Abstract
The non-Hodgkin's lymphoma (NHLs) is a diverse group of lymphoid neoplasms, prevalence of which increased since three decades. NHL is diverse in the manner of presentation, response to various treatment and prognosis. NHL usually involves not only lymph nodes but also extranodal sites. Usually, oral manifestation of NHL is secondary to the widespread involvement throughout the body. Oral NHL is relatively rare and difficult to diagnose in clinical setting as it presents as local swelling, pain, discomfort and mimics pyogenic granuloma, periodontal disease, osteomyelitis and other malignancies. Sometimes, oral lesion may present as the early disease (primary site). Careful evaluation of patient and proper investigations is required for correct diagnosis so that patient will receive the treatment in early stage which has a good prognosis. Here, we are presenting the case of low-grade B-cell NHL of palate of a 92-year-old man.
Keywords: B-lymphocytes, non-Hodgkin's lymphoma, t-lymphocytes
INTRODUCTION
Malignant lymphoma is the generic term given to tumors of the lymphoid system and these are divided into two major categories: Hodgkin's lymphoma (HL) and non-HL (NHL).[1] NHL usually involves the lymph nodes but can involve extranodal sites and can occur in the stomach, skin, lung, salivary glands and rarely in the mouth.[2] Oral lesions of NHL may develop in the soft tissues or within the jaws centrally. Oral lesions appear as nontender swellings commonly affecting the vestibule, gingiva or posterior hard palate and develops slowly, mimicking a dental abscess of endodontic or periodontal origin.[3] NHL collectively rank fifth above 75 years of age and[4] sixth[5] overall in cancer incidence and mortality among malignant neoplasms. The incidence was increasing at a rate of 3–4% per year before the advent of acquired immunodeficiency syndrome [AIDS] epidemic-associated lymphomas. Most physicians and many oncologists find the disorder arcane.[5]
NHL is a heterogeneous group of malignancies characterized by an abnormal clonal proliferation of T-cells, B-cells or both. The majority of the adult NHLs are of B-cell origin.[6] Recognized since the 1950s as a distinct group of diseases, NHLs range from indolent malignancies (low-grade histologies) to rapidly growing and highly aggressive tumors (high-grade histologies). The overall median age at presentation is 42 years (58 years for low-grade) and the incidence increases with advancing age. The majority of NHLs are of B-cell origin, with more than 90% of patients expressing CD20 antigen. In general, low-grade or follicular NHL is assumed to have an indolent course when compared with intermediate- and high-grade NHL.[7] Present case reports palatal swelling following extraction of 13, 14 and presents as the first manifestation of low-grade B-cell NHL of extranodal site.
CASE REPORT
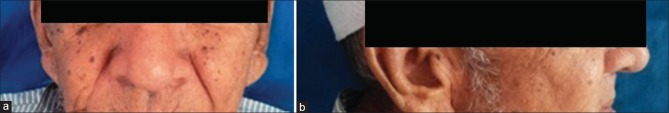
A 92-year-old patient reported to the Dental college and Research Center with a complaint of swelling in the upper right posterior region at recent extraction site and discomfort on eating food since 15 days. Patient had little pain in the swelling. Mild extraoral swelling is seen in the middle 3rd of the face on the right side [Figure 1a and b].
Figure 1.
(a and b) Extraoral photograph showing mild extraoral swelling seen in the middle 3rd of the face on the right side
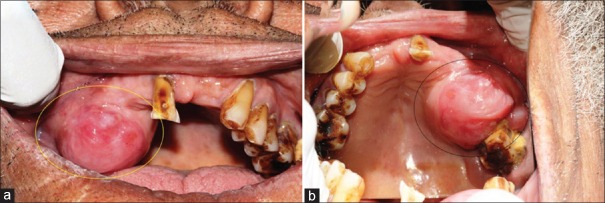
Intraorally single large, round swelling measuring approximately 3 cm × 2 cm × 2 cm [Figure 2a and b] was seen on the right maxillary ridge extending from 13 to 17, buccally extending in the vestibule and palatally for 1 cm (not crossing midline). Overlying mucosa is reddish pink with some bluish tinge. Swelling has sessile base, well-defined borders, reddish pink color and is firm in consistency. Lymph nodes were not palpable. Routine hemogram analysis, urine analysis and X-ray chest are normal. Patient was negative for HIV and hepatitis B virus.
