Abstract
Salivary duct carcinoma (SDC) is a high-grade adenocarcinoma arising from the ductal epithelium of salivary glands which rarely involves the minor salivary glands. The varied histological presentation in the minor salivary gland tumors makes them the most heterogeneous group of neoplasms, making the diagnosis a challenge. This report highlights the importance of immunohistochemical markers in the definitive diagnosis of SDC.
Keywords: Minor salivary gland, salivary duct carcinoma, salivary gland tumors
INTRODUCTION
Salivary gland neoplasms are clinically and histomorphologically diverse group of lesions that are uncommon and most often benign. Salivary gland malignancies comprise <1% of all malignancies and about 5% of head and neck cancers.[1] Minor salivary gland tumors are infrequent accounting for about 10–15% of the salivary gland neoplasms and about 80% of these are malignant.[2,3] The multifarious histological types of minor salivary gland tumors make them the most heterogeneous group of neoplasms in the upper aerodigestive tract and the omnipresence of minor salivary glands further complicates their diagnosis and management.[4]
Salivary duct carcinoma (SDC) is a high-grade adenocarcinoma arising from the ductal epithelium of salivary glands, which is clinically characterized by an aggressive behavior with early metastasis, local recurrence and significant mortality.[5,6] Although many tumors originate from the salivary duct system demonstrating ductal structures, the term SDC should be restricted for tumors that histologically resemble ductal carcinomas of the breast.[7] Here, we report a case of SDC of minor salivary gland in a 60-year-old male patient. The role of immunohistochemical markers in the definitive diagnosis of SDC is also highlighted.
CASE REPORT
A 60-year-old male reported with the chief complaint of swelling in the lower anterior jaw region of 15 days duration. Patient related this finding to an episode of extraction which was carried out 2 days before the development of the lesion. The patient confirmed that the swelling was smaller initially and grew progressively to its present dimension of approximately 3 cm × 4 cm associated with a gnawing pain. There was no history of bleeding or pus discharge and mouth opening was within acceptable limits. History of tobacco use suggested that the patient was pan chewer and bidi smoker for over 20 years, with abstinence from these habits only in the last 1 year. Patient's oral hygiene was poor and the submental lymph nodes were palpable. General examination revealed that the patient was well built and nourished with no reported weight loss in the recent past.
The intraoral examination showed an irregular, solitary, sessile growth on the lower anterior alveolar region extending from the mandibular left first premolar region to mandibular right lateral incisor region with the involvement of the lower lingual vestibule [Figure 1]. The swelling was erythematous and tender on palpation with mucosal ulceration and sloughing on its inferoposterior aspect.
Figure 1.
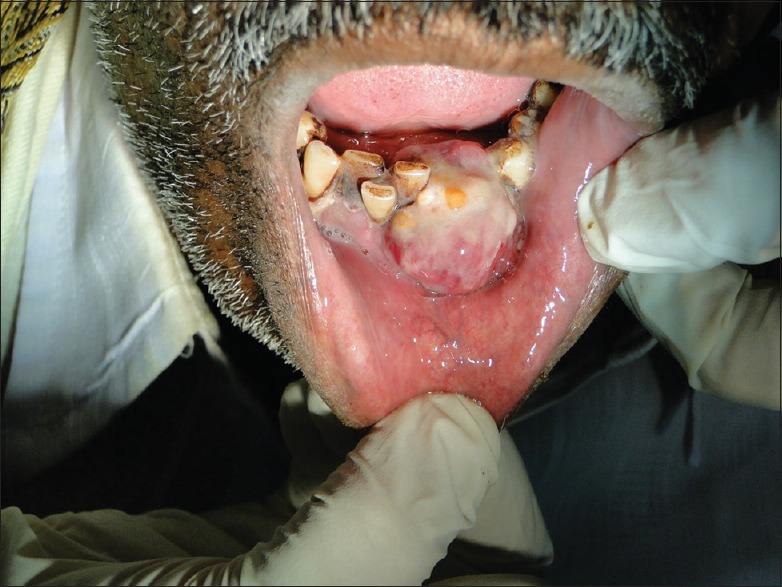
Irregular mass on the lower anterior alveolar region
Intraoral periapical radiograph of the lower anterior region revealed an irregular radiolucency in the symphyseal region with root resorption of the associated teeth. The mandibular occlusal radiograph showed slight expansion of the buccal cortical plate. The digital orthopantomogram suggested a diffuse radiolucent area extending from the roots of mandibular right canine to the left premolar region with resorption in relation to mandibular right lateral incisor [Figure 2]. A provisional diagnosis of intraosseous malignancy was made, and the patient was advised biopsy.
