Abstract
Alterations in gap junctions underlie the etiologies of syndromic deafness (KID) and Charcot-Marie Tooth disease (CMTX). Functional gap junctions are composed of connexin molecules with N-termini containing a flexible turn around G12, inserting the N-termini into the channel pore allowing voltage gating. The loss of this turn correlates with loss of Connexin 32 (Cx32) function by impaired trafficking to the cell membrane. Using 1H NMR we show the N-terminus of a syndromic deafness mutation Cx26G12R, producing “leaky channels”, contains a turn around G12 which is less structured and more flexible than wild-type. In contrast, the N-terminal structure of the same mutation in Cx32 chimera, Cx32*43E1G12R shows a larger constricted turn and no membrane current expression but forms membrane inserted hemichannels. Their function was rescued by formation of heteromeric channels with wild type subunits. We suggest the inflexible Cx32G12R N-terminus blocks ion conduction in homomeric channels and this channel block is relieved by incorporation of wild type subunits. In contrast, the increased open probability of Cx26G12R hemichannels is likely due to the addition of positive charge in the channel pore changing pore electrostatics and impairing hemichannel regulation by Ca2+. These results provide mechanistic information on aberrant channel activity observed in disease.
Keywords: NMR, connexin, peptides, structure-function, deafness
INTRODUCTION
Connexin proteins, which in humans are encoded by a gene family of 21 members, form both voltage-gated plasma membrane channels (undocked hemichannels) and by the head to head docking of two hemichannels - intercellular channels that aggregate to form gap junctions. Connexins are tetraspan membrane proteins with intracellular N- and C-termini, 2 extracellular loops and 1 intracellular loop. A hemichannel is formed by six connexin subunits surrounding a large central aqueous pore, ~ 15 Å in diameter. The crystal structure of a Cx26 intercellular (gap junction) channel at 3.5 Å (PDB:2ZW3)[1], its refinement by all-atom molecular dynamics (MD) simulation, and incorporation of co- and post-translational modifications identified by tandem mass spectroscopy provided an atomic model that arguably closely represents the physiological open state of the Cx26 hemichannel [2, 3].1
The roles of intercellular channels in propagation of electrical signals through electrical synapses, modulation of neuronal network activity and synaptic strength, intercellular molecular signaling in tissue homeostasis and development are well established [4–6]. Undocked hemichannels provide a direct aqueous communication path between intracellular and extracellular compartments and play an important role in autocrine/paracrine signaling mediated by signaling molecules including ATP and glutamate [7]. Some appear to modulate membrane potential in response to change in extracellular calcium [8], in a manner similar to the phylogenetically related CALHM1 channel [9, 10].
Connexins are mutational targets for at least 8 human diseases. These include syndromic and nonsyndromic deafness, where more than 200 different Cx26 mutations have been described. Cx26 mutations account for 50% of the ~1:650 individuals with inherited deafness [11]. Dominant Cx26 mutations underlying syndromic deafness associated with severe often fatal skin disorders exemplified by keratitis-ichthyosis-deafness (KID) syndrome, Vohwinkel syndrome, and Bart-Pumphrey syndrome are less common. 25 syndromic mutations have been identified, most of which map to the N-terminus and to the region of the channel pore formed by the first transmembrane helix (TM1) and first extracellular loop (E1) [12, 13]. Mutations of Cx32 cause X-linked Charcot-Marie-Tooth (CMT-X) disease, a late onset peripheral neuropathy [14], which in some cases has central nervous system involvement [15, 16]. More 2than 400 different Cx32 mutations have been described, causing CMT-X in 1 in 2500 individuals [17]. In myelinating Schwann cells, Cx32 can form gap junctions between adjacent loops of noncompact myelin. These intracellular channels, termed reflexive gap junctions shorten an intracellular communication pathway between the periaxonal cytoplasm and the peripheral cytoplasm where the cell nucleus is located.
Loss of gap junction communication appears to underlie the etiology of nonsyndromic deafness and manifestations of CMT-X in the peripheral nervous system. Such loss of function has been shown to arise by a number of different mechanisms including, defects in synthesis, assembly, membrane insertion and docking of hemichannels, large shifts in voltage dependence that close intercellular channels under conditions at which they would normally be open, structural changes that alter permeability of intercellular channels to key signaling molecules such as cAMP, ATP, and/or Ca2+ [13, 18, 19]. In contrast, in syndromic deafness the primary defect appears to be a consequence of “gain of function” Cx26 mutations [20]. It has been proposed that this gain of function arises because of increased open probability of Cx26 undocked hemichannels in cells of affected individuals. This can result from shifts in the voltage-dependence of loop-gating and/or reduced sensitivity to closure by extracellular [Ca2+] resulting in opening of undocked hemichannels under conditions where they would normally be closed. Several groups have shown that Ca2+ acts both by stabilization of the loop-gate closed state and destabilization of the open state by interaction with residues that likely form the loop-gate permeability barrier in Cx26 [21, 22]. This region of the Cx26 intercellular channel pore (residues 41–50) has shown to coordinate Ca2+ by X-ray crystallography [23] . NMR studies have shown that Cx26 and Cx32 N-terminal peptides have different structural features, but both are characterized by an open flexible turn formed in the vicinity of the 12th residue. Significantly, specific interactions leading to the formation and stabilization of the turn differ in the two peptides. Therefore, mutation of the same locus in the N-terminus of Cx26 and Cx32 may produce different structural defects and disease etiologies. It has been reported that the Cx26G12R mutation, a syndromic deafness gain of function mutation, forms functional but “leaky” hemichannels in Xenopus oocytes [24]. Here, we solve the N-terminal structure of this gain of function mutation, Cx26G12R, and compare it to the full length wild-type Cx26 N-terminus (residues 1–22) using 1H 2D NMR. We also investigate whether Cx32G12R, a mutation at the loci of CMTX mutations, forms functional hemichannels. We solve the structure of the Cx32G12R N-terminal peptide to compare it to the Cx26G12R N-terminus as well as with that of the wild-type Cx32 N-terminus. All structures are compared with previously solved Connexin 32 loss of function mutation structures to understand structure-function relationships of Connexin molecules in health and disease [25, 26].
MATERIALS AND METHODS
Peptide synthesis
N-terminal peptides of Cx26 (MDWGTLQTILGGVNKHSTSIGK), Cx26 mutant G12R and Connexin 32 mutant G12R (MNWTGLYTLLSRVNRHSTAIGR) were synthesized by the New England Peptide Company. The synthesis method was standard Fmoc chemistry [27] and the samples were purified by HPLC and sequence confirmed by electron ionization spectrometry. The peptides contained acetylated N termini and free C termini. For NMR data collection the peptides were dissolved in 100 mM KCL (ionic strength of 0.1 M) in 10% 2H2O / 90% H2O (pH 7.0) at a concentration of 1.0–1.2 mM with 100 µM 3-(trimethylsilyl)propionic acid (TSP) as a chemical shift reference.
1H and 13C 2D NMR
All 1H and 13C NMR spectra were collected on a Varian INOVA 600 MHz spectrometer equipped with a cryoprobe. Data sets in H2O were collected at 278, 283, 288 and 293 K to aid in proton assignment as well as for calculation of temperature coefficients. Water suppression in all 2D experiments was accomplished with Watergate [28]. The data were processed using NMRPipe [29], extended using linear prediction and zero filling and analyzed using NMRView [30].
A natural abundance 13C-H HSQC experiment was collected at 600 MHz to obtain Cα chemical shifts. The 13Cα secondary conformational shifts, (Δδ13C = δobs - δrandom coil,, ppm) were calculated using random coil values at pH 7 and 15°C [31–33].
All proton resonances were assigned by standard procedures [34]. For proton assignments both short (15 ms) and long (80 ms) mixing time TOCSY [35] experiments were used and proton-proton distance constraints were obtained from a 225 ms mixing time NOESY experiment at 288 K. Spin diffusion is not expected for a peptide of this size (22 amino acids) and there was no evidence of spin diffusion in comparisons of crosspeaks from NOESY spectra collected at 100 and 225 ms mixing times. NOESY crosspeak intensities were classified into very strong (3.0 Å), moderately strong (3.4 Å), and weak (5.0 Å) ranges. Amide proton temperature coefficients were calculated from the proton chemical shift change from TOCSY spectra collected in increments of 5° in the temperature range of 278–293 K. Any amide proton which shifted less than 6.0 ppb/K was considered likely to be hydrogen bonded. Initial structures were calculated using relevant NOE constraints by simulated annealing and torsional angle dynamics as applied in the program CNS 1.3 (Crystallography and NMR Systems) [36]. The previously solved wild-type Connexin 32 structure [25] was recalculated and further refined using CNS 1.3 as well. A hydrogen bond analysis was performed on all structures using the program MolMol [37]. A hydrogen bond was considered to occur if it was present in greater than 70% of the structures and if the donor proton had a reduced temperature coefficient. Since no hydrogen bonds satisfied these criteria, they were not used in the final structure calculations. The 20 lowest energy structures were energy minimized using CNS, evaluated with AQUA and PROCHECK-NMR [38] and displayed using MOLMOL [37].
The coordinates and NMR restraints for the 3 structures reported in this paper have been deposited in the Protein Data Bank. The accession numbers are 5KJ3 (Cx26), 5KJG (Cx26G12R) and 5KK9 (Cx32G12R).
Molecular Biology
Cx32*43E1 G12R was produced with a QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies) and confirmed by DNA sequencing. DNA was linearized with HindIII, and RNA was synthesized from this template with a mESSAGE mACHINE T7 promoter kit (Life Technologies; Ambion) using ~ 1 µg of DNA template and a 4 hour reaction. RNA was purified using a Megaclear kit (Life Technologies; Ambion).