Figure 2.
(a) Intraorally single large, round swelling is seen on the right maxillary ridge extending from 13 to 17, buccally extending in the vestibule and palatally for 1 cm (not crossing midline). (b) Mirror image of intraoral swelling
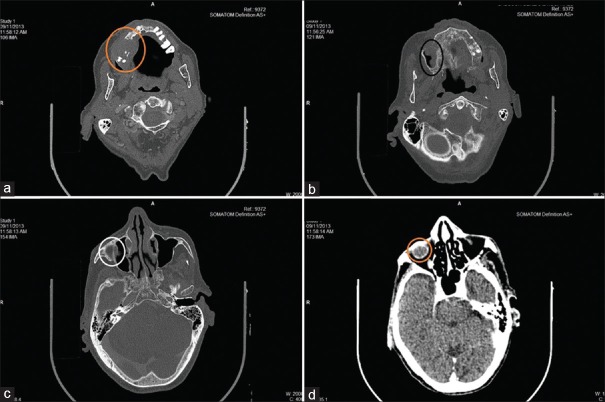
Plain axial and coronal computed tomography (CT) scan of paranasal sinuses revealed 2.8 cm × 1.7 cm × 2.4 cm (ML × AP × CO) sized soft tissue mass lesion involving superior alveolar process of the right maxillary bone involving canine, premolar and 1st molar, causing local destruction. Superiorly, the lesion involved the right maxillary sinus, inferomedially extending into the oral cavity and laterally extending into the right cheek [Figure 3a–d].
Figure 3.
(a) Soft tissue density mass lesion involving a superior alveolar process of the right maxillary bone involving canine, premolar and 1st molar, causing local destruction and laterally extending into the right cheek. (b and c) Superiorly lesion is involving the right maxillary sinus and causing local destruction. (d) Lesion extending till orbital region
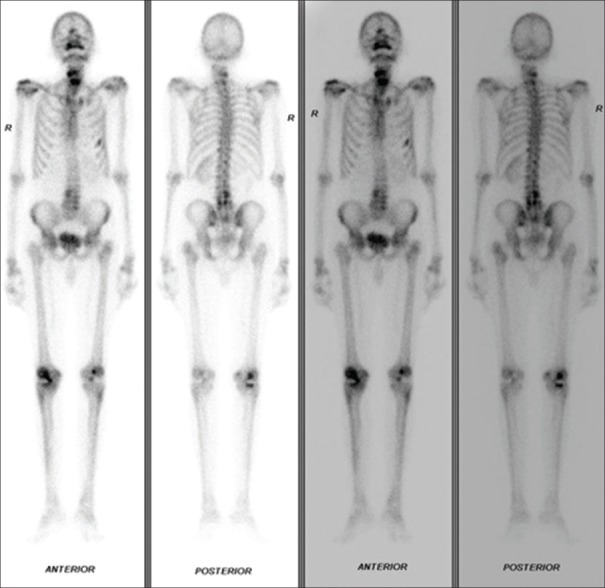
Bone scan revealed preserved tracer uptake by the skeletal system for the patient's age. Single-photon emission CT of cervical and lumbar region acquired increased radiotracer concentration involving –the left 5th and 6th rib and trochanteric region of left femur (could represent metastasis? stress factor?), multiple cervical vertebrae and multiple lumbar vertebrae (could represent degenerative etiology). Degenerative changes are seen involving bilateral shoulder (right > left) and bilateral knees (right > left) [Figure 4].
Figure 4.
