression of MT in ameloblastoma and found its higher expression, suggesting its role as a reservoir for zinc, promitotic and anti-apoptotic features in tumor.[31]
Molecular markers involved in cell proliferation
P 16 (cyclin-dependent kinase inhibitor) is a tumor suppressor protein encoded by a CDKN2A gene, which acts as a negative regulator of proliferation of normal cells. Various studies have suggested that methylation of CDKN2A gene functions as a major mechanism of tumorigenesis in many odontogenic tumors including ameloblastoma. Suzuki et al. found the highest expression of p16 in follicular ameloblastoma followed by acanthomatous and the minimum reactivity was recorded in plexiform type. Furthermore, correlation between p16 expression and rate of recurrence was also established such as, in the plexiform ameloblastoma, the rate of recurrence was lowest and the expression of p16 would be lowest.[32]
Ki-67 is a nonhistone nuclear protein and is considered to be the most reliable marker of cellular proliferation. It is present at all cellular phases of cell cycle except at resting phase G0. Ki-67-positive nuclei in ameloblastoma are mainly located in peripheral ameloblast-like cells in the follicular as well as in the plexiform areas of solid ameloblastoma and in the basal cells of unicystic ameloblastoma. Ki-67 labeling index was high in the tumor related to the recurrence of ameloblastomas.[33] Florescu et al. in their study observed an increased expression of Ki-67 in peripheral cells of tumor islands when compared to central cells, suggesting that peripheral cells are more proliferative.[34]
Cyclin D1, a member of G1 cyclins, controls the cell cycle from G1 to S phase. The dysregulation and overexpression of cyclin D1 has been correlated with rapid growth and proliferative activity, histologic aggressiveness, tumor invasiveness and poor prognosis. Ameloblastoma exhibits diverse histologic patterns, and the overexpression of cyclin D1 is found in some plexiform ameloblastomas. The peripheral columnar and central stellate reticulum-like cells of ameloblastomas exhibit a immunoreaction of cyclin D1 which is not seen in squamous and granular cells of ameloblastomas.[35]
Telomerase is a DNA polymerase that synthesizes telomeric DNA which compensates for its loss with each cell division, thereby stabilizing chromosomal structure. Telomerase reverse transcriptase (TERT) is a catalytic subunit of telomerase whose expression is correlated with telomerase activity. Kumamoto in his study estimated telomerase activity by the telomeric repeat amplification protocol assay and examined the immunohistochemical expression of TERT and c-myc protein in 21 ameloblastoma tissues. All ameloblastoma samples were positive for telomerase activity and TERT expression was detected in the nuclei of neoplastic cells but not in those of stromal cells. Numerous peripheral columnar or cuboidal cells, sporadic central polyhedral cells and some granular cells in ameloblastomas reacted with anti-TERT antibody. The results suggested that telomerase activity is associated with the oncogenesis or proliferative potential of odontogenic epithelium. The expression of c-myc protein showed a similar distribution pattern to that of TERT, suggesting that c-myc protein might induce telomerase activity in ameloblastomas.[36] In another study, it was reported that high expression of human telomerase RNA and h TERT was closely related to the clinical behavior of ameloblastoma and regulated by p53.[37]
In some studies, the proliferative activity was assessed by proliferating nuclear cell antigen (PCNA) labeling. It is 36 kD nuclear protein and a polymerase expressed during late G1 and S phases. It is a good marker of aggressiveness, recurrence and malignant potential of ameloblastoma. Maya et al. in their study found highest PCNA proliferative index for plexiform ameloblastoma[38] whereas Salehinejad et al. found the highest mean index of positivity for PCNA in acanthomatous.[39]
Molecular markers involved in tumor growth and angiogenesis
