Abstract
Subarachnoid hemorrhage secondary to rupture of a circumferential dissecting aneurysm continues to be a treatment dilemma. Vessel sacrifice, when possible, continues to be the safest option but in certain cases this is not possible due to lack of collateral supply. In such cases, coil assisted endovascular flow diversion has become a potential option but the requirement for dual antiplatelet therapy in an unsecured intracranial aneurysm continues to raise concern.
We present a 48-year-old man with a World Federation of Neurological Surgeons grade 5 subarachnoid hemorrhage, secondary to a ruptured intradural left vertebral artery dissecting aneurysm, who was treated successfully with a pipeline embolization device with Shield technology using aspirin and a single intravenous loading dose of abciximab. To our knowledge, this is the first case of an acute flow diversion performed using only aspirin as the sole oral antiplatelet agent.
Keywords: Aneurysm, Flow Diverter, Platelets, Hemorrhage
Background
Subarachnoid hemorrhage secondary to rupture of a circumferential dissecting aneurysm continues to be a difficult lesion to treat with the techniques available today. Vessel sacrifice, when possible, whether open or endovascular, continues to be the safest option but in certain cases this is not possible due to lack of a collateral supply.
In such cases, coil assisted endovascular flow diversion has become a potential option, as the dissected aneurysmal wall is jailed and flow is allowed to bypass the area of abnormality through the lumen of the flow diverter construct. However, the requirement for dual antiplatelet therapy in an unsecured intracranial aneurysm continues to raise concern.
The pipeline embolization device (PED) with Shield technology (PED-SHIELD; Covidien-Medtronic, Irvine, California, USA) is a new modification of the surface of the flow diverter which has lower thrombogenicity in vitro compared with the last iteration of the device, the PED Flex. Given this, we report a case where the PED-SHIELD was used with a single dose of abciximab and regular aspirin with no imaging or clinical evidence of significant thromboembolic complications.
Case presentation
A 48-year-old man presented to the emergency department after being found unresponsive. CT demonstrated extensive subarachnoid hemorrhage within the posterior fossa and basal cisterns, and intraventricular hemorrhage. CT angiography revealed a circumferential dissecting aneurysm of the intradural left vertebral artery just proximal to the vertebrobasilar confluence (figure 1).
Figure 1.
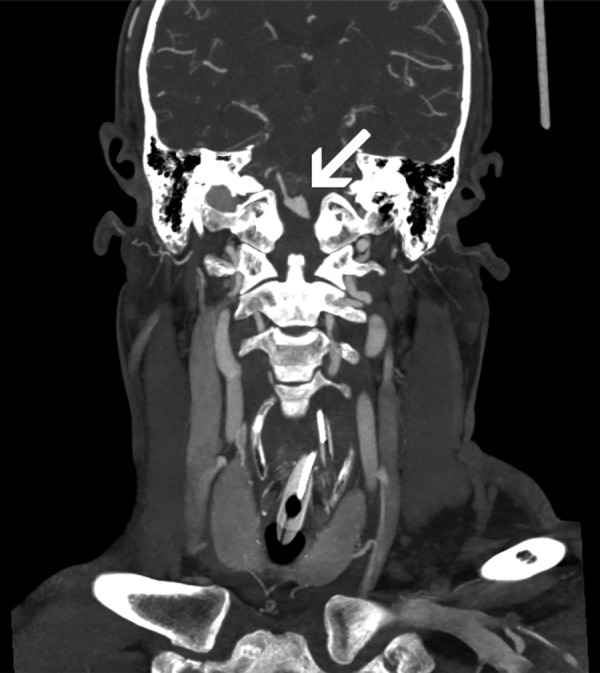
CT angiogram of the carotids and circle of Willis; coronal maximum intensity projection demonstrates the circumferential fusiform dissecting aneurysm of the left vertebral artery (arrow).