Increased radiotracer concentration is seen involving - left 5th and 6th rib and trochanteric region of the left femur, multiple cervical vertebrae and multiple lumbar vertebrae. Degenerative changes are seen involving bilateral shoulder (right > left) and bilateral knees
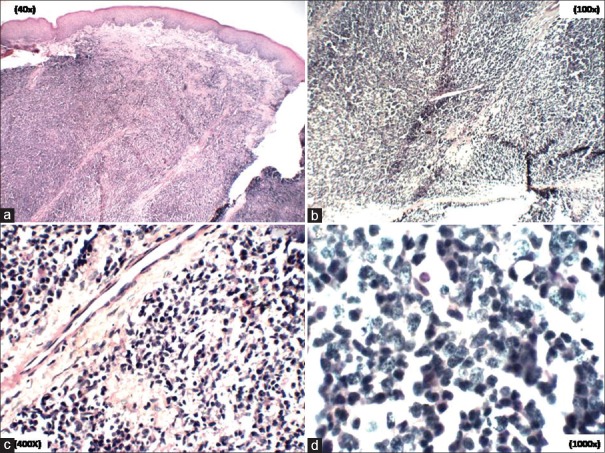
Incisional biopsy revealed squamous mucosa with underlying connective tissue composed of diffuse, uniform monotonous proliferation of medium-sized lymphocytes in loose fibrocellular stroma suggestive of lymphoproliferative disease [Figure 5a–d].
Figure 5.
(a-d) Photomicrographs showing squamous mucosa with underlying connective tissue composed of diffuse, uniform monotonous proliferation of medium-sized lymphocytes in loose fibrocellular stroma [H&E stain, a) ×40, b) ×40, c) ×100, d) 400]
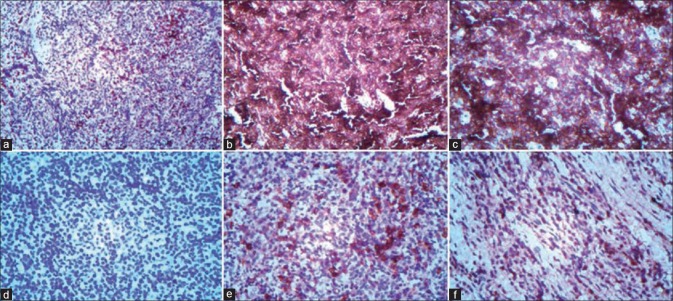
Immunohistochemistry (IHC) was performed. Markers used were CD3, CD20, MB1, CD138 and CD56, which revealed [Figure 6a–f].
Figure 6.
(a) CD3 m (Clone PS1) - Stains reactive T-cells (IHC stain, ×40). (b and c) CD20 (Clone L26) - Positive in tumor cell [IHC stain, b) ×100, c) ×400]. (d) CD56 (Clone 123 C 3) – Negative (IHC stain, ×400). (e) E-CD138 (Clone MI 15) - Positive in few cells (IHC stain, ×400). (f) F-MIB-1 (Clone BGX297) - 15–20% positive (IHC stain, ×400)
CD3 m (Clone PS1) - Stains reactive T-cells
CD20 (Clone L26) - Positive in tumor cell
CD56 (Clone 123 C 3) - Negative
CD138 (Clone MI 15) - Positive in few cells
MB-1 (Clone BGX297) – 15–20% positive.
Based on above findings, a diagnosis of low-grade B-cell NHL was made.