Expression in Xenopus oocytes
An aliquot of 50–100 ηl of purified RNA (~ 0.2 – 0.5 ηg/nl) was injected into Xenopus oocytes obtained from Xenopus 1 (Dexter, MI). Oocytes were cultured in media containing (in mM): 88 NaCl, 1 KCl, 1.8 CaCl2, 1 MgCl2, and 10 Hepes, pH 7.6, at 12°C. Connexin currents were recorded 1 to 2 days post-injection.
Electrophysiological Recording
Membrane currents were recorded with a CA-1B high-performance oocyte clamp (Dagan Corporation) at room temperature. Currents were digitized at a sampling frequency of 2 kHz and filtered at 200 Hz with a low-pass Bessel filter for gap free recordings and 5 kHz and 200 Hz for waveform recordings. Bath solution contained (in mM): 100 cesium methane sulfonate (CsMes), 1.8 MgCl2, 10 HEPES, pH 7.6. Recording pipette solutions contained 3 M CsCl and 10 mM HEPES, pH 7.6. Pipette resistances were between 0.1 and 0.25 MΩ. A separate ground chamber containing 3 M CsCl was connected to the bath chamber with an agar bridge containing 3 M CsCl.
Protein purification and Western blots
10–15 oocytes were briefly sonicated (10-1second pulses) at low power in 1.0 ml of homogenization buffer - 0.5 M NaCl, 10 mM HEPES pH 7.5, 5% glycerol and protease inhibitors (Roche complete EDTA free) followed by centrifugation at 16,000 g for 10 min in an Eppendorf benchtop centrifuge. The pellet was re-suspended in 500 µl homogenization buffer and solubilized with 1% DDM (n-dodecyl-β-D-maltopyranoside) with rotation at room temperature for 30 min. Following centrifugation at 16,000 g for 15 minutes at room temperature, SDS was added to the supernatant to a final concentration of 2%, DTT to a final concentration of 50 mM, and glycerol containing 0.1% bromphenol blue to a final concentration of 10%. 20 µl was electrophoresed through a Novex, 4–20% gradient Bis-Tris polyacrylamide gel (Invitrogen). Heteromeric hemichannels comprised of wild type his-tagged connexin protein subunits with the C-terminus truncated to 247 were isolated and solubilized in 1% DDM as described above. His-tagged channels were purified with a His-tagged Purification Miniprep Kit (Clontech Laboratories, Catalogue number 635710). The wash buffer was the same as the solubilization buffer but contained 5 mM imidazole. Bound proteins were eluted with 100 µl of solubilization buffer containing 500 mM imidazole and an aliquot was electrophoresed as described above. Following electrophoresis, proteins were transferred to Immobilon PVDF membranes (Millipore Corporation) by semi-dry electroblotting (Owl Scientific) following Millipore’s recommended procedures. Western blots were performed using a 1:1000 dilution of a mouse monoclonal antibody directed against the cytoplasmic loop of Cx32 (Sigma Chemical, C6344) and a western breeze alkaline phosphatase detection kit (Life Technologies).
RESULTS
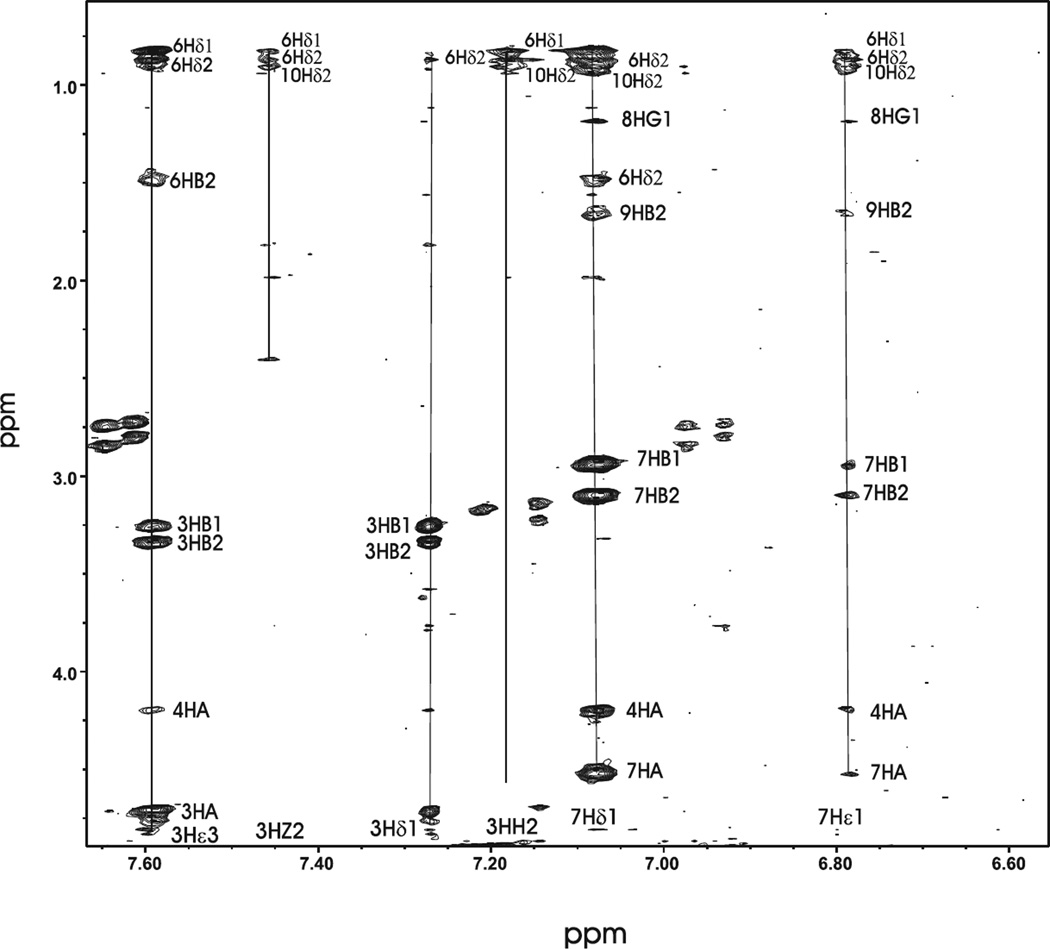
The aromatic to aliphatic section of the NOESY for the N-terminal mutant Cx32G12R is shown in Figure 1. The figure shows a number of medium range NOE connectivities between W3 aromatic protons and aliphatic protons of L6 and Y7 aromatic protons and aliphatic protons of T8, L9 and L10 in the NOESY spectrum of the Cx32G12R mutant. This portion of the spectrum indicates the peptide has a number of hydrophobic side chain interactions.
Figure 1.
The two-dimensional 1H NMR spectrum of the Cx32G12R mutant peptide showing labeled NOE interactions between aliphatic and aromatic side chain protons.
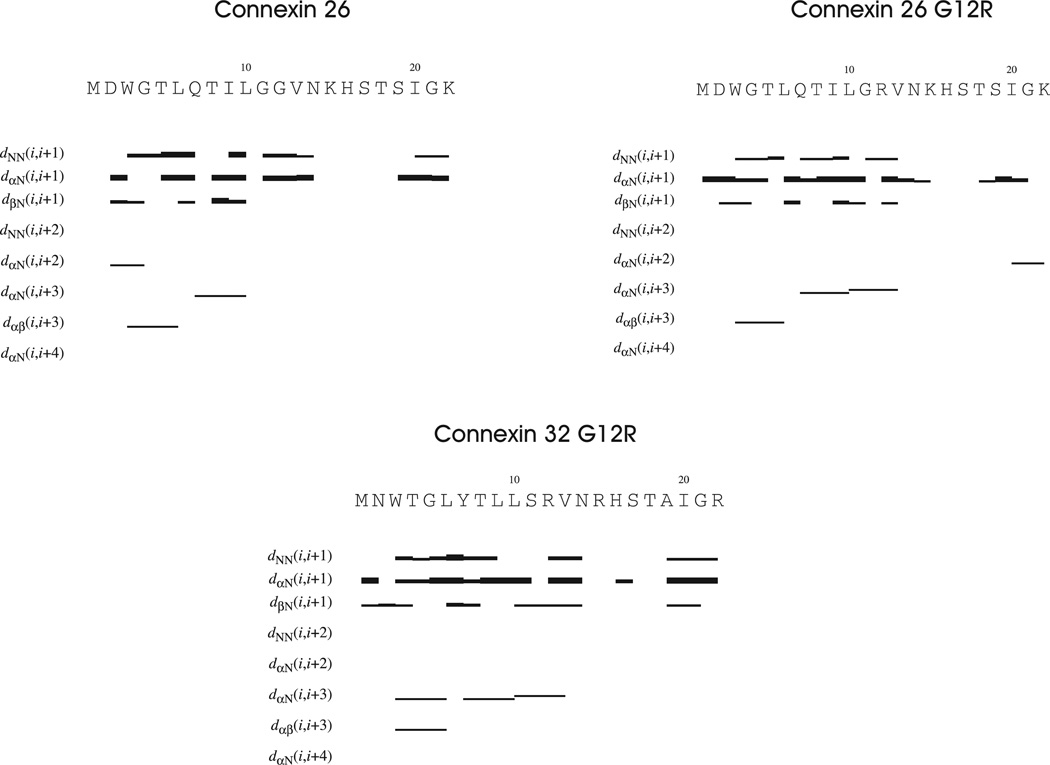
Figure 2 shows a summary of NOE patterns for the Cx26, Cx26G12R and Cx32G12R mutant peptides. The wild-type Cx26 as well as both mutants contain dαN(i,i+3) and dαβ(i,i+3) NOES within the first 13 residues of the N-terminus suggesting some helical structure or helical like turns in this portion of the molecules. There are also dαN(i,i+2) NOE patterns, indicative of turns, located in the beginning of the WTCx26 and at the end of the Cx32G12R peptides.
