Abstract
Osteomyelitis is defined as inflammation of the medullary cavities, haversian system and adjacent cortex of bone. Osteomyelitis involving maxilla is quite rare when compared to that of mandible. Fungal osteomyelitis is very rarely seen and documented in the maxillofacial area. It is devastating to patients if it is invasive in nature. A prospective study was undertaken from December 2011 to December 2013. Biopsied hard tissue bits were decalcified and sections were stained with H&E, periodic acid Schiff and Grocott methenamine silver. Culture sensitivity was carried out in all cases. Surgical intervention followed by antifungal therapy was advocated to treat the patients. The current series showed five cases of fungal osteomyelitis which included candidal osteomyelitis, aspergillosis and mucormycosis with slight predominance of maxilla over mandible. Osteomyelitis of facial bones needs to be investigated thoroughly as there is no difference in clinical presentation between bacterial and fungal osteomyelitis. The diagnostic workup with biopsy and culture sensitivity helps to identify the pathogen at the earliest. Appropriate antifungal treatment needs to be advocated for successful treatment.
Keywords: Aspergillosis, fungal infection, mucormycosis, osteomyelitis
INTRODUCTION
Osteomyelitis is defined as inflammation of the medullary cavities, haversian system and adjacent cortex of bone.[1] In the present era of antibiotics, osteomyelitis of facial bones is a rare condition. Osteomyelitis involving maxilla is quite rare compared to that of mandible because of the extensive vascularity and strut like nature seen in maxilla.[1,2]
Fungal osteomyelitis is very rarely seen and documented in the maxillofacial area. Here, we highlight five cases of fungal osteomyelitis presenting with diverse clinical presentation. The diagnostic workup with emphasis on special staining in histopathological sections to identify the fungal nature has been discussed.
CASE SERIES
A prospective study was undertaken from December 2011 to December 2013. Patients who reported for osteomyelitis were thoroughly screened and only five cases were obtained which showed characteristics of fungal osteomyelitis.
All five cases included in the series were primarily intraosseous with evidence of radiographic changes in bone. All cases had relevant clinical [Figure 1], radiological [Figure 2] and histopathological [Figures 3, 4 and 5] data. Biopsied tissue sections were stained with H&E, Periodic acid Schiff (PAS) and Grocott methenamine silver (GMS) stain. In all cases, microbiological confirmation by culture was obtained except in one case.
Figure 1.
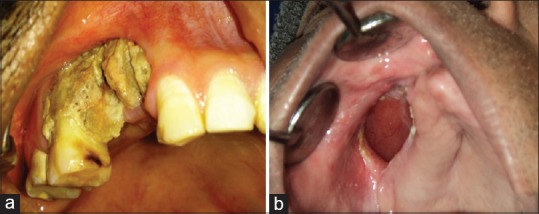
(a) Case 3-Clinical photographs showing exposed necrotic bone i.r.t. 14, 15 and 16; (b) Case 5-Clinical photograph showing oro-antral fistula in right maxillary vestibule, measuring 4×3 cm
Figure 2.

(a) Case 1-Radiographs showing radiopaque interdental septum i.r.t. 46 and 47 region in orthopantomogram. (b) Case 3- CT scan showing permeative bone destruction in anterior hard palate. (c) Case 5- Orthopantomograph shows necrotic bone i.r.t. 14,15 and 16 with haziness in right maxillary sinus in case 5
Figure 3.
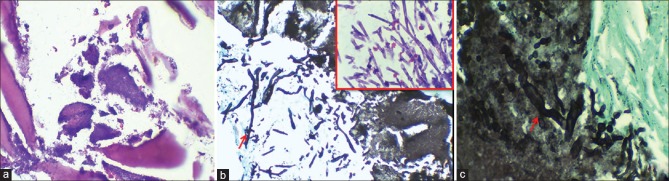
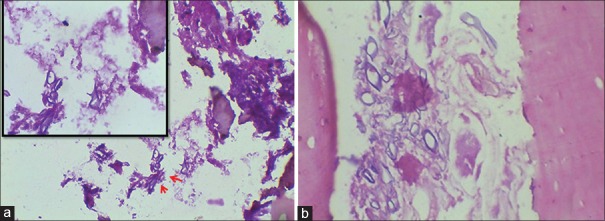
(a) Case 2- Section showing candidal pseudoseptate hyphae [Periodic acid Schiff (PAS) stain, ×400]. (b) Case 4- Section showing empty lacunae in bone and candidal pseudoseptate hyphae [PAS stain, ×40]. Inset: Higher magnification showing candidal pseudohypae [PAS, ×400]
Figure 4.