DISCUSSION
Lymphoma is a general term for a complex group of malignancies of the lymphoreticular system. These malignancies initially arise within the lymphatic tissues and may progress to an extranodular mass (NHL) or to a nontender mass or masses in a lymph node region (HL) that later may spread to other lymph node groups and involve the bone marrow. Lymphoma in the oral soft tissues usually presents as an extranodal, soft to firm asymptomatic lesion although the mass may also be painful.[8] Oral involvement by lymphoma may represent a local disease process but more frequently is part of a widespread disease that may also involve the lymph nodes of head and neck region.[8] Primary lymphomas in the head and neck region represent the second most[1,8] /third most[9] common malignancies after squamous cell carcinomas and salivary gland tumors and constitutes 2.2% of all malignancies of the head-neck and 3.5% of intraoral malignancies.[1]
Hodgkin's disease and NHLs are considered major types in comparison with Burkitt's lymphoma and mycosis fungoides (T-cell lymphoma involving the skin), which are rare forms of the disease. The HL and NHL are hematologic tumors, which are usually diagnosed by a biopsy of an enlarged lymph node or mass. A local mass or pain is the most common initial complaint of the majority of patients with extranodal lymphoma.[8]
NHL has long been recognized as heterogeneous group of disorders based on clinical presentation, morphological appearance and response to therapy. In recent years, the use of immunological and molecular biological techniques has led to important advances in our knowledge of lymphocyte differentiation and has provided the basis for a better understanding of the cellular origin and pathogenesis of NHL. Currently, various types of NHL are thought to represent neoplastic cells arrested at various stages in the normal differentiation scheme although the key events in malignant transformation may actually occur in cells at an earlier stage of differentiation.[10]
The NHLs are a diverse group of lymphoid neoplasms that collectively rank fifth in cancer incidence and mortality.[4] The prevalence of NHL has been increasing during the last two decades.[4] Recognized since the 1950s as a distinct group of diseases, NHLs range from indolent malignancies (low-grade histologies) to rapidly growing and highly aggressive tumors (high-grade histologies). The overall median age at presentation is 42 years (58 years for low-grade) and the incidence increases with advancing age.[7] The majority of NHLs are of B-cell origin[11] with more than 90% of patients expressing CD20 antigen.[12] In general, low-grade or follicular NHL is assumed to have an indolent course when compared with intermediate- and high-grade NHL.[7]
Oral involvement of NHL is rare,[1] the incidence of NHL has increased since 1950, with much of the increase due to large cell histologic types, and the most recent increase has been attributed at least in part to the HIV epidemic.[8] Many a times, lymphoma presents in the oral cavity as the first identifiable evidence of the disease. Sometimes, these may also present as squamous cell carcinoma and need to be considered in the differential diagnosis.[1]