Given the appearances on CT, the patient was loaded with 300 mg of aspirin 2 hours prior to transfer to the neurointerventional suite. Cerebral angiography demonstrated a dissecting aneurysm with a visible intimal flap and a clear rupture point (figure 2). A coiling microcatheter was jailed into the aneurysmal lumen by a 3.5 mm×20 mm PED-SHIELD deployed across the aneurysm, with heparin 5000 units administered intravenously just prior to stent deployment. Twelve detachable coils were then deployed via the coiling catheter into the jailed aneurysmal lumen, resulting in near stasis (figure 3). On completion, 20 mg of abciximab were administered intravenously. The patient was started on a 5 day heparin infusion (with an activated partial thromboplastin time targeted at 40–50 s) and continued daily aspirin antiplatelet therapy.
Figure 2.
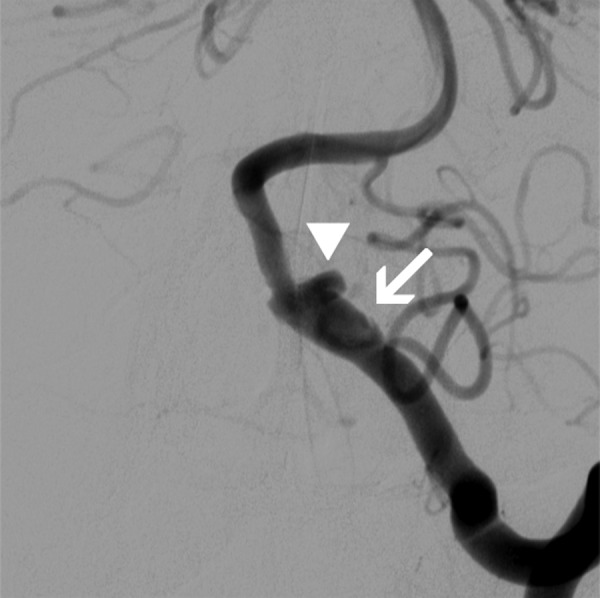
Catheter cerebral angiogram, left vertebral artery injection; posteroanterior projection demonstrates the fusiform dissecting aneurysm with the dissection flap (arrow) and the rupture point (arrowhead).
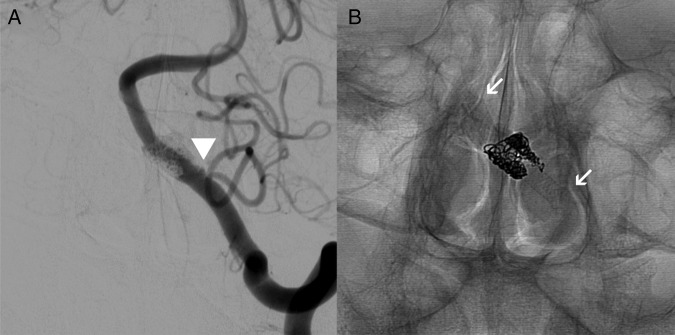
Figure 3.
Catheter cerebral angiogram, left vertebral artery injection, posteroanterior projections. (A) Subtracted angiogram with minimal filling of the sac proximally after coil assisted flow diversion (arrowhead). (B) The stent construct, with arrows marking the proximal and distal ends of the stent.
Outcome and follow-up
The patient was extubated in the subsequent week although he required prolonged rehabilitation. A repeat cerebral angiogram 1 week later demonstrated a patent flow diverter construct with stasis in the perfused portion of the aneurysm sac. Follow-up MRI with gadolinium enhanced angiography demonstrated no evidence of residual aneurysm by 2 weeks. A single small left cerebellar hemispheric diffusion weighted imaging abnormality was noted. MRI was repeated at 6 weeks, where the aneurysm remained occluded, the construct remained patent, and no further infarcts were identified. The patient was seen clinically at 7 weeks where he was still in rehabilitation but was independent. Aspirin was continued.