Figure 2.
A summary of NOE restraints for the Cx26 WT, Cx26G12R and Cx32G12R mutant N-terminal peptides. The horizontal lines indicate backbone NOES with the thickness representing intensity.
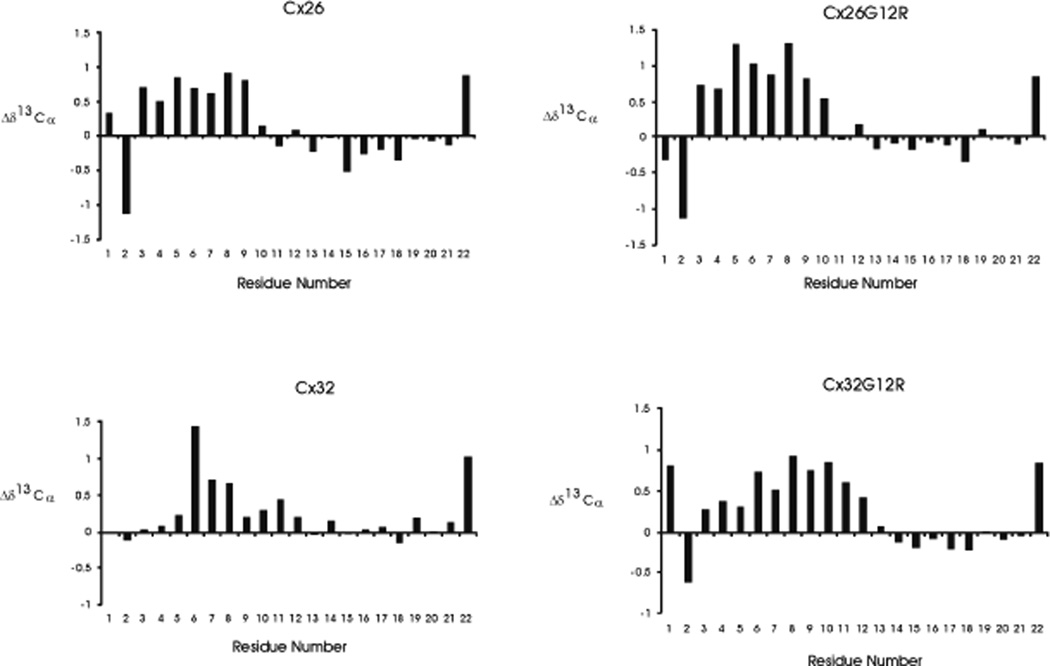
Figure 3 shows plots of Δd 13Cα secondary shifts (Δd 13Cα= δobs - δrandom coil,, ppm) for the Cx26, Cx26G12R, Cx32G12R and Cx32 N-terminal peptides. The 13C α conformational shifts are sensitive to secondary structure since they are mainly determined by the Φ and Φ backbone dihedral angles [39] and are used in conformational studies of flexible peptides [40]. The positive values of 13C α secondary shifts indicate α-helical structure, negative values indicate β sheets with lower values representing more flexible structures and values close to zero (random coil chemical shifts) representing residues within flexible, unconstrained regions [40]. Plots of 13Cα secondary shifts for WT Cx26 and Cx26G12R suggest rather similar structures with values indicating helical propensity within residues 3–9 for Cx26 and 3–10 for Cx26G12R. The residues following this region show no strong propensity for secondary structure and have very low values near zero suggesting a very flexible region in both peptides. More of a difference is observed between the secondary shift plots of WT Cx32 and Cx32G12R. In the WT Cx32 peptide, residues 6–8 show a propensity for helical structure with residues 9–12 exhibiting lower values suggesting a more flexible helical turn with remaining residues showing very low values indicating a very flexible region. In contrast the Cx32G12R N-terminal peptide showed helical propensity for residues 3–12 with higher values indicating a stronger helical propensity for residues 6–12 and a more flexible unstructured region from residue 13 and beyond. The Cx26, Cx26G12R and Cx32 peptides all show a more flexible region around residue 12 than that shown for the Cx32G12R peptide. In addition we have calculated secondary structural propensities plots for the 4 peptides using the difference between the 13Ca and 13Cβ chemical shifts and listed all 1H ,13Cα and 13Cβ chemical shift assignments in reference [41].
Figure 3.
Plots of 13Cα secondary chemical shifts (Δd13C = δobs - δrandomcoil,, ppm) for Cx26, Cx26G12R, Cx32 and Cx32G12R peptides.
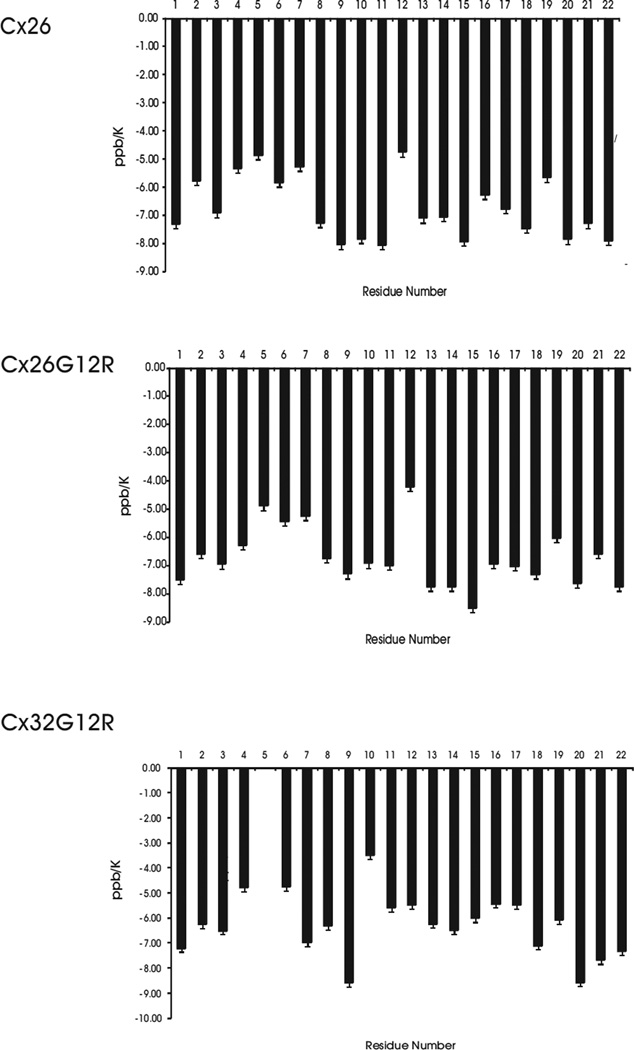
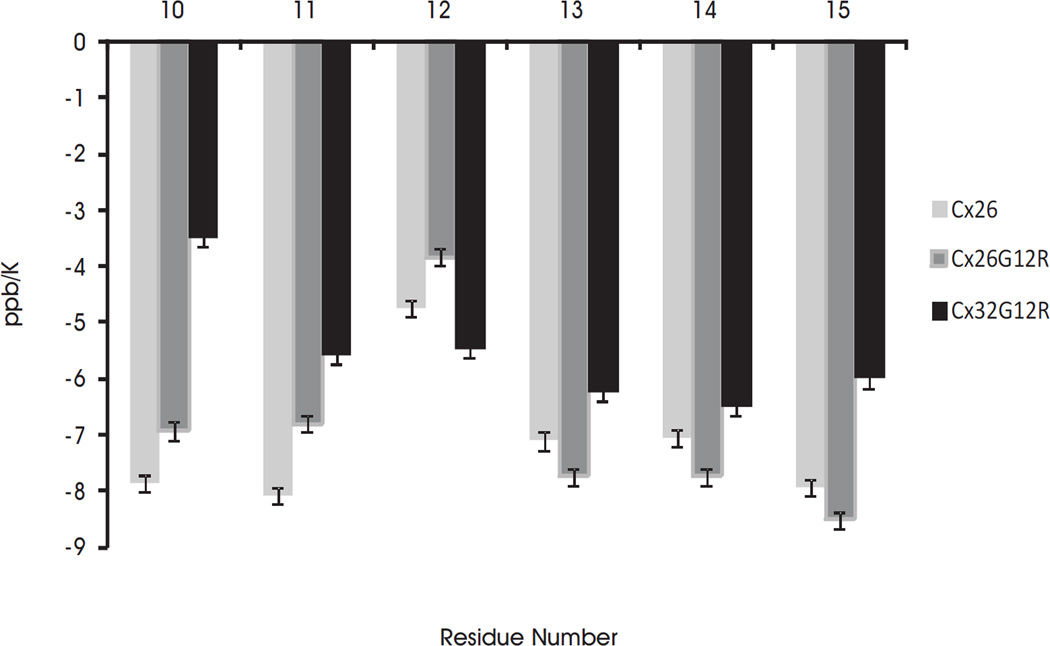
Temperature coefficients give structural information for amide protons and generally agree with the 13Cα chemical shifts. The temperature coefficients for the amide protons of WT Cx26, Cx26G12R and Cx32G12R peptides are shown in Figure 4a . The extent to which a temperature coefficient for an amide proton is lowered, from the solvent exposed value of 8 ppb K−1, is a measure of the degree of protection from the solvent [42]. A later study reported random coil temperature coefficients for solvent exposed amide protons for each amino acid [43]. The random coil values for amide proton temperature coefficients for amino acids used in our study range from −6.43 to −9.32 ppb/K depending on the amino acid [43]. Temperature coefficients of less than 6 ppb/K are suggestive of hydrogen bonding [42]. Figure 4a shows temperature coefficients calculated for residues whose NH proton was visible at all temperatures. In addition, we have plots of temperature coefficients normalized to their random coil value (see Figure 2 in reference [41]). Figure 4a shows that in Cx26 wild-type and Cx26G12R mutant the amino acid residues 3–9 (WGTLQTI) have temperature coefficients which are lower than their random coil values (W-7.98, T-7.40, G-7.02, L-8.42, Q-7.65, I-8.35) with residues 4–8 being particularly reduced. In Cx26 wild-type and Cx26G12R mutant, the reduced temperature coefficients were most likely due to hydrophobic interactions between W3, L6, I9 and L10, which stabilize the helical like turns and are expected to provide solvent protection to these protons and/or the reported effect of an aromatic ring in close proximity to the glycine amide proton [43]. Additionally in the Cx26G12R some structures show a H-bond between either T5 NH or L6 NH protons and the backbone carboxyl group of D2. In Cx32G12R, the N-terminus forms a U shaped structure around residues 1–16. The lower temperature coefficients for residues 2 through 8 are due to the folded nature of the structure and hydrophobic interactions between sidechains of residues 3, 6, 7 and 9 which stabilize the folded structure and limit solvent exposure. Some structures show a H-bond between the NH proton of L6 and the backbone carboxyl group of W3 and the NH proton of T8 and the CO of G5, which may also contribute to the reduced temperature coefficient and stabilize the structure. However, as shown in Figure 4b, there is a difference in the temperature coefficients in the turn region within residues 11–15 between the wild-type Cx26, the Cx26G12R mutant and the Cx32G12R mutant peptides. The temperature coefficient for G12 in Cx26 and R12 in Cx26G12R is lower than the random coil values for these residues (7.02 and 7.64 respectively). Since there are almost no structures showing a H-bond with the NH proton from these residues the lowered temperature coefficient would therefore suggest this proton is in a folded, less solvent exposed region of the molecule. The other residues in this region mostly show NH temperature coefficients for the wild-type Cx26 and the Cx26G12R mutant peptides