Growth factor and their receptors also play a important role in tumor development and progression. Myoken et al. developed a serum-free culture system for ameloblastoma cells and added fibroblast growth factor (FGF) 1 and FGF2. Ameloblastoma-like cells and stellate reticulum-like cells presented high expression of FGF1 whereas FGF2 was identified in basement membrane. Hence, FGF 1 has autocrine mechanism of tumor growth and FGF 2 has a role in tumor growth and also in invasion through induction of proteases.[40] In another study, Vered et al. evaluated the role of epidermal growth factor receptor (EGFR) level in ameloblastomas as they were considered to promote tumorigenesis. Anti-EGFR agents were found to reduce the size of tumors.[41] The expression of FGF1, FGF2, FGFR2 and FGFR3 in ameloblastoma has been previously reported, but in a study by Nakao et al., it was found that FGF7 and FGF10 are also expressed in ameloblastomas and have a role in the growth of ameloblastomas through MAPK pathway.[42]
Angiogenesis is a fundamental event in the process of metastatic dissemination. Vascular endothelial growth factor (VEGF) is one of the key regulators of this process. Activation of VEGF receptor pathway triggers a series of processes that promote endothelial cell growth, migration and survival from pre-existing vasculature. It also mediates vessel permeability and has been associated with tumor angiogenesis. VEGF was assessed by anti-CD-34 antibody in benign and malignant ameloblastomas to clarify its role in the angiogenesis of odontogenic tumors. It was found that VEGF expression was low in keratinizing cells in acanthomatous ameloblastomas and granular cells in granular cell ameloblastomas and acanthomatous ameloblastomas showed the lowest VEGF reactivity among the subtypes of ameloblastomas.[43] Platelet-derived growth factor (PDGF) and its receptor (PDGF-α) levels were more in malignant ameloblastomas.[44]
Midkine is a heparin-binding growth factor expressed during tooth development. This protein is usually overexpressed in ameloblastomas and ameloblastic carcinomas as ameloblastomas arise from odontogenic apparatus. This protein gave growth advantage to ameloblasts through upregulation of the MAP kinase (MAPK) and Protein kinase B (Akt) pathways and midkine expression in majority of ameloblastomas, especially solid/multicystic lesions may suggest a role of the protein in development, progression and behavior of the tumor.[4]
Apoptotic markers
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor are diffusely expressed in ameloblastomas and are possibly involved in the neoplastic transformation of odontogenic epithelium. Caspase 3, an enzyme in the apoptosis inducing protease, is associated with cell death. FAS, FAS ligand and caspase 3 are expressed in ameloblastomas.[45] Bcl-2 and bax are regarded as the most important apoptotic regulators. The excess of Bcl-2 homodimers favors cell survival and excess of bax favors cell death. Bcl-2 was found mainly in the peripheral basal cell layer of ameloblastoma. Survivin, member of IAP family of antiapoptotic proteins, inhibits apoptosis but also promotes cell proliferation in malignant tumors. It is expressed during G2/M phase of cell cycle and can be detected in nuclei of tumor cells, which may indicate a poor prognosis in several malignant tumors.[46]
Tumor suppressor genes
p53 is situated on chromosome 17 p13 and is one of the frequently altered genes in the tumors. Mutations and loss of heterozygosity of p53 gene have been associated with increased cellular proliferation and malignant potential. Kumamoto et al. observed that expression of p53 was higher in plexiform cases than in follicular cases. The results suggested that alteration of p53 cascade leads to oncogenesis or malignant transformation of odontogenic epithelium.[47] Hypermethylation of p16 tumor suppressor gene was observed in ameloblastoma and ameloblastic carcinomas.[48]
The PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor is a PI (phosphoinositide) 3-phosphatase that can inhibit cellular proliferation, survival and growth by inactivating PI 3-kinase-dependent signaling. Recently, in a study by Scheper et al., allelic loss of PTEN was shown to occur in ameloblastomas. In carcinogenesis, loss of PTEN allows for overactivity of the phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) pathway inducing an upregulation of mammalian target of rapamycin (mTOR) and its downstream effector ribosomal subunit-6 kinase (S6K); allowing for uncontrolled cell proliferation, apoptosis inhibition and cell cycle deregulation. Aberrant signaling in the PI3K/AKT/mTOR pathway may be the cause of aggressiveness of ameloblastomas.[49]
Molecular markers involved in extracellular matrix degradation