Case 4- Section showing actinomycotic foci within bone (H&E stain, ×200), (b) Grocott methenamine silver (×200) and H&E (Inset, ×400) stained section showing septate and dichotomous hyphae of aspergillosis, (c) Grocott methenamine silver stain showing aseptate broad, ribbon shaped hyphae of mucormycosis (×400)
Figure 5.
(a) Case 1- Section showing necrotic bone with aseptate hyphae (H&E stain, ×200), (b) Case 3- Sections showing broad aseptate hyphae and spores (H&E stain, ×400)
The clinical, radiological, histopathological and treatment data are illustrated in Table 1.
Table 1.
Clinical and radiological features with histopathological diagnosis and treatment details for cases 1-5

DISCUSSION
Osteomyelitis is an inflammatory condition of bone and bone marrow content which originates from chronic infection. Etiopathogenesis includes trauma, surgical intervention, bacteremia, fungal infection or a contiguous infectious focus and systemic diseases among the innumerable causes. It is further influenced by the immobility of stagnant blood which acts as nidus for the development of infection.[3]
Fungal osteomyelitis is very rare and generally presents in an indolent fashion.[4] Fungal infections are devastating to patients if it is invasive in nature. These are opportunistic infections which frequently enter the body due to a decrease in host defense or through an invasive gateway, such as a dental extraction. Candidal infection is more often encountered when compared to other fungal infection, i.e. mucormycosis, aspergillosis etc.[5] Osteomyelitis mostly results from bacterial infection. However, fungi, parasites and viruses can also affect bone and bone marrow.[6]
Since fungal infections involving bone occur infrequently, it can pose a diagnostic and therapeutic dilemma for those who are not familiar with its clinical presentation, hence leading to ineffective treatment or resolution. The clinical presentation of fungal osteomyelitis would be similar to the bacterial osteomyelitis. Exposed bone with history of varying pain intensity would be predominantly encountered.[3] Specific delineating features would be the involvement of maxillary sinus with a complaint of sinusitis in maxillary fungal osteomyelitis. An associated history of diabetes would generally be present in such cases.[7]
Diabetes mellitus is usually a propagating factor for maxillary osteomyelitis.[8] Out of five cases, three were involving the maxilla with a history of diabetes in two out of three cases. Altered immunity and compromised vascularity are well established and are commonly encountered facts related to uncontrolled diabetes mellitus (UDM).[7] The mechanism which facilitates bone infection includes diminished leukocyte chemotaxis, phagocytosis and life span; and diminished vascularity, thus reducing tissue perfusion. Defective glucose utilization causes delayed wound healing.[9] An incidence of 45.1% of maxillary osteomyelitis has been reported by Koorbusch et al. among UDM patients in a rural Indian population.[10]
Collateral blood supply, porous nature and thin cortices of maxilla, reduces the chance of osteomyelitis in maxilla when compared to the mandible.[1] However, the current series showed a slight predominance of fungal osteomyelitis in maxilla over mandible. According to Niranjan et al., 52% cases of fungal osteomyelitis and 48% of nonfungal osteomyelitis were observed in a 10 year study conducted on North Karnataka population. Maxillary involvement was seen in 80.76% of cases with male predominance associated with diabetes mellitus.[11] A thorough review of literature showed that the incidence ratio of maxilla: Mandible osteomyelitis was 1.07:1 by Peravali et al., whereas the incidence of 1:6.5 and 1:6 was noted by Koorbusch et al. and Rangne and Ruud.[7,10,12]
The diagnostic workup is of paramount importance to differentiate between bacterial and fungal osteomyelitis. The biopsied bony tissues on decalcified sections show irregular bony trabeculae with empty osteolytic lacunae. The presence of fungal hyphae within the bone would essentially highlight the fungal nature. Culture sensitivity should be followed in all cases irrespective of its nature.