which are very close to or exceed their solvent exposed random coil values (7.02, 7.02, 8.35, 7.02 and 7.87) for GGVNK or (7.02, 7.64, 8.35, 7.02,and 7.87) for GRVNK respectively. Also the majority of NH temperature coefficients for the Cx26G12R protons in this region are a little higher, closer to their random coil values as compared to those of the wild-type Cx26 suggesting the turn in the mutant may be more flexible and unstructured. On the other hand, the NH temperature coefficients of the Cx32G12R mutant peptide are significantly lower than the random coil values for residues 11–15 (7.02, 7.64, 8.35, 7.02 and 7.64) for SRVNR respectively. These values are close to or less than a value of 6 suggesting the presence of hydrogen bonds within these residues. Indeed a large number of structures show the placement for a couple of H bonds in this region suggesting a more folded less flexible structure. The temperature coefficients in this turn region are also significantly lower than those previously reported for those in the wild-type Cx32 [25]. This data suggests less solvent exposure for residues 11–15 in the mutant Cx32G12R and a more constrained region in this peptide as compared to the wildtype Cx32, wildtype Cx26 and the Cx26G12R mutant.
Figure 4.
a) Plots of temperature coefficients vs. residue number for the Cx26 WT (top) and mutants Cx26G12R (middle) and Cx32G12R (lower). Temperature coefficients were only calculated for residues whose NH proton was observable at all temperatures. b) Temperature coefficients of residues 11–15 for the wild-type Cx26, mutant Cx26G12R and Cx32G12R peptides. The error bars represent errors of 0.15 ppb/K.
The Cx26, Cx26G12R and Cx32G12R mutant structures were calculated in CNS using 224 (Cx26), 152 (Cx26G12R) and 233 (Cx32G12R) distance constraints obtained by the NOESY experiments. Structural statistics for the 20 converged structures and atomic root-mean-square differences (RMSDs) are given in Table 1. A medium range NOE is defined as a NOE between 2 protons which are 2–4 residues away. A long range NOE is defined as a NOE between 2 protons which are 5 or more residues away. There were no NOE violations greater than 0.5 Å and no angle violations greater than 5°. Deviations from idealized covalent geometry are low and the Ramachandran statistics indicate that neither bad contacts nor distortions exist in the converged structures.
Table I.
Structural Statistics
| Values for 20 converged structures | |||
|---|---|---|---|
| Cx26 | Cx26G12R | Cx32G12R | |
| Total Constraints | 224 | 152 | 233 |
| Intraresidue | 70 | 83 | 121 |
| Sequential | 81 | 50 | 72 |
| Medium range | 72 | 16 | 35 |
| Long range | 1 | 3 | 5 |
| Largest distance constraint violation (Å) | 0.5 | 0.5 | 0.5 |
| RMSD | |||
| Backbone (Å) (3–15) | 0.86 | 1.69 | 1.00 |
| Heavy atoms (Å) (3–15) | 1.63 | 2.91 | 1.93 |
| Deviations from idealized geometry | |||
| Bonds (Å) | 8.38 ×10−3 | 2.46 × 10−3 | 2.64 × 10−3 |
| Angles (deg) | 0.996 | 0.404 | 0.360 |
| Ramachandran | |||
| Most favorable | 37.2 % | 38.5% | 43.3% |
| Additionally or generously allowed regions |
59.7 % | 55% | 53% |
| Disallowed regions | 3.1 | 6.5% | 3.7% |
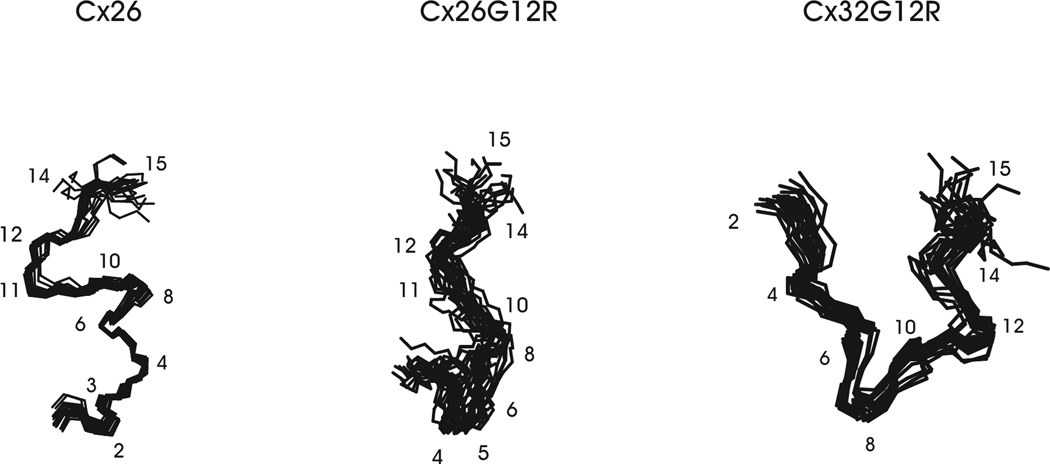
The 20 lowest energy structures that best fit the NMR data for wildtype Cx26, Cx26G12R and Cx32G12R mutant peptides are shown in Figure 5. Superposition of the backbone atoms for residues 3–15 for the Cx26 structures in Figure 5 gave an RMSD value of 0.86 Å. In the Cx26G12R mutant, superposition of backbone atoms for residues 3–15 gave an RMSD value of 1.69 Å. Superposition of the backbone atoms for residues 3–15 for the Cx32G12R mutant structures gave an RMSD value of 1.00 Å. These bundles show that the structures fit the NMR data well and that each molecule can be defined by a single conformation. They also show that the structural turns around and including residue 12 are well defined by the NMR data.
Figure 5.
The 20 lowest energy conformers of the Cx26 WT, Cx26G12R and Cx32G12R mutants are shown. Superpositions of the backbone atoms within residues 3–15 are shown for all the structures.
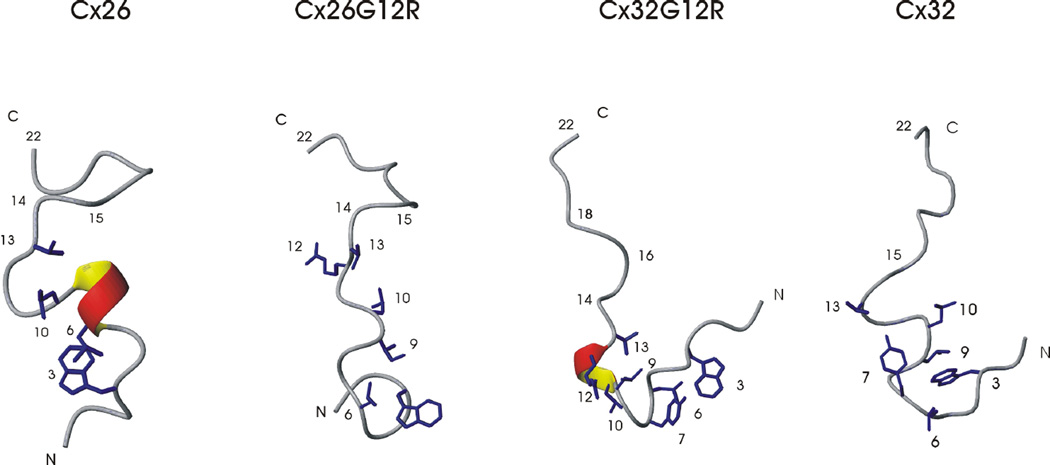
Representative low energy structures for Cx26, the Cx26G12R and Cx32G12R mutants and Cx32 N-terminal structures are shown in Figure 6. The structural data is supported by the 13Cα secondary shift and temperature coefficient data. The 22 amino acid Cx26 wild-type structure shows helical like turns in the beginning of the N-terminus (residues 2–6) with dihedral angles defining an α-helix within residues 6–9. Hydrophobic interactions occur among sidechains of residues 3–10 stabilizing the helical structure. The Cx26G12R structure has a similar overall fold to the Cx26 wildtype but with less well defined secondary structure. Despite having helical propensity, as shown by the 13Ca secondary shift plots, there were fewer NOES for this peptide suggesting it is a more dynamic and flexible structure. Hydrophobic sidechain interactions are seen between residues 3, 6, 9 and 10 as in the wild-type structure. Similar to Cx26, the internalization and interaction of the hydrophobic side chains between L10 and V13 stabilizes but does not constrict the turn within residues 10–15. A structural analysis revealed no H bonds within the turn encompassing residues 10–15 and temperature coefficients near those of the random coil values suggest an unconstrained, flexible turn in both structures.