Figure 2.
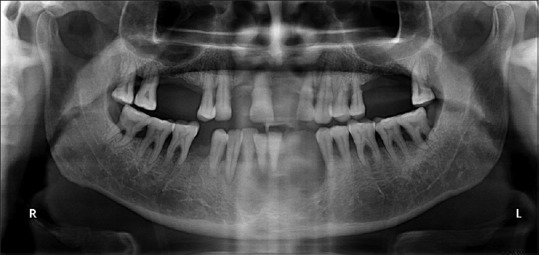
The digital orthopantomogram showing a diffuse radiolucent area in the lower anterior region
The tissue submitted for histological examination was pale white to brown in color, rubbery in texture and firm in consistency. Histopathologically, tumor cells arranged in the form of cords and islands were seen in the connective tissue stroma. Under higher magnification, multiple large ductal structures with cribriform growth pattern were seen [Figure 3]. In addition, ductal epithelium exhibited fenestrations resembling Roman bridge architecture [Figure 4]. Distinct necrosis was seen in the center which resembled comedo-like necrosis [Figure 5]. The invasive component was found to be dense with compressed small tumor islands and cords with cystic changes at places. The individual tumor cells were found to contain moderate amount of granular eosinophilic cytoplasm and large pleomorphic nuclei with coarse chromatin and prominent nucleoli [Figure 6]. The tissue was negative for both periodic acid-Schiff (PAS) and mucicarmine staining. Immunohistochemical staining showed luminal cells of ductal component and tumor cells in infiltrative component to be positive for HER-2/neu [Figure 7] and cytokeratin-7 (CK-7) [Figure 8].
Figure 3.
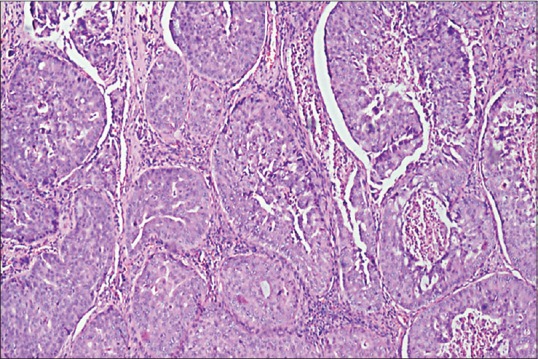
Photomicrograph showing multiple large ductal structures with cribriform-like growth pattern (H&E stain, ×40)
Figure 4.
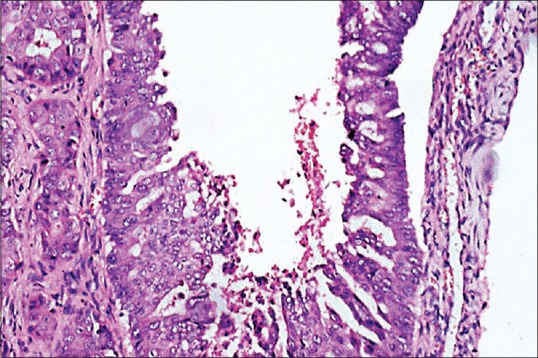
Photomicrograph showing ductal epithelium exhibiting fenestrations resembling Roman bridge architecture (H&E stain, ×200)
Figure 5.
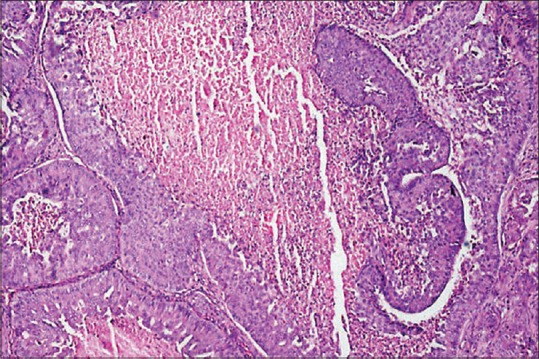
Photomicrograph showing necrosis seen in the center resembling comedo-like necrosis (H&E stain, ×100)
Figure 6.