The main symptom of both HL and NHL is swelling of lymph nodes in the neck, under the arms or in the groin. Other symptoms can include fever, night sweats, fatigue, abdominal pain and unexplained weight loss. Lymphomas usually are painless; lymph nodes may get larger slowly over a long time before the patient notices. Fever commonly associated with lymphoma may appear and disappear for several weeks.[13]
The signs and symptoms suggestive of lymphoma in the head and neck region are the presence of numbness, tooth mobility, swelling, unexplained dental pain or ill-defined lytic osseous changes. Other differential diagnosis includes a dental abscess, periodontal infection or benign reactive hyperplasia.[14]
The prevalence of NHL is increasing among those who are immunocompromised, receiving organ transplants, and those with autoimmune disorders such as Sjogren's syndrome or rheumatoid arthritis. An increased incidence of NHL has also been recognized in patients suffering from AIDS,[15] and oral lesions of NHL have presented as the first manifestation of AIDS.[1] During the last decade, the frequency of AIDS-related NHLs has been rising steadily.[16] Recent studies show a possible link between lymphoma and exposure to certain chemicals, herbicides and insecticides. Studies indicate that patients with certain genetic (inherited) immunodeficiency disorders, such as Wiskott–Aldrich syndrome, may have an increased risk of developing lymphoma. Viral infections such as the Epstein–Barr virus, AIDS patients, also are more likely to get both HL and NHL.[17]
NHL rarely manifests as a primary malignancy in the head and neck region (>1%) and may give an important clue for undiagnosed HIV infection, which accounts for 2% of oral neoplasms in patients with AIDS.[18] It more commonly affects the middle-aged and the elderly with a slight male preponderance.[19] The gingival and palate regions are commonly affected.[5] In the era that preceded the introduction of highly active antiretroviral therapy in 1996, NHL was diagnosed approximately 60 times more often in the HIV-positive population than in the general population.[15]
Histopathologically, NHL presents either as nodular (neoplastic cells are clustered into identifiable nodules) or diffuse (neoplastic cells spread diffusely) lesions.[20] NHL may originate in T- or B- (80–85%) cells. Within B-cell series resting cells have the appearance of typical small lymphocytes with a dark large nucleus, after antigenic challenge, follicular B-cells enlarge, their nucleus develops cleft and folds and nucleoli become prominent.[20] From purely morphologic observation, without using immunologic markers determination of cell of origin is difficult.[21] CD2, CD3, CD4, CD7 and CD8 are useful for identification of T-cell and their tumor. CD10, CD19, CD20 and surface IgG are a B-cell marker.[20]
The vast majority of lymphomas of the oropharyngeal region are of the B-cell rather than the T-cell type. The genetic profile for extranodal follicular (mixed type) lymphoma is as follows:
Characteristic - CD20+, CD3–
Immunoprofile - CD10+, CD5–
Most frequent - t(14:18)
Cytogenetic site - q32 q21