Figure 6.
Representative ribbon diagrams of the Cx26 WT, Cx26G12R, Cx32G12R and Cx32 N-terminal peptides.
In comparison, the Cx32G12R mutant has a very different structure. Residues 1–16 form a U shaped structure which is stabilized by multiple hydrophobic sidechain interactions along this segment. Interactions are seen between the sidechains of residues 3, 6, 7, 9, 10 and 13. There are helical like turns in the beginning of the N-terminus within residues 2–10 and the dihedral angles within residues 9–13 indicate an α-helical segment. The hydrophobic sidechain of I9 interacts with those of L6, Y7 and L10. The hydrophobic sidechain of I9 also interacts with V13 constricting the turn around residue R12. The interactions of the I9 sidechain with those of residues below it and above it in the sequence stabilizes the U shaped turn in the first half of the N-terminus and constrains the structure. The turn around residue 12 is very constricted in this structure due to hydrophobic interactions between I9, L10 and V13 as well as reduced temperature coefficients (≤ 6 ppb K−1) and H bonds in this region shown in many of the structures. A structural analysis of the Cx32G12R mutant peptide shows the likelihood of a hydrogen bond between S11 NH- L9 CO, R12 NH – L9 CO and V13 NH – L10 CO with the R12NH and V13NH hydrogen bond occurring in many of the models.
Figure 6 also shows the comparison of the refined wild-type Cx32 N-terminal structure with that of the mutant Cx32G12R structure solved in this study. The sidechain hydrophobic interactions in the wild-type structure occur mainly within residues 3–10 while residue V13 is not involved in hydrophobic associations with L9 or L10 but interacts with Y7. In addition, we have previously reported the wild-type Cx32 N-terminal peptide has NH temperature coefficients in the residues comprising the turn (10–15) that are very close to or even greater than their solvent exposed random coil values [25] indicating a flexible turn around G12 in this peptide In the Cx32G12R mutant both the structure and the turn are more constricted. All Connexin N-terminal structures show an unstructured region in the C-terminus from residues 16 to 22.
Functional studies of Cx32 G12R
Our past structure-function studies have shown a correlation between formation of an open unconstrained turn in N-terminus peptides and functional Cx32 channels. N-terminal peptides with mutations that fail to form a flexible turn do not form functional channels [25, 26]. The solution structure of the Cx26 N-terminal G12R peptide indicates formation of a highly flexible open turn, whereas that of Cx32G12R forms a highly structured more constrained turn. Cx26G12R is reported to express membrane currents as a homomeric undocked hemichannel with altered voltage-dependence and reduced sensitivity to closure by extracellular Ca2+ [24]. Because of the structural differences between Cx26G12R and Cx32G12R mutant peptides, we asked whether the G12R mutation would form functional hemichannels in Cx32 as it does in Cx26, or if formation of a more constrained turn would compromise Cx32 hemichannel function.
We perform these functional studies on the background of a chimeric connexin, Cx32*43E1, which we have shown provides a model system to probe Cx32 structure-function relations and to determine how mutations alter channel function [44, 45]. Cx32*43E1 is a chimera in which the first extracellular loop (E1) of Cx32 (residues 41–70) is replaced with that of Cx43. Hemichannels formed by the chimera express robust voltage-dependent membrane currents in the plasma membrane of Xenopus oocytes when they are not docked to another hemichannel [46] and consequently permit direct biophysical characterization of hemichannel voltage-dependent properties.
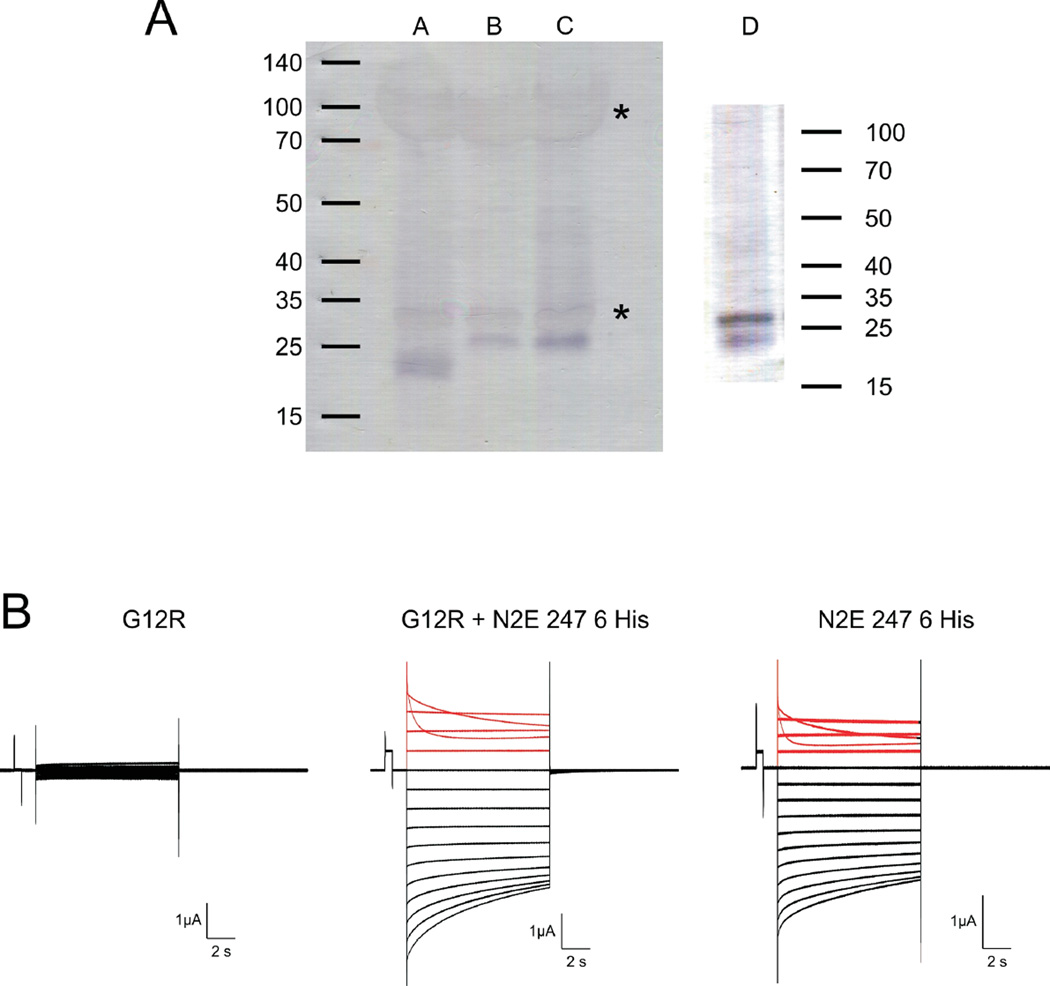
Unlike Cx26 G12R, Xenopus oocytes injected with Cx32*43E1 G12R RNA do not express membrane current attributable to an undocked connexin hemichannel (Fig 7B, n = 12 oocytes from 3 different frogs). This lack of expression does not result from improper protein folding and consequent degradation by cellular quality control systems [47, 48] or by failure of plasma membrane insertion, as western blots of DDM solubilized channels from enriched membrane preparations of Xenopus oocytes injected with Cx32*43E1 G12R RNA show that the mutant protein is the same size and expressed at levels comparable to wild type (Fig. 7A).
Figure 7.
Expression of G12R in Xenopus oocytes. Panel A. Western blot of Xenopus oocyte membrane proteins. Lane A. N2E Cx32*43E1 247 6 His. Lane B. Cx32*43E1 (wild type). Lane C. Cx32*43E1 G12R. Lane D. Oocytes coexpressing N2E Cx32*43E1 247 6 His and Cx32*43E1 G12R. Cx32*43E1 is a Cx32 chimera in which the first extracellular loop (E1, residues 41–70) has been replaced with that of Cx43 [46]. N2E Cx32*43E1 247 6 His denotes a Cx32*43E1 construct to which a hexa-histidine tag was attached at residue 247. Wild type Cx32 has an electrophoretic mobility that corresponds to ~ 27 kDa; the his-tagged truncation to ~ 23 kDa. Xenopus oocyte yolk proteins that bind trace amounts of either primary and/or secondary antibodies are marked by asterisks. The western blot in lane D corresponds to a “pull down” assay of heteromeric channels containing G12R subunits by IMAC affinity purification of channels containing his-tagged “wild type” (N2E Cx32*43E1 247 6 His) subunits. The presence of full length Cx32 (G12R) protein indicates formation of heteromeric hemichannels.