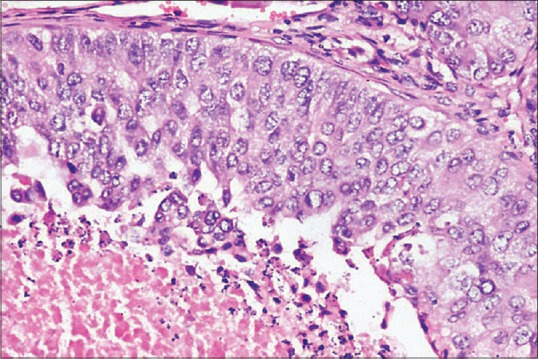
Photomicrograph showing tumor cells with moderate amount of granular eosinophilic cytoplasm and large, pleomorphic nuclei with coarse chromatin and prominent nucleoli (H&E stain, ×400)
Figure 7.
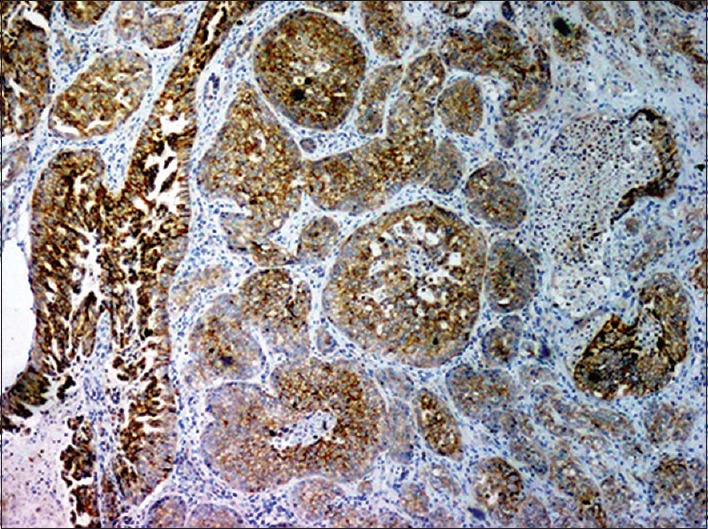
Luminal cells of ductal component and tumor cells in infiltrative component are positive for HER-2/neu (IHC stain, ×40)
Figure 8.
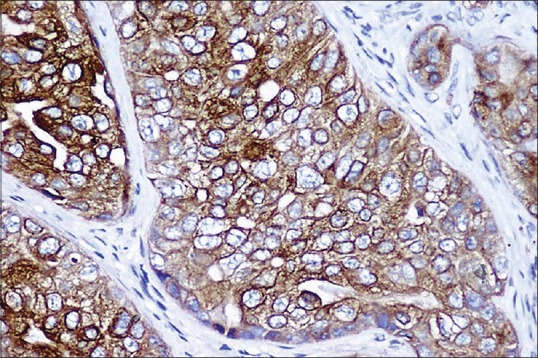
Luminal cells of ductal component and tumor cells in infiltrative component positive for cytokeratin 7 (IHC stain, ×400)
CLINICOPATHOLOGIC INTERPRETATION
The salivary gland tumors showing papillary cystic variantion or cribriform growth patterns such as high-grade mucoepidermoid carcinoma, a papillary cystic variant of acinic cell carcinoma, oncocytic carcinoma and metastatic breast carcinoma were considered in the differential diagnosis. Mucoepidermoid carcinoma usually lacks a cribriform growth pattern, is distinguished by its more variable cell population, including the presence of epidermoid and mucous cells. The mucous cells are demonstrated by PAS and mucicarmine stains. The papillary cystic variant of acinic cell carcinoma may exhibit architectural patterns similar to those of SDC. The cytoplasm of the cells in acinic cell carcinoma contains PAS-positive, diastase-resistant secretory granules. Histopathologically, oncocytic carcinoma comprises large epithelioid cells with round nuclei and greater amount of cytoplasm. A phosphotungstic acid hematoxylin stain will reveal intracytoplasmic granules indicative of mitochondria in oncocytic carcinoma. SDC can be differentiated from high-grade adenocarcinoma not otherwise specified by the characteristic intraductal growth patterns.[7] The characteristic histopathology and strong HER-2/neu expression in our case helped us rule out these malignancies. Metastatic breast carcinoma was ruled out on the basis of detailed clinical evaluation and gender of the patient. Squamous cell carcinoma involving the glands as metastasis or extension from adjacent sites was ruled out based on CK-7 positivity which is considered a marker for glandular origin. On the basis of histopathological and immunohistochemical findings, the final diagnosis of SDC was made.