Associated oncogene - B-cell lymphoma 2 (Bcl-2).
The differential diagnosis includes benign reactive hyperplasia and other types of lymphomas.[18]
Flow cytometry is useful in classifying the specific subtype of lymphoma present. Core flow cytometry panel for the investigation of surface antigen expression in suspected mature B-cell malignancies include surface immunoglobulin (Ig)-heavy and light chains, CD79a, CD19, CD20 and CD22. Additional antibodies (CD5, CD10, CD23 and cyclin D1) are used to further delineate the subtype of lymphoma.[13]
CD20 is the most widely used pan-B-cell marker and is expressed from the native B-cell until the final stages of B-cell development just before plasmacytic differentiation. Chronic lymphocytic leukemia/small lymphocytic lymphoma may be weakly positive or occasionally negative for CD20 by IHC. If an abnormal immature blast population is present or if the patient has received rituximab (anti-CD20 antibody) therapy, other antibodies to detect B-cell differentiation such as CD79a or transcription factor paired box gene 5 (Pax-5) should be included.[22]
CD3 is the most commonly used pan-T-cell antigen and is normally expressed at the second stage of thymic differentiation and beyond. CD3 may be lost in some T-cell neoplasms, particularly anaplastic large cell lymphoma. Natural killer cells can also express the € chain of CD3 detected by the commonly used paraffin-reactive anti-CD3 antibodies, so expression of cytoplasmic CD3 is not incontrovertible evidence of T-cell lineage.[22]
Ann Arbor staging classification, which is based on the anatomic extent of involvement, was initially introduced for HL and later adopted for classification of non-HLs. Classification is as follows:
Stage I - Involvement of a single lymph node region (I) or of a single extralymphatic organ or site (IE)
Stage II - Involvement of 2 or more lymph node regions on the same side of the diaphragm (II) or localized involvement of extralymphatic organ or site and of 1 or more lymph node regions on the same side of the diaphragm (IIE). An optional recommendation is that the number of node regions involved be indicated by a subscript [e.g., II3]
Stage III - Involvement of lymph node regions on both sides of the diaphragm (III), which may also be accompanied by localized involvement of extralymphatic organ or site (IIIE) or by involvement of the spleen (IIIS) or both (IIISE)
Stage IV - Diffuse or disseminated involvement of 1 or more extralymphatic organs or tissues with or without associated lymph node enlargement. The reason for classifying the patient as Stage IV should be identified further by defining site by symbols. Those with Stage 1 or Stage 2 disease have a more favorable prognosis than do those with Stage 3 or Stage 4.[23]
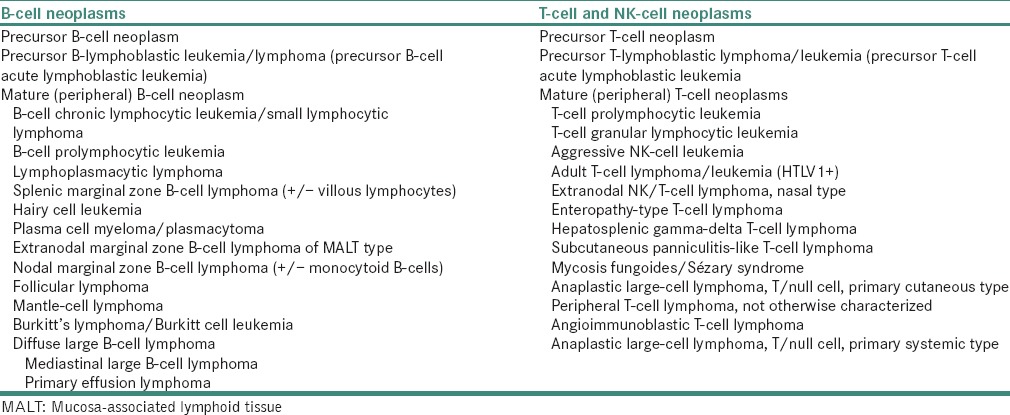
The currently used World Health Organization (WHO) classification system is based on morphology, molecular abnormalities, immunophenotype and clinical presentation. Evolution of NHL classification from the 1940s to proposed WHO in 1999 is presented in Tables 1 and 2 respectively.[24]
Table 1.
NHL classification from the 1940 to 1982
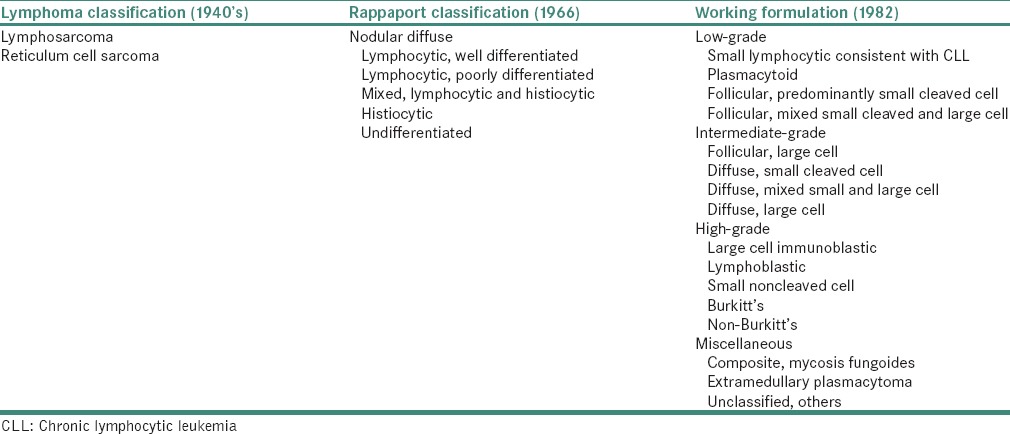
Table 2.
