Abstract
Introduction
With a rapidly ageing population, sarcopenic obesity, defined as decreased muscle mass and function combined with increased body fat, is a complex health problem. Although sarcopenic obesity contributes to a decline in physical function and exacerbates frailty in older adults, evidence from clinical trials about the effect of exercise and nutrition on this complex syndrome in Chinese older adults is lacking.
Methods and analysis
We devised a study protocol for a single-blind randomised controlled trial. Sarcopenia is described as age-related decline in muscle mass plus low muscle strength and/or low physical performance. Obesity is defined as a percentage of body fat above the 60th centile. Ninety-two eligible participants will be randomly assigned to a control group, nutrition group, exercise group and nutrition plus exercise group to receive an 8-week intervention and 12-week follow-up. The primary outcomes will be the change in short physical performance battery scores, grip strength and 6 m usual gait speed. The secondary outcomes will include basic activities of daily living scores, instrumental activity daily living scores, body composition and body anthropometric indexes. For all main analyses, the principle of intention-to-treat will be used.
Ethics and dissemination
This study was approved by the medical ethics committee of Zhejiang Hospital on 25 November 2015. The study will present data targeting the clinical effects of nutrition and exercise on physical function and body composition in a Chinese older population with sarcopenic obesity. The results will help to provide important clinical evidence of the role of complex non-pharmaceutical interventions for sarcopenic obese older people. The findings of this study will be submitted to peer-reviewed medical journals for publication and presented at relevant academic conferences.
Trial registration number
ChiCTR-IOR-15007501; Pre-results.
Keywords: elderly, sarcopenia, obesity, exercise, physical function
Strengths and limitations of this study.
This will be the first single-blind, randomised controlled trial examining the effectiveness of comprehensive and multicomponent interventions on sarcopenic obesity in a Chinese older population.
Objective and subjective measures of physical function will both be assessed.
A limitation of the study is that broader inferences cannot be drawn owing to the characteristics of the study sample.
Introduction
Age-related physiology changes in body composition occur that are characterised by loss of muscle mass and the presence of excess fat. Sarcopenic obesity is a complex health problem, with coexisting sarcopenia (decreased muscle mass and function) and obesity (increased body fat) in the same older adult.1 Previous research has reported the prevalence of sarcopenic obesity as between 4% and 50% in older adults.2–6 It is estimated that the number of the older adults in China will exceed 400 million by the year 2050, accounting for roughly one-third of the population.7 Clearly, the prevalence of sarcopenic obesity will continue to rise rapidly as the population ages. Sarcopenia and obesity may interact, and synergistically cause negative health outcomes, such as increased prevalence of chronic diseases and functional decline, which result in frailty, poor quality of life, physical disability and death.2 8–12
Previous studies have focused separately on the roles of exercise and nutrition in targeting sarcopenia or obesity, but fewer studies has been made of sarcopenic obesity.13–15 For sarcopenic obese elderly people, it is of utmost importance for interventions to maintain muscle mass and function as well as reduce excess fat.
As already reported, caloric restriction in obese elderly people reduced fat but led to loss of muscle mass, and therefore increased the risk of sarcopenia.16 17 Such repeated, long-term loss of muscle mass may have harmful effects by impairing physical function and exacerbating frailty.18 Diet protein supplementation and exercise may have a positive effect on maintaining muscle mass and strength in older adults, and protect against sarcopenia and frailty.19–22
In addition, evidence from intervention studies showed that exercise was beneficial for preventing and treating sarcopenia and obesity.13 23–25 A recent study conducted by Gadelha et al25 showed that resistance training might improve phenotypes related to sarcopenic obesity in older women.
Accordingly, given that inactivity and inappropriate food consumption are two important behavioural factors contributing to the development of sarcopenic obesity, it would not be surprising if combined exercise and nutrition interventions improved physical function to a greater extent than exercise or nutrition alone.15 25–28 For obese and frail older adults, complex intervention combining different exercise elements and a weight-loss diet had positive effects on body weight and physical function. One study reported that function increased by 21% from baseline in the group receiving combined treatment, whereas for the exercise-only group and the weight-loss-only group the increase was 15% and 12%, respectively.15
This study protocol aims at exploring the independent and combined effects of nutrition and exercise interventions on physical performance and body composition in older people with sarcopenic obesity, which is a significant contributor to the decline in physical function. Additionally, we will examine the effects of potential factors on the long-term response to different treatments.