Many studies have found the role of markers of ECM degradation in the invasion of ameloblastoma, and matrix metalloproteinases (MMPs) are zinc metalloenzymes involved in ECM remodeling. Overexpression of MMP-2 promotes invasion, metastasis and also induction of angiogenesis in the ameloblastoma.[50] Even interaction between MMP and osteonectin/secreted protein acetic and rich in cysteine (SPARC), a major noncollagenous constituent of bovine and human bone, occurs in response to tumor injury, growth and metastasis, contributing to aggressive behavior. Shen et al. studied immunohistochemical expression of osteonectin/SPARC and MMP-1, -2 and -9 in 23 cases of ameloblastoma to regulate tumor invasion. MMP2 and -9 degrade type IV collagen in basement membrane.[51] Heparanase is an endoglycosidase enzyme, cleaves heparan sulfate and plays an important role in ECM remodeling. It mainly contributes toward tumor invasiveness and metastasis. Nagatsuka et al. in their study found that the increased levels of heparanase contributes toward local invasiveness of ameloblastoma.[52]
Molecular markers involved in bone remodeling
Number of cytokines such as interleukin 1α, interleukin 1 β, interleukin 6 and TNF α possess osteolystic activity and they are been implicated in the growth and expansion of the ameloblastoma.[53] Ooya detected the expression of parathyroid hormone-related protein (PTHrP), osteoclast differentiation factor (ODF)/receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoclastogenesis inhibitory factor (OCIF)/osteoprotegerin (OPG) mRNA in all tooth germs and ameloblastoma samples. In ameloblastomas, PTHrP, reactivity in peripheral columnar cells was stronger than central polyhedral cells, and keratinizing cells showed increased PTHrP reactivity. ODF/RANKL and OCIF/OPG were expressed predominantly in mesenchymal cells rather than in odontogenic cells in both tooth germs and ameloblastomas. Epithelial ODF/RANKL and OCIF/OPG expression was slightly lower in ameloblastomas than in tooth germs. Tumor cells in plexiform ameloblastomas showed higher reactivity for PTHrH and ODF/RANK L than tumor cells in follicular ameloblastomas.[54]
Osteopontin (OPN) is a phosphorylated sialic acid-rich noncollagenous bone matrix protein. It has been implicated as a key factor in bone remodeling. In addition, CD 44 v6 expression was associated with migration and generation of metastatic tumors. OPN can trigger integrin-mediated signal transduction which, in turn, leads to osteoclastic activation. In addition, binding of OPN to osteoblastoma tumor cell membrane receptor CD 44 v6 can enhance tumor cell migration, invasion and spread. In ameloblastoma, high OPN expression and CD 44 v6 expression were found in both unicystic and multicystic ameloblastomas.[55]
Twist is a mesoderm determining factor and it is a highly conserved basic helix loop helix transcription protein essential in embryological morphogenesis. Its high level in tumor will promote bone metastasis by bone remodeling and its high expression is found in solid ameloblastoma than unicystic ameloblastoma.[56]
Molecular markers involved with the function of tumor stromal cells
The mean number of myofibroblasts is an important prognostic marker of aggressiveness of ameloblastoma. It is found to be high in solid ameloblastoma as proven in a study by Fregnani et al.[57]
Another recent approach to the mechanism of invasion of ameloblastoma was focused on CD 10, which is cell surface zinc-dependent metalloprotease glycoprotein with endopeptidase activity. CD 10 is associated with differentiation and growth of neoplastic cells. Its expression is found to be increased with the increase in tumor dysplasia. It has been reported that solid ameloblastoma had high immunoreactivity for CD 10 with high chances of recurrence. Hence, CD 10 immunostaining may be useful to identify areas with locally aggressive behavior even in low-risk ameloblastoma.[58]
CONCLUSION
Pathogenesis of ameloblastoma is multifactorial and involves numerous cellular pathways. Diverse type of molecules and gene alterations affect the development and progression of odontogenic epithelium and these characteristics appear to depend on diverse molecular mechanisms. Proper understanding of the pathogenic mechanism involved in ameloblastoma and its proliferation will help in the development of new therapeutic approaches such as molecular-targeted treatment for odontogenic tumors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B:86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 2.Jeddy N, Jeyapradha T, Anathalakshmi R, Jeeva S, Saikrishna P, Lakshmipathy P. The molecular and genetic aspects in the pathogenesis and treatment of ameloblastoma. J Dr NTR Univ Health Sci. 2013;2:157–61. [Google Scholar]