Discussion
Endovascular flow diversion has become a mainstream option for treatment of wide necked and fusiform aneurysms. Use of the PED has been described in the treatment of acutely ruptured blister aneurysms1 but concerns persist regarding the traditional requirement for dual antiplatelet therapy, typically aspirin and clopidogrel. Use of oral dual antiplatelet therapy requires some degree of preloading, rendering a period of time where the aneurysm is unprotected in the presence of multiple circulating antithrombotics, and also increasing the risk of external ventricular drain complications.2
The PED-SHIELD is a modification to the PED device developed by Medtronic which utilizes a phosphorylcholine (PC) coating bonded to the surface of the device, resulting in a thickness increase of <3 nm. The resultant effect is a physiologic mimicry of erythrocytes which normally are abundantly coated in PC. This has been demonstrated by thrombin generation testing to have significantly lower thrombogenicity compared with other flow diverting stents, and similar thrombogenicity to devices with less metal coverage (Solitaire AB, Covidien-Medtronic, Irvine, California, USA and LEO+, Balt Extrusion, Montmorency, France).3
Our patient's left vertebral artery could not be sacrificed as it was larger than the contralateral artery. Also, the patient had a large left posterior inferior cerebellar artery immediately proximal to the abnormal fusiform aneurysmal segment. Given the known in vitro effect of the PC coated PED-SHIELD device, we elected to utilize the effect to minimize the duration and extent of antithrombotic therapy.
The results of the sequential diffusion weighted imaging sequences serve to demonstrate in vivo the effectiveness of the PC coating for minimizing thrombogenicity, as the platelet effects of the abciximab administered as a bolus generally recover over 12 hours.4 Abciximab administration was elected by the primary operator to minimize the risk of acute thrombosis of the stent, but as endothelization does not occur for weeks, our case raises the possibility that in acute cases it may be reasonable to place the PED-SHIELD with only aspirin loading and full dose heparinization. Lower metal coverage stents may also benefit from PC coating, and it is not unforeseeable that in the future, stent assisted coiling may be routinely possible with single antiplatelet agent therapy. To our knowledge, this is the first case of acute or elective flow diverter therapy treated with aspirin as the sole oral antiplatelet agent.
Learning points.
Subarachnoid hemorrhage secondary to rupture of a circumferential dissecting aneurysm continues to be a treatment dilemma.
Vessel sacrifice, the traditional treatment, remains an effective and safe option when anatomically feasible.
When flow diversion is required to preserve perfusion, hemorrhagic complications secondary to dual antiplatelet loading need to be considered. The time the aneurysm is exposed to dual antiplatelet therapy and heparin should be minimized.
The pipeline embolization device (PED) with Shield technology (PED-SHIELD) has been demonstrated in vitro as well as our case to be effective in minimizing the potential for thromboembolic complications. In conjunction, intraprocedural loading with a single dose of abciximab may have a role.
Further studies are required to assess the thrombogenicity of the PED-SHIELD with aspirin only during treatment, as if this is possible, then the treatment of intracranial dissections may be revolutionized.
Footnotes
Contributors: AHYC and RR drafted the manuscript. JW and AC conceived the manuscript. All authors edited and reviewed the manuscript and give approval for the final version for publication.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chalouhi N, Zanaty M, Tjoumakaris S et al. . Treatment of blister-like aneurysms with the pipeline embolization device. Neurosurgery 2014;74:527–32. 10.1227/NEU.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 2.Kung DK, Policeni BA, Capuano AW et al. . Risk of ventriculostomy-related haemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. Clinical article. J Neurosurg 2011;114:1021–7. 10.3171/2010.9.JNS10445 [DOI] [PubMed] [Google Scholar]
- 3.Girdhar G, Li J, Kostousov L et al. . In-vitro thrombogenicity assessment of flow diversion and aneurysm bridging devices. J Thromb Thrombolysis 2015;40:437–43. 10.1007/s11239-015-1228-0 [DOI] [PubMed] [Google Scholar]
- 4.Tcheng J, Ellis SG, George BS et al. . Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high risk coronary angioplasty. Circulation 1994;90:1757–64. [DOI] [PubMed] [Google Scholar]