Proposed World Health Organization classification (1999)
Molecular techniques are of increasing practical importance in the analysis of NHLs, both for diagnosis and prognosis. In addition, clonal or disease-specific abnormalities can provide markers for the detection of minimal residual disease. This is possible because of an increasing number of largely complementary techniques including southern blotting, PCR amplification of DNA or RNA and FISH.[25]
The vast majority of NHLs have undergone physiological, clonal Ig or T-cell receptor (TCR) rearrangements. The identification of a clonal Ig/TCR rearrangement (lymphoid clonality) is therefore widely used at diagnosis and increasingly for follow-up.[25]
Chromosomal translocations involving antigen receptor genes are a central feature of many lymphoid neoplasms including the majority of NHLs as well as some acute leukemias and plasma cell neoplasms. These translocations characteristically juxtapose a cellular gene regulating cell growth, survival or differentiation with transcriptional enhancer elements for antigen receptor genes, leading to deregulated oncogene expression. While other genetic changes are required for malignant transformation, in many cases, the translocation is sufficient to initiate the tumor pathway, suggesting that it represents an early and essential step in lymphoid oncogenesis. Two components can, therefore, be considered in the pathogenesis of lymphoid malignancies: Generation of potentially oncogenic translocations and evolution of clinical malignancy as a result of growth or survival advantages and further genetic changes.[25]
Chromosomal translocations and molecular rearrangements are commonly used to confirm the diagnosis. The most common chromosomal abnormality in NHL is the translocation of t(14;18)(q32;q21) that is found in 85% of follicular lymphomas and 28% of diffuse large B cell lymphomas. t(8;14) or MYC in Burkitt's lymphoma, t(2;5) or anaplastic lymphoma kinase in anaplastic large-cell lymphoma, t(11;14) or bcl-1 in mantle cell lymphoma and trisomy 3 or trisomy 18 in marginal zone lymphomas are noticed.[13]
Treatment for NHL depends on the grade of lymphoma (low, intermediate or high), the stage of the disease and the age and health of the patient. In very early stages, low-grade (slow-growing) lymphomas sometimes can be cured with a combination of radiation and chemotherapy. Advanced-stage, low-grade lymphoma may be treated in a variety of ways, ranging from chemotherapy with or without radiation therapy to a bone marrow transplant. In recent clinical trials, radioimmunotherapy involves injecting antibodies with added radioactive iodine to treat advanced, higher-grade lymphomas or those that keep returning after treatment.[13]
There is no definitive way to prevent NHL. Absence of infection HIV may lower the risk. It is not known whether avoiding certain chemicals will prevent lymphoma.[13]
For patients with NHL, the chance of survival depends on the grade and stage of cancer, overall health and response to treatment. Between 50% and 80% of patients survive 5 years or more. The higher-grade aggressive types of lymphoma are more likely to be cured with chemotherapy, but this form can be fatal. Lower-grade lymphomas usually not curable and often have longer average survival times (mean 10 years) in some cases. Most children respond well to treatment, even though children tend to have the higher-grade, aggressive types of NHL. Seventy to ninety percent of children survive 5 years or more.[13]
CONCLUSION
Although NHL is disease of reticuloendothelial system involving lymph nodes, can also involve extranodal sites such as stomach, skin, lung, salivary glands and rarely affects the mouth. Oral NHL is relatively rare, and it presents as local swelling, pain, discomfort affecting the vestibule, gingiva or posterior hard palate and mimics pyogenic granuloma, periodontal disease, osteomyelitis, dental abscess of endodontic or periodontal origin and other malignancies. Sometimes, oral lesion may present as early disease (primary site). Although NHLs are hematologic tumors, they are usually diagnosed by a biopsy of an enlarged lymph node or mass. Careful evaluation of patient and proper investigations are required for correct diagnosis so that patient will receive the treatment in early stage which leads to a good prognosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Manjunatha BS, Gowramma R, Nagarajappa D, Tanveer A. Extranodal non-Hodgkin's lymphoma presenting as gingival mass. J Indian Soc Periodontol. 2011;15:418–20. doi: 10.4103/0972-124X.92584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolvius EB, van der Valk P, van der Wal JE, van Diest PJ, Huijgens PC, van der Waal I, et al. Primary extranodal non-Hodgkin lymphoma of the oral cavity. An analysis of 34 cases. Eur J Cancer B Oral Oncol. 1994;30B:121–5. doi: 10.1016/0964-1955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 2nd ed. Pennsylvania: WB Saunders; 1995. pp. 432–4. [Google Scholar]