Methods and design
Design
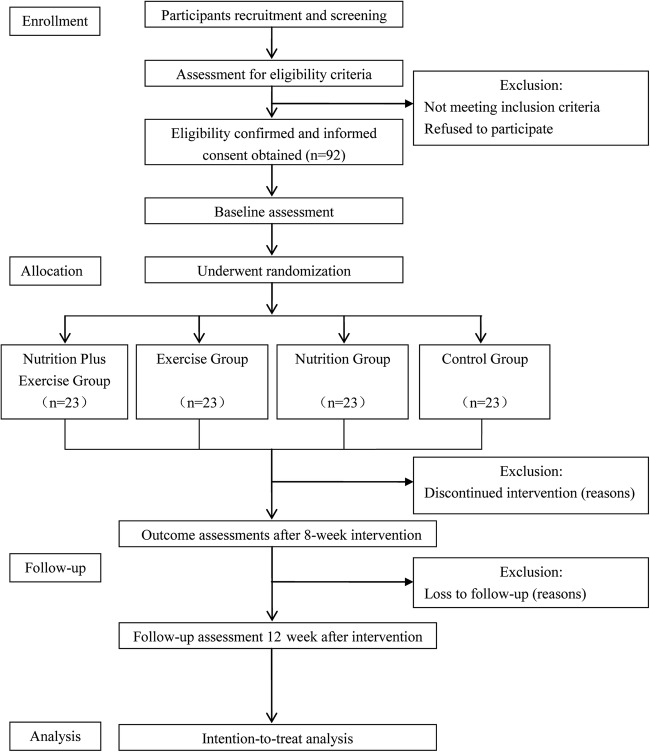
The study is a single-blind randomised clinical trial. The trial will be performed in Zhejiang Hospital in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement.29 The protocol was registered in the Chinese Clinical Trial Registry ChiCTR-IOR-15007501. Approval for the study was granted by the medical ethical committee of Zhejiang Hospital. Information about the study protocol, including the objectives, procedures, possible benefits and risks, will be provided at the beginning of the study and written informed consent will be obtained from each study participant. A flow diagram of the study design is presented in figure 1.
Figure 1.
Flow diagram of study design.
Participants
All 92 participants will be recruited through advertisement in the geriatric ward or clinic of Zhejiang Hospital. All potential participants will undergo comprehensive medical screening to confirm eligibility based on the following inclusion and exclusion criteria.
Inclusion criteria are as follows:
age ≥65 years;
diagnosis in accordance with the consensus for sarcopenia diagnosis by the Asian Working Group for Sarcopenia:30 low muscle mass plus low muscle strength and/or low physical performance; the cut-off values are shown in table 1;
obesity as defined by Baumgartner et al2: percentage of total body fat greater than the 60th centile of the study reference group; the cut-off values of obesity are 26.3% in men and 33.2% in women in reference to previous results;
volunteer to sign informed consent;
in a stable chronic disease state.
Table 1.
Cut-off values of sarcopenia by AWGS
| Assessment content | Assessment method | Cut-off values | |
|---|---|---|---|
| Low muscle mass | Height-adjusted appendicular skeletal muscle mass (ASM/height2, kg/m2) | Bioelectrical impedance analysis | Men: 7.0 kg/m2; women: 5.7 kg/m2 |
| Low muscle strength | Grip strength | Hand dynamometer | Men: <26 kg; women: <18 kg |
| Low physical performance | Usual gait speed | 6 m usual gait speed | ≤0.8 m/s |
ASM, appendicular skeletal muscle mass; AWGS, Asian Working Group for Sarcopenia.
Participants with any of the following conditions will be excluded:
taking nutritional supplements regularly or partaking in other clinical trials within the past 3 months;
having had an acute infection or acute cerebral organic disease in the past 6 months;
being equipped with a pacemaker;
having a serious heart disease, severe haemodynamic instability, severe bradycardia and tachycardia at rest;
having renal failure (creatinine clearance rate ≤60 mL/min/1.73 m2);
having severe cognitive impairment (Mini-Mental State Examination ≤17) or extremity disability, unable to complete gait speed and grip strength measurement;
having difficulty in swallowing or with oral intake;
having recently undergone major surgery or extremity surgery, unsuitable for taking part in the exercise intervention;
judged by the clinicians to be unsuitable to take part in the study for medical reasons.