Subsequent magnetic resonance imaging, bone scan and ultrasonography findings revealed multiple metastatic deposits in the scapula, humerus, pelvic bones and liver. The patient was subsequently lost to follow-up.
DISCUSSION
SDC was recognized as a distinct entity and included in the 2nd edition of the WHO classification of salivary gland tumors in 1991,[8] however, the first description of SDC by Kleinsasser et al. dates back to 1968.[9] According to the 2005 WHO classification, SDC is defined as “an aggressive adenocarcinoma which resembles high-grade breast duct carcinoma.”[10] It has also been termed cribriform salivary carcinoma of excretory ducts and infiltrating SDC.[11,12] The etiology remains unknown, although one case was found in a patient with long-standing chronic obstructive sialadenitis.[13] The parotid gland is the most common site of origin, but occasionally minor salivary glands may also be involved.[5,14] To the best of our knowledge, about 39 cases of SDC arising in the minor salivary glands have been reported, the most common site being the hard palate. In addition, cases have been reported in the posterior mandible arising from the retromolar glands or ectopic salivary glands.[15,16,17] It is a high-grade malignant tumor which has propensity for invasive spread, perineural invasion with early locoregional and distant metastasis.[5,6,11] To confirm the origin of SDC within the jaw (intraosseous), there should be an intact cortical bone with no connection between the oral mucosal surface and the lesion and metastasis from other primary lesions should be ruled out.[17] However, in the present case, although a complete workup ruled out metastasis from other primary lesions, the lesion was seen involving the oral mucosal surface.
Histologically, SDC bears a striking resemblance to ductal carcinoma of the breast with intraductal and invasive components. The intraductal component comprises expanded salivary ducts that lack a definite lobular arrangement with fenestrated (Roman bridge), solid, papillary, cribriform and comedo patterns. The invasive carcinoma consists of irregular glands and cords of cells that frequently elicit a prominent desmoplastic reaction.[6] Common histological variants of SDC are sarcomatoid,[18] mucin-rich[19] and invasive micropapillary[20] A low-grade SDC with predominant intraductal growth pattern has also been described.[21] However, it is recognized as a distinct entity according to the WHO classification 2005 and termed low-grade cribriform cystadenocarcinoma.[22]
SDC exhibits immunoreactivity for CKs, carcinoembryonic antigen, epithelial membrane antigen.[5,11,23] Prostate-specific antigen (PSA) and androgen receptor (AR) have been found to be positive[24] and high AR expression in SDC may play an important role in tumor progression.[25] The strong expression of AR and frequent demonstration of PSA may indicate that though SDC shares a histological similarity to ductal carcinoma of the breast, is rather immunophenotypically similar to prostatic carcinoma and may share a common mechanism of carcinogenesis. In addition, anti-androgen therapy as used in prostatic carcinoma might be beneficial in patients with metastatic SDC when other treatment modalities fail.[24] An association of p53 expression and patient outcome is not clear in SDC.[23,26] Ki-67 labeling index has been found to be high in SDC, thus reflecting an increased cellular activity and aggressive clinical behavior.[23,27] Analysis of p53 and HER-2/neu expression at initial diagnosis may aid in predicting the risk for the development of local recurrence and metastasis.[27]
HER-2/neu gene, also known as c-erbB-2, encodes a transmembranous tyrosine kinase growth factor receptor and its overexpression has been reported in various carcinomas, including breast and salivary gland tumors mostly SDCs.[28,29] However, unlike breast ductal carcinomas, the frequency of positivity for estrogen and progesterone receptors in SDC is considerably less.[30] HER-2/neu gene amplification or overexpression is demonstrated in approximately 15–20% of breast cancers.[29] HER-2/neu overexpression may significantly correlate with the clinical outcome in SDC.[27] Furthermore, this highly malignant tumor might respond to therapy with monoclonal antibody trastuzumab directed against the extracellular domain of the HER-2/neu protein.[31]