- 3.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classifications of tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC; 2005. [Google Scholar]
- 4.Scheper MA, Duarte EC, Intapa C, Zhang M, Nascimento LM, Almeida TP, et al. Expression of midkine in ameloblastomas and its correlation with clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:497–502. doi: 10.1016/j.oooo.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Lee SK, Kim YS. Current concepts and occurrence of epithelial odontogenic tumours: Ameloblastoma and adenomatoid odontogenic tumour. Korean J Pathol. 2013;147:191–202. doi: 10.4132/KoreanJPathol.2013.47.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Sathi GA, Tamamura R, Tsujigiwa H, Katase N, Lefeuvre M, Siar CH, et al. Analysis of immunoexpression of common cancer stem cell markers in ameloblastoma. Exp Ther Med. 2012;3:397–402. doi: 10.3892/etm.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumamoto H, Ohki K. Detection of CD133, Bmi-1, and ABCG2 in ameloblastic tumors. J Oral Pathol Med. 2010;39:87–93. doi: 10.1111/j.1600-0714.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumamoto H, Ohki K, Ooya K. Expression of Sonic hedgehog (SHH) signaling molecules in ameloblastomas. J Oral Pathol Med. 2004;33:185–90. doi: 10.1111/j.0904-2512.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 10.McMillan R, Matsui W. Molecular pathways: The hedgehog signaling pathway in cancer. Clin Cancer Res. 2012;18:4883–8. doi: 10.1158/1078-0432.CCR-11-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12(20 Pt 1):5924–8. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry Z, Rikani AA, Choudhry AM, Tariq S, Zakaria F, Asghar MW, et al. Sonic hedgehog signalling pathway: A complex network. Ann Neurosci. 2014;21:28–31. doi: 10.5214/ans.0972.7531.210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra P, Panda A, Bandyopadhyay A, Kumar H, Mohiddin G. Sonic hedgehog signalling pathway and ameloblastoma – A review. J Clin Diagn Res. 2015;9:ZE10–3. doi: 10.7860/JCDR/2015/15443.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tompkins K. Molecular mechanisms of cytodifferentiation in mammalian tooth development. Connect Tissue Res. 2006;47:111–8. doi: 10.1080/03008200600727756. [DOI] [PubMed] [Google Scholar]
- 15.Kumamoto H, Ooya K. Immunohistochemical detection of beta-catenin and adenomatous polyposis coli in ameloblastomas. J Oral Pathol Med. 2005;34:401–6. doi: 10.1111/j.1600-0714.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 16.Stolf DP, Karim AC, Banerjee AG. Genetic aspects of ameloblastoma: A brief review. Biotechnol Mol Biol. 2007;5:116–22. [Google Scholar]
- 17.Moghadam HG, Urist MR, Sandor GK, Clokie CM. Successful mandibular reconstruction using a BMP bioimplant. J Craniofac Surg. 2001;12:119–27. doi: 10.1097/00001665-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kumamoto H, Ooya K. Expression of bone morphogenetic proteins and their associated molecules in ameloblastomas and adenomatoid odontogenic tumors. Oral Dis. 2006;12:163–70. doi: 10.1111/j.1601-0825.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim TJ, Lee YS, Kim BK, Lee KY. Ameloblastoma associated with dentinogenic ghost cell tumor: A case report. Korean J Pathol. 2006;40:297–302. [Google Scholar]
- 20.Lee SK, Kim YS. Current concepts and occurrence of epithelial odontogenic tumors: I. Ameloblastoma and adenomatoid odontogenic tumor. Korean J Pathol. 2013;47:197–202. doi: 10.4132/KoreanJPathol.2013.47.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonoda A, Iwamoto T, Nakamura T, Fukumoto E, Yoshizaki K, Yamada A, et al. Critical role of heparin binding domains of ameloblastin for dental epithelium cell adhesion and ameloblastoma proliferation. J Biol Chem. 2009;284:27176–84. doi: 10.1074/jbc.M109.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes CC, Duarte AP, Diniz MG, Gomez RS. Review article: Current concepts of ameloblastoma pathogenesis. J Oral Pathol Med. 2010;39:585–91. doi: 10.1111/j.1600-0714.2010.00908.x. [DOI] [PubMed] [Google Scholar]
- 23.Alaeddini M, Etemad-Moghadam S, Baghaii F. Comparative expression of calretinin in selected odontogenic tumours: A possible relationship to histogenesis. Histopathology. 2008;52:299–304. doi: 10.1111/j.1365-2559.2007.02948.x. [DOI] [PubMed] [Google Scholar]