In the current study, two cases of mucormycosis were seen. All histopathological sections were stained with H&E, PAS and GMS. Special staining with GMS specially identifies the nature of hyphae whether septate or aseptate. Identification can be accurately done on histopathological sections itself and the culture will confirm the exact species.[13] Mucormycosis is an opportunistic fulminant fungal infection, which mainly infects immunocompromised patients.[14] The fungus invades the arteries leading to thrombosis that subsequently causes necrosis of hard and soft tissues.[15] Mucormycosis is frequent in diabetic patients because a favorable environment is created due to an excess of ketone bodies in diabetic patients. Rhizopus arrhizus, produces the enzyme ketoreductase, which allows them to utilize the patient's ketone bodies. The increase in the levels of free iron ions favors fungal growth.[16,17] Literature reveals that zygomycosis is less documented in mandible with only five cases of mandibular zygomycosis being reported until date.[18]
The evidence of candidal osteomyelitis is scarce in the reported literature. The current series showed few cases of candidal osteomyelitis. PAS stained histopathological sections showed pseudoseptate hyphae in clusters with budding at focal areas (case# 2 and case# 4). This was confirmed by culture sensitivity. Peravali et al. have analyzed 31 cases of osteomyelitis in which there appears to be only one case of candidal osteomyelitis, along with three cases of mucormycosis and one case of aspergillosis.[7]
It is extremely rare to find candidal osteomyelitis in the maxilla and because of nonspecific symptoms, diagnosis is very challenging.[19] Case#4 was a pediatric patient diagnosed with candidal osteomyelitis. It is very rare to find candidal osteomyelitis as compared to bacterial osteomyelitis. Incidence in children ranges from 1:5000 -1:10,000 as documented by Weichert et al. and Dahl et al.[20,21]
Aspergillosis is the second most common fungal infection after candida. It is usually invasive in nature when involving maxillary sinus though noninvasive forms have also been reported[22] and does not cause bone destruction when compared to mucormycosis.[17,23] Case#5 had a combination of mucormycosis, aspergillosis, actinomycotic foci and superimposed candidiasis. A thorough search in PubMed reveals only one case of trifungal osteomyelitis reported till date.[18] Case#5 in the current series appears to be the second case being reported. The identification of these hyphae are quite characteristic with aspergillosis showing narrow septate hyphae of 3–6 μm in size having dichotomous branching between 45° and 90°.[24] Mucormycosis which belongs to zygomycetes family typically shows broad aseptate hyphae of size 6–20 μm with branching at > 90°. Histopathological identification can be considered as the gold standard in such cases.[25]
Appropriate treatment of antifungal regime needs to be initiated as the culture sensitivity results could take up to 6 weeks. Early identification and prompt treatment with the identification of underlying systemic disease would lead to successful outcome. In the present study, two cases of candidal osteomyelitis (case#2 and case #4) healed with appropriate antifungal regime of itraconazole and fluconazole along with surgical debridement.
Case#3 was administered with amphotericin B, cefotaxime and metronidazole which was followed by the complete removal of maxillary sinus lining with excision and closure of oroantral fistula by the nasolabial flap. A similar drug regime was followed for case#1.
Case#5 underwent sequestrectomy with reconstruction with buccal fat pad and irrigation, followed by drugs, i.e. amoxicillin, clavulanic acid and itraconazole.
All cases were followed up for renal toxicity for 6 months with an interval of 1 month. There were no aberrant changes in serum creatinine and urea levels. However, case#5 was lost to follow-up.
CONCLUSION
Osteomyelitis of facial bones needs to be investigated thoroughly as there is no difference in clinical presentation between bacterial and fungal osteomyelitis unless accompanied by maxillary sinusitis. The diagnostic workup with biopsy and culture sensitivity helps to identify the pathogen at the earliest. Fungal osteomyelitis is very rare and appropriate treatment with antifungal regime and timely surgical intervention, i.e., debridement, curettage, sequestrectomy will lead to successful resolution of the disease process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hudson JW. Osteomyelitis of the jaws: A 50-year perspective. J Oral Maxillofac Surg. 1993;51:1294–301. doi: 10.1016/s0278-2391(10)80131-4. [DOI] [PubMed] [Google Scholar]