Panel B. Representative current traces obtained from oocytes voltage clamped to 0 mV and stepped from +50 to −100 mV in 10 mV increments. The currents shown in the left panel correspond to endogenous currents in oocytes expressing Cx32*43E1 G12R protein. No currents attributable to connexin channels are observed. The center panel shows currents from an oocyte co-injected with equal amounts of G12R and N2E Cx32*43E1 247 6 His RNA. The right panel shows currents from an oocyte expressing N2E Cx32*43E1 247 6 His protein. Currents elicited by depolarization from 0 mV are colored red (0 to 50 mV). Current relaxations at potentials more positive than 30 mV in the center and left panels result from closure of voltage-dependent Vj-gates and demonstrate expression of N2E Cx32*43E1 247 6 His subunits (see text). Current relaxations at inside negative potentials correspond to loop-gate closure in homomeric N2E Cx32*43E1 247 6 His channels (right panel).
To further investigate the cause underlying Cx32*43E1 G12R loss of function, we tested whether mixtures of G12R and “wild-type” (i.e. not containing the G12R substitution) subunits would form a conductive, voltage-gated heteromeric hemichannel. First, we verified heteromeric channel formation by western blots of purified channels following co-injection of mutant and wild-type RNA. The wild-type subunit was engineered with a C-terminal hexa-HIS-tag added to a Cx32*43E1 construct truncated to residue 247. The truncation does not alter Cx32*43E1 expression or voltage-dependence [49] in macroscopic recordings in Xenopus oocytes. The HIS tag allowed channel purification from enriched membrane preparations by immobilized metal affinity chromatography (IMAC). Truncation of the “wild-type” 43E1 subunit to residue 247 removes the last 36 residues and reduces the molecular weight by ~ 2kDa permitting resolution of “wild-type” from G12R subunits following SDS-PAGE and western blotting (Fig. 7A).
We interpret the observed co-purification of G12R subunits with wild-type HIS-tagged subunits in this IMAC based “pull-down” assay to indicate formation of heteromeric channels (Fig 7A lane D). In this set of experiments, the concentration of the G12R subunit was approximately twice that of the “wild-type” (N2E43E1-247-HIS) subunit. If we assume that subunits assemble randomly into hexameric hemichannels, then from the binomial distribution the probability of a hemichannel containing 0, 1, 2, 3, 4, 5, and 6 G12R subunits is 0.002, 0.02, 0.095, 0.236, 0.328, 0.244, and 0.075 respectively,. In the population of channels formed, nearly all (p = 0.998) will contain at least one G12R subunit and nearly all (p = 0.925) will contain at least one wild type subunit. In electrophysiological recordings of oocytes performed in parallel with this biochemical characterization, the mean peak current at 50 mV was roughly 3 times greater for heteromeric hemichannels; 3.14 ± 1.17 µA (n = 6), than for the homomeric “wild-type” hemichannels; 1.4 ± 0.14 µA (n = 3). The robust expression of membrane current suggests that most if not all permutations of heteromeric hemichannels form conductive hemichannels. This in turn suggests that the presence of one “wild-type” subunit may be sufficient to permit ion permeation in heteromeric channels. By similar reasoning, we also conclude that incorporation of a single G12R subunit into heteromeric channels does not abolish hemichannel function, i.e. G12R does not act as a dominant negative mutation, because if it did, one would observe very low or no current expression corresponding to the small proportion (0.002) of “wild type” hemichannels.
To monitor the formation of heteromeric channels in electrophysiological studies (Fig. 7), we substituted a glutamic acid residue for the asparagine at the second position (N2E) of the HIS tagged Cx32*43E1 truncated subunit (termed Cx32N2E-247-HIS for simplicity). The N2E substitution reverses the polarity of the Vj-gating (also termed fast-gating) from closure favored at inside negative potentials to closure favored at inside positive potentials [50]. It is well established that heteromeric channels containing at least one N2E subunit will display bi-polar Vj-gating. Closure of Vj-gates at inside positive membrane potentials is attributed to the action of N2E subunits and closure of Vj-gates at inside negative potentials is attributed to the action of subunits containing asparagine at the second position, in this case the G12R subunits [44]. Thus, in the macroscopic recordings of heteromeric channels shown in Fig. 7B, current relaxations observed at positive potentials (red traces) correspond to Vj-gating of hemichannels containing at least one Cx32N2E-247-HIS subunit.
In homomeric “wild-type” channels (Cx32N2E-247-HIS), current relaxations elicited by membrane polarizations to voltages more negative than −30mV (black traces, Fig. 7) report channel closure by a second distinct voltage-dependent process that is termed loop-gating or slow-gating [51, 52]. In contrast to Vj-gating, loop-gating is most likely a cooperative process that requires a concerted conformational change of all six hemichannel subunits to effect channel opening and closing [53]. In heteromeric hemichannels, the underpinnings of current relaxations at inside negative potentials are more complex: these relaxations result from the closure of Cx32 G12R subunits by Vj-gating in addition to closure of loop-gates. Recall that in heteromeric hemichannels containing a single G12R subunit, closure of Vj-gates will occur also at inside negative potentials, i.e. heteromeric channels containing both Cx32N2E and Cx32G12R subunits will display bi-polar Vj-gating, Cx32N2E subunits will close at inside positive potentials, Cx32G12R subunits will close at inside negative potentials [44].
Thus, although not directly comparable (because of the expected expression of Cx32G12R Vj-gating at inside negative potentials in heteromeric hemichannels), the marked similarity of the voltage-dependence of heteromeric and homomeric hemichannels is nonetheless significant because it strongly suggests that the loss of function of G12R homomeric hemichannels does not result from large shifts in voltage-dependence. Stated differently, there is no evidence to indicate that the presence of a Cx32 G12R subunit destabilizes the open state to promote either Vj- or loop-gate closure.
The simplest explanation for the failure of Cx32 G12R to produce conductive homomeric channels is that the N-terminus of this mutant has a larger, constrained turn, as shown by NMR, which allows the G12R N-terminus to extend into the channel pore and block ion conduction in the homomeric mutant hemichannel by reducing pore diameter. We propose that ion conductance is restored in heteromeric channels as the obstruction caused by G12R is relieved by successive addition of the more flexible WT N-termini.
DISCUSSION
In this study, we examine the structures of three peptides corresponding to the N-terminus (residues 1–22) of wild-type Cx26, a syndromic deafness mutation - Cx26G12R, and the corresponding Cx32 mutation - Cx32G12R in the context of their channel properties and relation to connexin diseases. Cx26G12R has been reported to express membrane currents as an undocked hemichannel as does Cx26 but not as an intercellular channel (gap junction). Changes in functional properties of the undocked Cx26G12R hemichannel, which include reduced sensitivity of hemichannel regulation by extracellular calcium, are believed to underlie the dominant gain of function KID phenotype, a severe often lethal skin disease associated with hereditary deafness. In contrast, in Cx32, the G12R mutation is a loss of function mutation that prevents expression of membrane currents in a chimeric Cx32 undocked hemichannel. Notably, but like other N-terminal Cx32 mutations, G12R expresses membrane currents when expressed as a heteromeric hemichannel comprised of wild type and mutant subunits [54]. The different effects of the G12R mutation in Cx26 and Cx32 are related to the differences in amino acid interactions determining N-terminus structure.
The structure of the 22 amino acid Cx26 peptide is similar to that of the 15 amino acid Cx26 wild-type peptide that we solved previously [55] . The shorter peptide had helical like turns in the beginning of the N-terminus (residues 2–10) and an open turn encompassing residues 12–15. The longer peptide has helical-like turns from residues 4–9 and then a looser turn encompassing residues 10–16. This peptide structure is very similar to the N-terminus in the x-ray crystal structure of the Cx26 gap junction channel [1] as well that of the Cx26 hemichannel refined by all-atom molecular dynamics simulation in a fully hydrated membrane system [2]. The agreement between the N-terminus in the Cx26 crystal and the peptide structure reported here is another example that small peptides from large proteins have structures resembling their conformation in the native protein [56–58]. Furthermore, the similarity suggests that the Cx26G12R peptide structure will inform the structure of the N-terminus in the mutant channel.
As shown in Figure 5, the Cx26 and Cx26G12R peptides have overall similar structure. Notably, the unconstrained turn around residues 10–15 is not disrupted by the Arg substitution, which indicates that the turn can accommodate a residue with a longer side chain, possibly because the very short side chain of the adjacent residue (G11) will minimize steric hindrance.
In contrast, the overall structure of the Cx32G12R mutant peptide dissimilar to the wild-type Cx32 N– terminal structure. It is less flexible and more constricted by hydrophobic interactions and has a more constrained helical turn around residues 9–13. This may be due to the additional polar sidechain of R12, along with S11, being surface oriented which would influence an inner orientation of hydrophobic sidechains of V13, L10 and L9. This facilitates their interaction as well as interaction with other hydrophobic sidechains in the peptide producing a more constricted structure.