- 24.Leocata P, Villari D, Fazzari C, Lentini M, Fortunato C, Nicòtina PA. Syndecan-1 and Wingless-type protein-1 in human ameloblastomas. J Oral Pathol Med. 2007;36:394–9. doi: 10.1111/j.1600-0714.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 25.Bologna-Molina R, Mosqueda-Taylor A, Lopez-Corella E, Almeida OP, Carrasco-Daza D, Garcia-Vazquez F, et al. Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol. 2008;44:805–11. doi: 10.1016/j.oraloncology.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s) Curr Opin Cell Biol. 2013;25:39–46. doi: 10.1016/j.ceb.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 27.González-González R, Molina-Frechero N, Damian-Matsumura P, Bologna-Molina R. Molecular markers of cell adhesion in ameloblastomas. An update. Med Oral Patol Oral Cir Buccal. 2014;19:e8–e14. doi: 10.4317/medoral.19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modolo F, Martins MT, Loducca SV, de Araújo VC. Expression of integrin subunits alpha2, alpha3, alpha5, alphav, beta1, beta3 and beta4 in different histological types of ameloblastoma compared with dental germ, dental lamina and adult lining epithelium. Oral Dis. 2004;10:277–82. doi: 10.1111/j.1601-0825.2004.01028.x. [DOI] [PubMed] [Google Scholar]
- 29.Bello IO, Soini Y, Slootweg PJ, Salo T. Claudins 1, 4, 5, 7 and occludin in ameloblastomas and developing human teeth. J Oral Pathol Med. 2007;36:48–54. doi: 10.1111/j.1600-0714.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 30.González-Alva P, Tanaka A, Oku Y, Miyazaki Y, Okamoto E, Fujinami M, et al. Enhanced expression of podoplanin in ameloblastomas. J Oral Pathol Med. 2010;39:103–9. doi: 10.1111/j.1600-0714.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro AL, Nobre RM, Rocha GC, de Souza Lobato IH, de Melo Alves Junior S, Jaeger RG, et al. Expression of metallothionein in ameloblastoma. A regulatory molecule? J Oral Pathol Med. 2011;40:516–9. doi: 10.1111/j.1600-0714.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Sugimura H, Kitayama Y, Uchiyama Y, Masumoto K, Tanaka H, et al. Expression of p16INK4A in ameloblastoma: Immunohistochemical and FISH analysis. Oral Med Pathol. 2010;14:99–105. [Google Scholar]
- 33.Bologna-Molina R, Mosqueda-Taylor A, Paes de Almeida O, Victor Toral R, Gullermo Martines M. Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol. 2008;44:805–11. doi: 10.1016/j.oraloncology.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Florescu A, Simionescu C, Ciurea R, Pitru A. P53, Bcl-2 and Ki67 immunoexpression in follicular solid ameloblastomas. Rom J Morphol Embryol. 2012;53:105–9. [PubMed] [Google Scholar]
- 35.Kumar H, Vandana R, Kumar G. Immunohistochemical expression of cyclin D1 in ameloblastomas and adenomatoid odontogenic tumors. J Oral Maxillofac Pathol. 2011;15:283–7. doi: 10.4103/0973-029X.86685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumamoto H, Kinouchi Y, Ooya K. Telomerase activity and telomerase reverse transcriptase (TERT) expression in ameloblastomas. J Oral Pathol Med. 2001;30:231–6. doi: 10.1034/j.1600-0714.2001.300407.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhong M, Zhang LH, Wang J, Zhang B, Hou L. Expression of telomerase and p53 in ameloblastoma of the jaw. Int Chin J Dent. 2004;4:27–33. [PubMed] [Google Scholar]
- 38.Maya R, Sekar B, Murali S. Comparative evaluation of expression of proliferating cell nuclear antigen in variants of ameloblastoma and ameloblastic carcinoma. Indian J Dent Res. 2012;23:15–9. doi: 10.4103/0970-9290.99031. [DOI] [PubMed] [Google Scholar]
- 39.Salehinejad J, Zare-Mahmoodabadi R, Saghafi S, Jafarian AH, Ghazi N, Rajaei AR, et al. Immunohistochemical detection of p53 and PCNA in ameloblastoma and adenomatoid odontogenic tumor. J Oral Sci. 2011;53:213–7. doi: 10.2334/josnusd.53.213. [DOI] [PubMed] [Google Scholar]
- 40.Myoken Y, Myoken Y, Okamoto T, Sato JD, Takada K. Immunohistochemical localization of fibroblast growth factor-1(FGF-1) and FGF-2 in cultured human ameloblastoma epithelial cells and ameloblastoma tissues. J Oral Pathol Med. 1995;24:387–92. doi: 10.1111/j.1600-0714.1995.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 41.Vered M, Shohat I, Buchner A. Epidermal growth factor receptor expression in ameloblastoma. Oral Oncol. 2002;31:28–34. doi: 10.1016/s1368-8375(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 42.Nakao Y, Mitsuyasu T, Kawano S, Nakamura N, Kanda S, Nakamura S. Fibroblast growth factors 7 and 10 are involved in ameloblastoma proliferation via the mitogen-activated protein kinase pathway. Int J Oncol. 2013;43:1377–84. doi: 10.3892/ijo.2013.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumamoto H, Ohki K, Ooya K. Association between vascular endothelial growth factor (VEGF) expression and tumor angiogenesis in ameloblastomas. J Oral Pathol Med. 2013;71:62–5. doi: 10.1046/j.0904-2512.2001.10061.x. [DOI] [PubMed] [Google Scholar]