The primary tumors of various origin and histologic type may metastasize or invade into the salivary glands, posing significant diagnostic challenge.[32] However, carcinomas largely reserve the CK profile of their epithelium of origin, thus making the differential immunohistochemical staining aid in the appropriate diagnosis.[33] A vast majority of adenocarcinomas including lung, ovary, uterus, breast, salivary gland are positive for CK-7; however, adenocarcinoma of the prostate and colon are rarely positive. CK-7 immunostaining is considered a valuable marker in the diagnostic workup of a carcinoma. The neoplasms of lung, breast, ovary, endometrium, bladder, thymus are positive for CK-7.[34] Most of the salivary gland malignancies including SDC are positive for CK-7.[33] Thus, CK-7 may add valuable information for the differential diagnosis of the primary salivary gland carcinomas and metastatic tumors and also in the diagnosis of metastatic salivary gland malignancies.[32,33]
Minor SDCs are rare and account for very few percentage in salivary gland carcinomas. Histopathologically, SDCs resemble various other tumors of the salivary gland. Immunohistochemical markers can be of value in diagnosing rare salivary gland malignancies including SDCs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8:229–40. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 2.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51–8. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 3.Jansisyanont P, Blanchaert RH, Jr, Ord RA. Intraoral minor salivary gland neoplasm: A single institution experience of 80 cases. Int J Oral Maxillofac Surg. 2002;31:257–61. doi: 10.1054/ijom.2002.0223. [DOI] [PubMed] [Google Scholar]
- 4.Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: Review of 213 cases. J Oral Maxillofac Surg. 2005;63:805–10. doi: 10.1016/j.joms.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Delgado R, Vuitch F, Albores-Saavedra J. Salivary duct carcinoma. Cancer. 1993;72:1503–12. doi: 10.1002/1097-0142(19930901)72:5<1503::aid-cncr2820720503>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Barnes L, Rao U, Krause J, Contis L, Schwartz A, Scalamogna P. Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1994;78:64–73. doi: 10.1016/0030-4220(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 7.Gnepp DR, Henley JD, Simpson RH, Eveson J. In: Salivary and lacrimal glands. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Gnepp DR, editor. Philadelphia: Saunders Elsevier; 2009. pp. 497–503. [Google Scholar]
- 8.Seifert G, Sobin LH. Histological typing of salivary gland tumours. World Health Organization International Histological Classification of Tumours. 2nd ed. New York: Springer-Verlag; 1991. [Google Scholar]
- 9.Kleinsasser O, Klein HJ, Hübner G. Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192:100–5. [PubMed] [Google Scholar]
- 10.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 11.Brandwein MS, Jagirdar J, Patil J, Biller H, Kaneko M. Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts).A clinicopathologic and immunohistochemical study of 12 cases. Cancer. 1990;65:2307–14. doi: 10.1002/1097-0142(19900515)65:10<2307::aid-cncr2820651024>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen KT, Hafez GR. Infiltrating salivary duct carcinoma. A clinicopathologic study of five cases. Arch Otolaryngol. 1981;107:37–9. doi: 10.1001/archotol.1981.00790370039008. [DOI] [PubMed] [Google Scholar]
- 13.Hogg RP, Ayshford C, Watkinson JC. Parotid duct carcinoma arising in bilateral chronic sialadenitis. J Laryngol Otol. 1999;113:686–8. doi: 10.1017/s002221510014486x. [DOI] [PubMed] [Google Scholar]
- 14.Huh KH, Heo MS, Lee SS, Choi SC. Three new cases of salivary duct carcinoma in the palate: A radiologic investigation and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:752–60. doi: 10.1067/moe.2003.246. [DOI] [PubMed] [Google Scholar]
- 15.Gondak RO, Mariano FV, Alves FA, Almeida OP, Lopes MA. Salivary duct carcinoma in minor salivary glands: Report of two cases with different clinical behavior. J Clin Exp Pathol. 2014;4:164. [Google Scholar]