Randomisation, allocation concealment and blinding
After initial assessment, a total of 92 eligible subjects will be randomly allocated into one of four groups in a ratio of 1:1:1:1 according to a computer-generated randomisation list. The allocation sequence will be concealed in sealed opaque envelopes, which will not be opened until the baseline assessment has been completed. The participants will be blinded to the group allocation (but not blinded to the programme they are taking part in), and will receive an 8-week intervention and a 12-week follow-up. Moreover, a trained geriatric nurse will assess the intervention outcome and a professional will perform the statistical analysis; both of them will be blinded to the group assignment of study participants.
Interventions
For this 20-week study, the participants will be randomly assigned to the following four groups: a group who will continue with their daily diet and exercise habits, and will be instructed not to participate in a nutrition or exercise programme (control group); a group who will follow a nutrition programme, with energy restriction and high-quality protein intake (nutrition group); a group who will follow an exercise programme, including flexibility exercise, aerobic exercise, balance and progressive resistance training (PRT) (exercise group) and a group who will follow both the nutrition and exercise programmes (nutrition plus exercise group).
Control group
Participants in the control group will not receive any advice about changing their usual diet or physical activity habits during the study period, and will be instructed not to participate in any nutrition or exercise programme. They will receive telephone calls or face-to-face interviews once a week. During these weekly visits, health problems, functional problems and medication use will be recorded by a trained researcher. At the same time, the researcher will reinforce the obligation to maintain their usual diet and activity habits.
Nutrition intervention programme
Prior studies reported that a nutrition programme with a moderate energy restriction of 500–750 kcal/day, while assuring a daily protein intake of 1.0–1.5 g/kg and appropriate intake of micronutrients, was advisable and seemed to be beneficial in the treatment of sarcopenic obesity in older people.27 31 In this study, at first, a specialised dietician will calculate the daily energy requirement of each participant according to his/her characteristics and daily physical activity. Participants in the nutrition group will be provided with a diet with an energy deficit of 500 kcal/day based on their daily energy requirement. This nutrition programme contains approximately 1.2 g of high-quality protein per kilogram of body weight per day, 20–30% of energy from intake of fats and appropriate intake of micronutrients. Each participant will be instructed to complete a food diary, and meet weekly with a dietitian for adjustments of the nutrition programme.
Exercise intervention programme
Participants assigned to the exercise intervention programme will be instructed by an experienced physical therapist (PT). The programme, consisting of 10 min of flexibility exercise, 20 min aerobic exercise, 10 min balance training and 20 min PRT in sequence, will be for 8 weeks, with 60-min sessions three times a week. Each exercise session will begin with 3–5 min warm-up involving elastic bands, stretching exercise and cycle ergometer. During the exercise sessions, blood pressure and heart rate will be measured by a portable ambulatory ECG monitor, and fear of falling and rating of perceived exertion32 will be self-reported. The PT will adjust the intensity of exercise according to the above objective and subjective measures.
The flexibility exercise will be performed at a moderate intensity of stretching activities for the neck, shoulder, elbow, wrist, hip, knee and ankle. Aerobic exercise will be performed at a moderate and high intensity of exercise using a cycle ergometer. The PT will prescribe a cycle ergometer at 60–80% of the age-predicted maximum heart rate as recommended by the American College of Sports Medicine (200−age)×(60 to 80%) as exercise therapy for cardiovascular diseases. Balance exercise will include feet in a side-by-side stance, semi-tandem stance, tandem stance, tandem walk, backward walking, standing from a sitting position and standing with eyes closed. PRT will comprise lower extremity exercise with an elastic band for stretching and squatting and upper extremity exercise with lifting of dumbbells. One repetition maximum (1-RM) for each participant will be evaluated before the PRT session, and will be performed every 4 weeks.
Exercises will be performed with one or two sets of 8–12 repetitions using 65% of their 1-RM in the preliminary stage of training, and then gradually increasing the intensity to two to three sets of 6–8 repetitions at 80% of 1-RM. A minimum frequency of 75% of exercise intervention will be adopted and, finally, the effect analysed. If a participant has any uncomfortable symptoms or adverse events during the exercise session, the PT will immediately stop that session and take action accordingly, then re-evaluate the participant's overall health and determine whether to continue the programme. At the same time, the PT will give researchers and the ethics committee feedback.