We and others have shown that the N-termini of Cx26 and Cx32 contain an unconstrained turn around G12 that positions the first 10 amino acids into the channel pore [25, 54, 55] allowing it to play a key role in voltage-gating, channel conductance and charge selectivity. Our past studies have shown that formation of an open flexible turn in Cx32 N-terminal peptides correlates with channel expression. Cx32 peptides containing substitutions G12S, Y7D and W3D do not form the open turn initiated by G12 and these mutations fail to produce functional channels [25, 26, 54]. In the case of G12S, the loss of expression is a consequence of a trafficking/assembly defect [19], and it is likely that the same is true for Y7D and W3D. In contrast, the Cx32 G12P peptide forms a flexible open turn [25] and the mutation forms intercellular channels in homotypic and in heterotypic pairings with Cx26 and Cx32 that are nearly identical to those formed with wild-type Cx32 [55].
Here, we show that the Cx32 G12R N-terminal peptide forms a highly structured inflexible turn encompassing residues 1–14. Unlike, G12S, Y7D and W3D, the G12R turn could position the N-terminus within the channel pore allowing for assembly and membrane insertion of G12R channels, consistent with the biochemical characterizations performed in this study. Therefore, the failure of homomeric G12R homomeric undocked hemichannels to express currents in Xenopus oocytes is not a consequence of defects in channel assembly and/or trafficking to the plasma membrane. Notably, heteromeric hemichannels containing mixtures of G12R and “wild-type” subunits express robust membrane currents with voltage-dependencies similar to wild-type homomeric channels. This indicates that lack of expression of membrane currents is not caused by large shifts in voltage-dependent gating, such that homomeric G12R hemichannels are closed over the voltage range examined in this study. The simplest structural explanation is that the inflexible turn observed in G12R peptides causes the N-terminus to project into the channel pore and block ionic currents. We propose that incorporation of wild-type subunits in heteromeric channels progressively relieves this block. Rescue of mutant Cx32 channel function by formation of heteromeric channels may provide a viable strategy to treat some CMT-X mutations by insertion and expression of wild-type Cx32 in affected cells.
In contrast, the Cx26 N-terminal G12R peptide forms a highly flexible open turn that,like wild-type Cx26, allows positioning of the N-terminus into the channel pore. Cx26 G12R is reported to express membrane currents as a homomeric undocked hemichannel in Xenopus oocytes but with reduced sensitivity to closure by voltage and extracellular Ca2+ (20). Experimental studies have shown that the loop-gate permeability barrier and computational studies strongly suggest that the voltage-sensor is formed by residues located in the channel pore at the border of the first transmembrane domain and first extracellular loop, termed the 310 helix[1] or parahelix [53]. The Cx26 gap junction crystal structure solved in 20 mM Ca2+ indicates that residues in this region of the channel pore also coordinate Ca2+ [23] . Although it would reside at some distance from the loop-gate permeability barrier, introduction of a positive charge at the 12th position will change pore electrostatics. An increase in positive charge in the channel pore is expected to decrease Ca2+ occupancy in the pore and consequently favor open state occupancy. Changes in the electric field within the channel pore may also decrease Cx26 channel sensitivity to applied voltage and favor channel opening at more negative potentials than wild type hemichannels.
It has been proposed that this voltage and Ca2+ misregulation of Cx26G12R accounts for the dominant gain of function KID disease phenotype by disruption of cell homeostasis (20). For reasons not yet understood, Cx26G12R does not form functional intercellular channels in pairs of Xenopus oocytes, an apparent loss of function that, along with increased hemichannel activity, may underlie the loss of hearing phenotype of the mutation. It seems unlikely that the very flexible N-terminus would alter the structure of the structurally stable extracellular loops to prevent intercellular channel formation. Others [59] have suggested that the syndromic deafness disease etiology is a consequence of the ability of some dominant gain of function syndromic mutations (including Cx26G12R) to form heteromeric hemichannels with wild-type Cx43. The gain of function of hemichannels in cochlear cells and in keratinocytes as well as nonfunctional intercellular transmembrane channels may be ascribed to this novel hemichannel formation [59]. Possibly the increased flexibility and open turn in the Cx26G12R N-terminus plays a role in its ability to form heteromeric hemichannels and allow hemichannel oligomerization [59].
Given that Cx26G12R forms functional hemichannels, the substitution of a positive charge at the 12th locus must change Cx26 hemichannel function to cause disease. Several KID mutations, including G11E, G12R, N14K, A40V, G45E, D50N/A [20, 22, 60–62] have been reported to produce “leaky” Cx26 hemichannels resulting from impaired hemichannel regulation by extracellular Ca2+. It has been shown that Ca2+ favors Cx26 hemichannel closure by both destabilization of the open state [21] and stabilization of the voltage-dependent loop-gate closed state [22, 63]. At the resting potential of cells (reported to lie between −25 and −40 mV, [64]) Cx26 hemichannels will reside in the “loop-gate” closed state. Decreased sensitivity to Ca2+ caused by KID mutations would increase Cx26 hemichannel opening in epithelial and supporting cells in the cochlea and in keratinocytes. This would perturb homeostasis by collapsing membrane potential and increase Ca2+ entry or exit of ATP and other signaling molecules and loss of cellular metabolites leading to cell death [65] of both cochlear and skin cells contributing to deafness and fatal skin disease [66].
In conclusion, the determinants of Cx26 and Connexin 32 N-terminal structure differ in the two connexins. The same mutation, G12R, alters each structure differently and produces different functional effects in the respective channels. The Cx32G12R structure is altered from wild-type Cx32 and had decreased flexibility which can be directly related to loss of channel function. In contrast, the Cx26G12R mutation results in a more subtle structural change and an increase in flexibility which is similar to wild-type Cx26. The Cx26G12R mutant leads to a gain of function phenotype which can be explained by electrostatic changes in the pore rather than overt structural changes in the N-terminus. The different structural and functional effects of the G12R mutation in Cx32G12R and Cx26G12R lead to different diseases, Charcot Marie Tooth disease and syndromic deafness (KID disease) respectively. The data presented in this study sheds light on how differences in the structure of Cx26 and Cx32 channels are related to the etiology of these two diseases and why the same mutation at the G12 locus has markedly different effects on functional properties.
Connexin26G12R, a gain of function, syndromic deafness mutation (KID) produces functional hemichannels.
Chimeric Connexin32G12R hemichannels do not express membrane currents.
Differences in function correlate with 1H NMR structures of Cx26G12R (less constrained) and Cx32G12R (more constrained).
Function of Cx32G12R chimeric hemichannels could be rescued by formation of heteromeric channels with WT Connexin 32 chimera.
Positive charge in pore of Cx26G12R may change pore electrostatics impairing hemichannel regulation by Ca2+.
Acknowledgments
The authors would like to thank Drs. Mark Girvin, Sean Cahill and Richard Harris for helpful discussions and technical advice. This work was supported by NIH grant GM098584 to T.A.B. and T.L.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cx26 – Connexin 26, Cx32- Connexin 32
REFERENCES
- 1.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5Å resolution. Nature. 2011;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 2.Kwon T, Harris AL, Rossi A, Bargiello TA. Molecular dynamics simulations of the Cx26 hemichannel: Evaluation of structural models with Brownian dynamics. J Gen Physiol. 2011;138:475–493. doi: 10.1085/jgp.201110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke D, Bian S, Li H, Harris AL. Post-translational modifications of connexin26 revealed by mass spectrometry. Biochem J. 2009;424:385–398. doi: 10.1042/BJ20091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett MVL, Zukin RS. Electrical Coupling and Neuronal Synchronization in the Mammalian Brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 5.Potolicchio I, Cigliola V, Velazquez-Garcia S, Klee P, Valjevac A, Kapic D, Cosovic E, Lepara O, Hadzovic-Dzuvo A, Mornjacovic Z, Meda P. Connexin-dependent signaling in neuro-hormonal systems. (BBA) - Biomembranes. 2012;1818:1919–1936. doi: 10.1016/j.bbamem.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox. Signal. 2009;11:283–295. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MVL, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retamal MA, Alcayaga J, Bultynck G, leybaer L, Sáez PJ, Fernandez R, León LE, Saez JC. Opening of pannexin and connexin based-channels increases the excitability of nodose ganglion sensory neurons. Front. Cell. Neurosci.ence. 2014;8 doi: 10.3389/fncel.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Siebert AP, Cheung K-H, Lee RJ, Johnson B, Cohen AS, Vingtdeux V, Marambaud P, Foskett JK. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. P. Natl.l Acad. Sci. 2012;109:E1963–E1971. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Structural and Functional Similarities of Calcium Homeostasis Modulator 1 (CALHM1) Ion Channel with Connexins, Pannexins, and Innexins. J. Biol. Chem. 2013;288:6140–6153. doi: 10.1074/jbc.M112.409789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snoeckx RL, Huygen PLM, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A, Ploski R, Murgia A, Orzan E, Castorina P, Ambrosetti U, Nowakowska-Szyrwinska E, Bal J, Wiszniewski W, Janecke AR, Nekahm-Heis D, Seeman P, Bendova O, Kenna MA, Frangulov A, Rehm HL, Tekin M, Incesulu A, Dahl H-HM, du Sart D, Jenkins L, Lucas D, Bitner-Glindzicz M, Avraham KB, Brownstein Z, del Castillo I, Moreno F, Blin N, Pfister M, Sziklai I, Toth T, Kelley PM, Cohn ES, Van Maldergem L, Hilbert P, Roux A-F, Mondain M, Hoefsloot LH, Cremers CWRJ, Löppönen T, Löppönen H, Parving A, Gronskov K, Schrijver I, Roberson J, Gualandi F, Martini A, Lina-Granade G, Pallares-Ruiz N, Correia C, Fialho G, Cryns K, Hilgert N, Van Heyningde Heyning P, Nishimura CJ, Smith RJH, Van Camp G. GJB2 Mutations and Degree of Hearing Loss: A Multicenter Study. Amer. J. Hum. Genet. 2005;77:945–957. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levit NA, White TW. Connexin hemichannels influence genetically determined inflammatory and hyperproliferative skin diseases. Pharmacol. Res. 2015;99:337–343. doi: 10.1016/j.phrs.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott CA, Tattersall D, O’Toole EA, Kelsell DP. Connexins in epidermal homeostasis and skin disease. (BBA) - Biomembranes. 2012;1818:1952–1961. doi: 10.1016/j.bbamem.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Bergoffen J, Scherer S, Wang S, Scott M, Bone L, Paul D, Chen K, Lensch M, Chance P, Fischbeck K. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- 15.Abrams C, Freidin M. GJB1-associated X-linked Charcot-Marie-Tooth disease, a disorder affecting the central and peripheral nervous systems. Cell Tissue Res. 2014:1–15. doi: 10.1007/s00441-014-2014-6. [DOI] [PubMed] [Google Scholar]