- 16.Suzuki H, Hashimoto K. Salivary duct carcinoma in the mandible: Report of a case with immunohistochemical studies. Br J Oral Maxillofac Surg. 1999;37:67–9. doi: 10.1054/bjom.1998.0285. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi Y, Hirota M, Iwai T, Aoki S, Chikumaru H, Kawabe R, et al. Salivary duct carcinoma in the mandible: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e41–6. doi: 10.1016/j.tripleo.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Henley JD, Seo IS, Dayan D, Gnepp DR. Sarcomatoid salivary duct carcinoma of the parotid gland. Hum Pathol. 2000;31:208–13. doi: 10.1016/s0046-8177(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 19.Simpson RH, Prasad AR, Lewis JE, Skálová A, David L. Mucin-rich variant of salivary duct carcinoma: A clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2003;27:1070–9. doi: 10.1097/00000478-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Nagao T, Gaffey TA, Visscher DW, Kay PA, Minato H, Serizawa H, et al. Invasive micropapillary salivary duct carcinoma: A distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28:319–26. doi: 10.1097/00000478-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–67. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Brandwein-Gensler M, Gnepp D, Skalova A, Nagano T. In: Tumors of the salivary glands. World Health Organization Classification of Tumors. Pathology and Genetics. Head and Neck Tumors. Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Lyon, France: IARC Press; 2005. pp. 233–4. [Google Scholar]
- 23.Martinez-Barba E, Cortes-Guardiola JA, Minguela-Puras A, Torroba-Caron A, Mendez-Trujillo S, Bermejo-Lopez J. Salivary duct carcinoma: Clinicopathological and immunohistochemical studies. J Craniomaxillofac Surg. 1997;25:328–34. doi: 10.1016/s1010-5182(97)80035-2. [DOI] [PubMed] [Google Scholar]
- 24.Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: An immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24:579–86. doi: 10.1097/00000478-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Hoang MP, Callender DL, Sola Gallego JJ, Huang Z, Sneige N, Luna MA, et al. Molecular and biomarker analyses of salivary duct carcinomas: Comparison with mammary duct carcinoma. Int J Oncol. 2001;19:865–71. doi: 10.3892/ijo.19.4.865. [DOI] [PubMed] [Google Scholar]
- 26.Felix A, El-Naggar AK, Press MF, Ordonez NG, Fonseca I, Tucker SL, et al. Prognostic significance of biomarkers (c-erbB-2, p53, proliferating cell nuclear antigen, and DNA content) in salivary duct carcinoma. Hum Pathol. 1996;27:561–6. doi: 10.1016/s0046-8177(96)90162-8. [DOI] [PubMed] [Google Scholar]
- 27.Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Löning T. Clinical and immunohistologic typing of salivary duct carcinoma: A report of 50 cases. Cancer. 2005;103:2526–33. doi: 10.1002/cncr.21116. [DOI] [PubMed] [Google Scholar]
- 28.Skálová A, Stárek I, Kucerová V, Szépe P, Plank L. Salivary duct carcinoma – A highly aggressive salivary gland tumor with HER-2/neu oncoprotein overexpression. Pathol Res Pract. 2001;197:621–6. doi: 10.1078/0344-0338-00136. [DOI] [PubMed] [Google Scholar]
- 29.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 30.Barnes L, Rao U, Contis L, Krause J, Schwartz A, Scalamogna P. Salivary duct carcinoma. Part II. Immunohistochemical evaluation of 13 cases for estrogen and progesterone receptors, cathepsin D, and c-erbB-2 protein. Oral Surg Oral Med Oral Pathol. 1994;78:74–80. doi: 10.1016/0030-4220(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 31.Skálová A, Stárek I, Vanecek T, Kucerová V, Plank L, Szépe P, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology. 2003;42:348–56. doi: 10.1046/j.1365-2559.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 32.Meer S, Altini M. CK7+/CK20- immunoexpression profile is typical of salivary gland neoplasia. Histopathology. 2007;51:26–32. doi: 10.1111/j.1365-2559.2007.02728.x. [DOI] [PubMed] [Google Scholar]
- 33.Nikitakis NG, Tosios KI, Papanikolaou VS, Rivera H, Papanicolaou SI, Ioffe OB. Immunohistochemical expression of cytokeratins 7 and 20 in malignant salivary gland tumors. Mod Pathol. 2004;17:407–15. doi: 10.1038/modpathol.3800064. [DOI] [PubMed] [Google Scholar]
- 34.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol. 2000;13:962–72. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]