- 4.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 5.Aisenberg AC. Coherent view of non-Hodgkin's lymphoma. J Clin Oncol. 1995;13:2656–75. doi: 10.1200/JCO.1995.13.10.2656. [DOI] [PubMed] [Google Scholar]
- 6.Jayakrishnan R, Thomas G, Kumar A, Nair R. Non-Hodgkin's lymphoma of the hard palate. J Oral Maxillofac Pathol. 2008;12:85–7. [Google Scholar]
- 7.Czuczman MS, Grillo-López AJ, White CA, Saleh M, Gordon L, LoBuglio AF, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–76. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JB, Epstein JD, Le ND, Gorsky M. Characteristics of oral and paraoral malignant lymphoma: A population-based review of 361 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:519–25. doi: 10.1067/moe.2001.116062. [DOI] [PubMed] [Google Scholar]
- 9.Jaradat JM, Potluri A, Bilodeau EA. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma: Report of a case in the oral cavity. Indian J Dent Res. 2013;24:384–6. doi: 10.4103/0970-9290.118016. [DOI] [PubMed] [Google Scholar]
- 10.Weisenburger DD. Pathological classification of non-Hodgkin's lymphoma for epidemiological studies. Cancer Res. 1992;52(19 Suppl):5456s–62s. [PubMed] [Google Scholar]
- 11.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. Lymphoma classification proposal: Clarification. Blood. 1995;85:857–60. [PubMed] [Google Scholar]
- 12.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: A model of human B cell differentiation. Blood. 1984;63:1424–33. [PubMed] [Google Scholar]
- 13.Sathiya M, Muthuchelian K. Significance of immunologic markers in the diagnosis of lymphoma. Acad J Cancer Res. 2009;2:40–50. [Google Scholar]
- 14.Vinoth PN, Selvan SM, Sahni L, Krishnaratnam K, Rajendiran S, Anand CV, et al. Primary extra nodal non-Hodgkin's lymphoma of the oral cavity in a young girl. Natl J Maxillofac Surg. 2012;3:187–9. doi: 10.4103/0975-5950.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powles T, Matthews G, Bower M. AIDS related systemic non-Hodgkin's lymphoma. Sex Transm Infect. 2000;76:335–41. doi: 10.1136/sti.76.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidano G, Carbone A, Dalla-Favera R. Pathogenesis of AIDS-related lymphomas: Molecular and histogenetic heterogeneity. Am J Pathol. 1998;152:623–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan H. Text Book of Pathology. 6th ed. New Delhi: Jaypee brothers medical publisher (p) ltd; 2010. [Google Scholar]
- 18.Ramani P, Ahmed S, Janaki VR. Primary extranodal non-Hodgkin's lymphoma of the oral cavity. Indian J Dermatol Venereol Leprol. 2004;70:172–4. [PubMed] [Google Scholar]
- 19.Clifford A, Devi P, Jyoti B. Maxillary sinus lymphoma with vision loss and intraoral presentations: A case report. Pak Oral Dent J. 2010;30:341–3. [Google Scholar]
- 20.Kumar Vinay, Cotran Ramzi, Robbins Stanley., editors. Robbins Basic Pathology. 6th ed. Noida: Harcourt India Private ltd; 1999. [Google Scholar]
- 21.Rajendran R, Shivpathasundaram B. Shafer's Text Book of Oral Patholoigy. 7th ed. New Delhi: Elsevier a division of Reed Elsevier India Private ltd; 2012. [Google Scholar]
- 22.Higgins RA, Blankenship JE, Kinney MC. Application of immunohistochemistry in the diagnosis of non-Hodgkin and Hodgkin lymphoma. Arch Pathol Lab Med. 2008;132:441–61. doi: 10.5858/2008-132-441-AOIITD. [DOI] [PubMed] [Google Scholar]
- 23.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 24.Vasef MA, Lin Y, Dick F. Another lymphoma classification. Lab Med. 2000;31:679–84. [Google Scholar]
- 25.Macintyre E, Willerford D, Morris SW. Non-Hodgkin's lymphoma: Molecular features of B Cell lymphoma. Hematology Am Soc Hematol Educ Program. 2000:180–204. doi: 10.1182/asheducation-2000.1.180. [DOI] [PubMed] [Google Scholar]