- 16.Abrams CK, Scherer SS. Gap junctions in inherited human disorders of the central nervous system. (BBA) - Biomembranes. 2012;1818:2030–2047. doi: 10.1016/j.bbamem.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleopa KA, Abrams CK, Scherer SS. How do mutations in GJB1 cause X-linked Charcot-Marie- Tooth disease? Brain Res. 2012;1487:198–205. doi: 10.1016/j.brainres.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh S, Ri Y, Bennett MV, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997;19:927–938. doi: 10.1016/s0896-6273(00)80973-3. [DOI] [PubMed] [Google Scholar]
- 19.Deschênes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered Trafficking of Mutant Connexin32. J. Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JR, DeRosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J.Invest. Dermatol. 2009;129:870–878. doi: 10.1038/jid.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez W, Gonzalez J, Liu Y, Harris AL, Contreras JE. Insights on the mechanisms of Ca2+ regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y) J.Gen. Physiol. 2013;142:23–35. doi: 10.1085/jgp.201210893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 2010;136:47–62. doi: 10.1085/jgp.201010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett BC, Purdy MD, Baker KA, Acharya C, McIntire WE, Stevens RC, Zhang Q, Harris AL, Abagyan R, Yeager M. An electrostatic mechanism for Ca2+-mediated regulation of gap junction channels. Nat Commun. 2016;7 doi: 10.1038/ncomms9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JR, Derosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J Invest Dermatol. 2009;129:870–878. doi: 10.1038/jid.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalmatsky BD, Bhagan S, Tang Q, Bargiello TA, Dowd TL. Structural studies of the N-terminus of Connexin 32 using 1H NMR spectroscopy. Arch Biochem Biophys. 2009;490:9–16. doi: 10.1016/j.abb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalmatsky BD, Batir Y, Bargiello TA, Dowd TL. Structural studies of N-terminal mutants of connexin 32 using (1)H NMR spectroscopy. Arch Biochem Biophys. 2012;526:1–8. doi: 10.1016/j.abb.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrifield RB. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;5:2149–2154. [Google Scholar]
- 28.Piotto M, Saudek V, Sklenář V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 29.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BA, Blevins RA. NMR View: A computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 31.Kjaergaard M, Brander S, Poulsen FM. Random coil chemical shift for intrinsically disordered proteins: effects of temperature and pH. J. Biomol. NMR. 2011;49:139–149. doi: 10.1007/s10858-011-9472-x. [DOI] [PubMed] [Google Scholar]
- 32.Kjaergaard M, Poulsen FM. Sequence correction of random coil chemical shifts: correlation between neighbor correction factors and changes in the Ramachandran distribution. J.Biomol. NMR. 2011;50:157–165. doi: 10.1007/s10858-011-9508-2. [DOI] [PubMed] [Google Scholar]
- 33. spin.niddk.nih.gov/bax/nmrserver/Poulsen_rc_cs.
- 34.Wuthrich K. NMR of Proteins and Nucleic Acids. New York: John Wiley and Sons; 1986. [Google Scholar]
- 35.Bax A, Davis DG. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 1985;65:355–360. [Google Scholar]
- 36.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D. Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 37.Koradi R, Billeter M, Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 38.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 39.Spera S, Bax A. Empirical correlation between protein backbone conformation and C.alpha. and C.beta. 13C nuclear magnetic resonance chemical shifts. J. Am. Chem. Soc. 1991;113:5490–5492. [Google Scholar]
- 40.Wishart DS, Sykes BD. Chemical shifts as a tool for structure determination. Methods Enzymol. 1994;239:363. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- 41.Batir Y, Bargiello TA, Dowd TL. 1H and 13C NMR Chemical Shift and Temperature Coefficient Analysis for Connexin 32 and Connexin 26 N-Terminal Peptides. Arch Biochem Biophys (DIB) 2016 [Google Scholar]
- 42.Dyson HJ, Satterthwait AC, Lerner RA, Wright PE. Conformational preferences of synthetic peptides derived from the immunodominant site of the circumsporozoite protein of Plasmodium falciparum by proton NMR. Biochemistry. 1990;29:7828–7837. doi: 10.1021/bi00486a008. [DOI] [PubMed] [Google Scholar]
- 43.Merutka G, Dyson HJ, Wright PE. Random coil 1H chemical shifts obtained as a function of temperature and trifluoroethanol concentration for the peptide series GGXGG. J. Biomol. NMR. 1995;5:14–24. doi: 10.1007/BF00227466. [DOI] [PubMed] [Google Scholar]
- 44.Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J. Gen. Physiol. 2000;116:13–31. doi: 10.1085/jgp.116.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfahnl A, Zhou XW, Werner R, Dahl G. A chimeric connexin forming gap junction hemichannels. Pflugers Arch. 1997;433:773–779. doi: 10.1007/s004240050344. [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Retzlaff M, Roos T, Frydman J. Cellular Strategies of Protein Quality Control. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babst M. Quality control at the plasma membrane: One mechanism does not fit all. J. Cell Biol. 2014;205:11–20. doi: 10.1083/jcb.201310113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon T, Tang Q, Bargiello TA. Voltage-dependent gating of the Cx32*43E1 hemichannel: Conformational changes at the channel entrances. J. Gen. Physiol. 2013;141:243–259. doi: 10.1085/jgp.201210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related onnexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 51.Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proceedings of the National Academy of Sciences. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukauskas FF, Verselis VK. Gap junction channel gating. (BBA) - Biomembranes. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon T, Roux B, Jo S, Klauda Jeffery B, Harris Andrew L, Bargiello Thaddeus A. Molecular Dynamics Simulations of the Cx26 Hemichannel: Insights into Voltage-Dependent Loop-Gating. Biophys J. 2012;102:1341–1351. doi: 10.1016/j.bpj.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purnick PEM, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys. J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purnick PEM, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 56.Adler M, Seto MH, Nitecki DE, Lin JH, Light DR, Morser J. The structure of a 19-residue fragment from the C-loop of the fourth epidermal growth factor-like domain of thrombomodulin. J. Biol. Chem. 1995;270:23366–23372. doi: 10.1074/jbc.270.40.23366. [DOI] [PubMed] [Google Scholar]
- 57.Katragadda M, Alderfer JL, Yeagle PL. Solution structure of the loops of bacteriorhodopsin closely resembles the crystal structure. (BBA)-Biomembranes. 2000;1466:1–6. doi: 10.1016/s0005-2736(00)00167-x. [DOI] [PubMed] [Google Scholar]
- 58.Katragadda M, Alderfer JL, Yeagle PL. Assembly of a polytopic membrane protein structure from the solution structures of overlapping peptide fragments of bacteriorhodopsin. Biophys J. 2001;81:1029–1036. doi: 10.1016/S0006-3495(01)75760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.García IE, Maripillán J, Jara O, Ceriani R, Palacios-Muñoz A, Ramachandran J, Olivero P, Perez-Acle T, González C, Sáez JC. Keratitis-ichthyosis-deafness syndrome-associated cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type cx43. J.l Invest. Dermatol. 2015;135:1338–1347. doi: 10.1038/jid.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. - Cell Ph. 2007;293:C337–C345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 61.Terrinoni A, Codispoti A, Serra V, Didona B, Bruno E, Nisticò R, Giustizieri M, Alessandrini M, Campione E, Melino G. Connexin 26 (GJB2) mutations, causing KID Syndrome, are associated with cell death due to calcium gating deregulation. Biochem. Biophy.l Res. Co. 2010;394:909–914. doi: 10.1016/j.bbrc.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez HA, Villone K, Srinivas M, Verselis VK. The D50N mutation and syndromic deafness: altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions. J. Gen. Physiol. 2013;142:3–22. doi: 10.1085/jgp.201310962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez HA, Bienkowski R, Slavi N, Srinivas M, Verselis VK. Altered Inhibition of Cx26 Hemichannels by pH and Zn2+ in the A40V Mutation Associated with Keratitis-Ichthyosis-Deafness Syndrome. J. Biol. Chem. 2014;289:21519–21532. doi: 10.1074/jbc.M114.578757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koegel H, Alzheimer C. Expression and biological significance of Ca2+-activated ion channels in human keratinocytes. FASEB J. 2001;15:145–154. doi: 10.1096/fj.00-0055com. [DOI] [PubMed] [Google Scholar]
- 65.Martinez AD, Acuna R, Figueroa V, Maripillan J, Nicholson B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid Redox Signal. 2009;11:309–322. doi: 10.1089/ars.2008.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez HA, Verselis VK. Aberrant Cx26 hemichannels and keratitis-ichthyosis-deafness syndrome: insights into syndromic hearing loss. Front. Cell. Neurosci. 2014;8 doi: 10.3389/fncel.2014.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]