- 2.Burduk PK, Skorek A, Stankiewicz C, Dalke K. Chronic, recurrent, progressive osteomyelitis of the maxilla caused by methicillin-resistant Staphylococcus epidermidis: A therapeutic dilemma. J Oral Maxillofac Surg. 2010;68:2012–5. doi: 10.1016/j.joms.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Prasad KC, Prasad SC, Mouli N, Agarwal S. Osteomyelitis in the head and neck. Acta Otolaryngol. 2007;127:194–205. doi: 10.1080/00016480600818054. [DOI] [PubMed] [Google Scholar]
- 4.Kohli R, Hadley S. Fungal arthritis and osteomyelitis. Infect Dis Clin North Am. 2005;19:831–51. doi: 10.1016/j.idc.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, et al. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670–4. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: A review of clinical features, therapeutic considerations and unusual aspects (second of three parts) N Engl J Med. 1970;282:260–6. doi: 10.1056/NEJM197001292820507. [DOI] [PubMed] [Google Scholar]
- 7.Peravali RK, Jayade B, Joshi A, Shirganvi M, Bhasker Rao C, Gopalkrishnan K. Osteomyelitis of maxilla in poorly controlled diabetics in a rural Indian population. J Maxillofac Oral Surg. 2012;11:57–66. doi: 10.1007/s12663-011-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfowitz BL. Osteomyelitis of the maxilla. S Afr Med J. 1971;45:632–3. [PubMed] [Google Scholar]
- 9.Baltensperger MM, Eyrich GK. Textbook on Osteomyelitis of the Jaws. New York: Springer Publications; 2009. pp. 5–50. [Google Scholar]
- 10.Koorbusch GF, Fotos P, Goll KT. Retrospective assessment of osteomyelitis. Etiology, demographics, risk factors, and management in 35 cases. Oral Surg Oral Med Oral Pathol. 1992;74:149–54. doi: 10.1016/0030-4220(92)90373-x. [DOI] [PubMed] [Google Scholar]
- 11.Niranjan KC, Sarathy N, Alrani D, Hallekeri K. Prevalence of fungal osteomyelitis of the jaws associated with diabetes mellitus in North Indian population: A retrospective study. Int J Cur Res. 2016;8:27705–10. [Google Scholar]
- 12.Rangne A, Ruud A. Osteomyelitis of the jaws. Int J Oral Surg. 1978;7:523–7. doi: 10.1016/s0300-9785(78)80068-4. [DOI] [PubMed] [Google Scholar]
- 13.Limongelli WA, Clark MS, Saglimbene R, Baden E, Washington JA, Williams AC. Successful treatment of mucocutaneous mucormycosis after dental extractions in a patient with uncontrolled diabetes. J Oral Surg. 1975;33:705–12. [PubMed] [Google Scholar]
- 14.Sridhara SR, Paragache G, Panda NK, Chakrabarti A. Mucormycosis in immunocompetent individuals: An increasing trend. J Otolaryngol. 2005;34:402–6. doi: 10.2310/7070.2005.34607. [DOI] [PubMed] [Google Scholar]
- 15.Butala A, Shah B, Cho YT, Schmidt MF. Isolated pulmonary mucormycosis in an apparently normal host: A case report. J Natl Med Assoc. 1995;87:572–4. [PMC free article] [PubMed] [Google Scholar]
- 16.Damante JH, Fleury RN. Oral and rhinoorbital mucormycosis: Case report. J Oral Maxillofac Surg. 1998;56:267–71. doi: 10.1016/s0278-2391(98)90883-7. [DOI] [PubMed] [Google Scholar]
- 17.Singh J, Prasanna NM. Phycomycosis in an apparently normal host. J Otolaryngol. 1977;6:37–42. [PubMed] [Google Scholar]
- 18.Lador N, Polacheck I, Gural A, Sanatski E, Garfunkel A. A trifungal infection of the mandible: Case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:451–6. doi: 10.1016/j.tripleo.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Bali R, Sharma P, Gupta P, Gaba S. Chronic candidal osteomyelitis of mid face: A therapeutic dilemma. J Oral Biol Craniofac Res. 2013;3:151–3. doi: 10.1016/j.jobcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weichert S, Sharland M, Clarke NM, Faust SN. Acute haematogenous osteomyelitis in children: Is there any evidence for how long we should treat? Curr Opin Infect Dis. 2008;21:258–62. doi: 10.1097/QCO.0b013e3283005441. [DOI] [PubMed] [Google Scholar]
- 21.Dahl LB, Høyland AL, Dramsdahl H, Kaaresen PI. Acute osteomyelitis in children: A population-based retrospective study 1965 to 1994. Scand J Infect Dis. 1998;30:573–7. doi: 10.1080/00365549850161124. [DOI] [PubMed] [Google Scholar]
- 22.Dreizen S, Bodey GP, McCredie KB, Keating MJ. Orofacial aspergillosis in acute leukemia. Oral Surg Oral Med Oral Pathol. 1985;59:499–504. doi: 10.1016/0030-4220(85)90091-x. [DOI] [PubMed] [Google Scholar]
- 23.Peterson KL, Wang M, Canalis RF, Abemayor E. Rhinocerebral mucormycosis: Evolution of the disease and treatment options. Laryngoscope. 1997;107:855–62. doi: 10.1097/00005537-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Eliashar R, Resnick IB, Goldfarb A, Wohlgelernter J, Gross M. Endoscopic surgery for sinonasal invasive aspergillosis in bone marrow transplantation patients. Laryngoscope. 2007;117:78–81. doi: 10.1097/01.mlg.0000245941.03953.5d. [DOI] [PubMed] [Google Scholar]
- 25.Ferry AP, Abedi S. Diagnosis and management of rhino-orbitocerebral mucormycosis (phycomycosis).A report of 16 personally observed cases. Ophthalmology. 1983;90:1096–104. doi: 10.1016/s0161-6420(83)80052-9. [DOI] [PubMed] [Google Scholar]