- 44.Sulzbacher I, Wick N, Pichlhofer B, Mazal PR. Expression of platelet-derived growth factor-AA and platelet-derived growth factor-alpha receptor in ameloblastomas. J Oral Pathol Med. 2008;37:235–40. doi: 10.1111/j.1600-0714.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 45.Kumamoto H, Kimi K, Ooya K. Immunohistochemical analysis of apoptosis-related factors (Fas, Fas ligand, caspase-3 and single-stranded DNA) in ameloblastomas. J Oral Pathol Med. 2001;30:596–602. doi: 10.1034/j.1600-0714.2001.301004.x. [DOI] [PubMed] [Google Scholar]
- 46.Luo HY, Yu SF, Li TJ. Differential expression of apoptosis-related proteins in various cellular components of ameloblastomas. Int J Oral Maxillofac Surg. 2006;35:750–5. doi: 10.1016/j.ijom.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Kumamoto H, Izutsu T, Ohki K, Takahashi N, Ooya K. p53 gene status and expression of p53, MDM2, and p14 proteins in ameloblastomas. J Oral Pathol Med. 2004;33:292–9. doi: 10.1111/j.0904-2512.2004.00044.x. [DOI] [PubMed] [Google Scholar]
- 48.Khojasteh A, Khodayari A, Rahimi F, Ghaderian MH, Jafarian M, Nayebi A, et al. Hypermethylation of p16 tumor-suppressor gene in ameloblastic carcinoma, ameloblastoma, and dental follicles. J Oral Maxillofac Surg. 2013;71:62–5. doi: 10.1016/j.joms.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Scheper MA, Chaisuparat R, Nikitakis NG, Sauk JJ. Expression and alterations of the PTEN/AKT/mTOR pathway in ameloblastomas. Oral Dis. 2008;14:561–8. doi: 10.1111/j.1601-0825.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 50.Pinheiro JJ, Freitas VM, Moretti AI, Jorge AG, Jaeger RG. Local invasiveness of ameloblastoma. Role played by matrix metalloproteinases and proliferative activity. Histopathology. 2004;45:65–72. doi: 10.1111/j.1365-2559.2004.01902.x. [DOI] [PubMed] [Google Scholar]
- 51.Shen LC, Chen YK, Hsue SS, Shaw SY. Expression of osteonectin/secreted protein acidic and rich in cysteine and matrix metalloproteinases in ameloblastoma. J Oral Pathol Med. 2010;39:242–9. doi: 10.1111/j.1600-0714.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- 52.Nagatsuka H, Han PP, Tsujigiwa H, Siar CH, Gunduz M, Sugahara T, et al. Heparanase gene and protein expression in ameloblastoma: Possible role in local invasion of tumor cells. Oral Oncol. 2005;41:542–8. doi: 10.1016/j.oraloncology.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Pripatnanont P, Song Y, Harris M, Meghji S. In situ hybridisation and immunocytochemical localisation of osteolytic cytokines and adhesion molecules in ameloblastomas. J Oral Pathol Med. 1998;27:496–500. doi: 10.1111/j.1600-0714.1998.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 54.Kumamoto H, Ooya K. Expression of parathyroid hormone-related protein (PTHrP), osteoclast differentiation factor (ODF)/receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoclastogenesis inhibitory factor (OCIF)/osteoprotegerin (OPG) in ameloblastomas. J Oral Pathol Med. 2004;33:46–52. doi: 10.1111/j.1600-0714.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang YP, Liu BY. Expression of osteopontin and its receptors in ameloblastomas. Oral Oncol. 2009;45:538–42. doi: 10.1016/j.oraloncology.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Feng Y, Zhou YM, Hua CG, Tang XF, He DQ. Expression of Twist in different subtype of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:565–70. doi: 10.1016/j.tripleo.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 57.Fregnani ER, Sobral LM, Alves FA, Soares FA, Kowalski LP, Coletta RD. Presence of myofibroblasts and expression of matrix metalloproteinase-2 (MMP-2) in ameloblastomas correlate with rupture of the osseous cortical. Pathol Oncol Res. 2009;15:231–40. doi: 10.1007/s12253-008-9110-4. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Aziz A, Amin MM. EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: Possible role in local recurrence. Diagn Pathol. 2012;7:14. doi: 10.1186/1746-1596-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