Baseline assessments
Physical function
The Chinese version of the short physical performance battery (SPPB) is used to assess physical performance, comprising 4 m at usual pace gait, chair stand test and three standing balance tests (side-by-side stance, semi-tandem stance and tandem stance).33 The SPPB score ranges from 0 to 12, and a score >8 indicates good physical performance. Grip strength is measured three times to assess muscle strength every 5 min using a hand dynamometer, with the participants sitting straight with elbows at a 90° angle.34 The maximum of the three trials will be used for the final analysis. A 6 m usual gait speed is also timed by a stopwatch, indicating current physical performance. In addition, a basic activities of daily living score35 measured with the Barthel Index and an instrumental activities of daily living score36 are used to evaluate daily functional status through special questionnaires.
Body composition.
Body composition is measured to evaluate segmental body fat and muscle mass by a bioelectrical impedance analysis method (Inbody 230, Biospace, Seoul, Korea).37 First, all participants will be asked to fast for at least 2 hours, urinate, defecate, and then stand for about 5 min before measurement. Second, all participants will remove their shoes and socks, and wear light clothing, then stand on the device while body weight is measured. Third, the participants' age, sex and height will be entered into the bioelectrical impedance analysis device. Finally, impedance will be measured with the participant standing still and holding the handles that are slightly abducted. Thereafter, details of total and segmental (arms, legs and trunk) body fat (kg), total body fat percentage (%), total and segmental muscle mass (kg) and fat-free mass (kg) will be output. From the above data, appendicular skeletal muscle mass (ASM, kg) and height-adjusted ASM (ASM/height2, kg/m2) will be calculated. ASM is defined as the sum of the muscle mass of four limbs. The 60th centile of total body fat percentage reflects the current degree of fat accumulation. The cut-off values are listed in table 1.
Additionally, body anthropometric indexes include body weight (kg), body height (m), calf circumference (cm), upper arm circumference (cm) and waist-to-hip ratio. The body mass index (kg/m2) is calculated as body weight divided by body height squared.
Blood examination
Venous blood samples will be collected after a 12-hour overnight fast and all the samples will be measured in the experiment department of Zhejiang Hospital. Haemoglobin will be determined by a spectrophotometric method. Fasting blood glucose will be assessed using the glucose oxidase method. Bromocresol green method will be used to measure albumin level. Total cholesterol, triglyceride, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol will be measured in serum samples quantitatively by the cyclophorase method using an Olympus AU 5400 auto biochemistry analyser. Serum 25-hydroxyvitamin D will be tested by an electrochemiluminescence method using a Roche 6000 device. Serum transferrin will be determined using immunonephelometry. A turbidimetric latex agglutination method will be used to determine serum hypersensitive C-reactive protein.
Assessment of adverse events
At each visit during the trial, study participants will be asked to report any expected or unexpected adverse events. The evaluator will confirm the validity of the adverse events and then record details of the date of occurrence, the seriousness of the event, the causal relationship between the event and the intervention and possible treatments for it. If any adverse event occurs during the intervention, the researchers will immediately discontinue the programme and treat the event. The relevant records will be immediately conveyed to the ethics committee. The ethics committee will determine whether or not to revise the study protocol or remove the participants from the trial.
Adherence assessment
Adherence to the intervention, including intervention programme completion and satisfaction, will be recorded. If the study participants have poor compliance with the programme, the evaluator will record the reasons for this.
Follow-up assessments
All baseline assessments will be repeated on weeks 8 and 20, with the exception of demographic data which will be assessed only at baseline.
Outcome measures
The primary outcomes consist of the longitudinal changes in SPPB scores, grip strength and 6 m usual gait speed in the groups at the three assessment points. The changes of basic activities of daily living scores, instrumental activities of daily living scores, body composition and body anthropometric indexes will be the secondary outcomes of this study. In addition, the above-mentioned venous blood samples are also outcome evaluation indexes.
Quality control
To ensure reliability and consistency of the data, a series of quality control measures will be implemented. First, all staff will be required to receive standardisation training before implementation of the study. Case screening and recruitment, inclusion and exclusion criteria, intervention methods, outcome assessments and data management will be included in the training programme. Second, all data will be collected and recorded on a printed case report form. Third, the quality supervisor will periodically review data from the ongoing study, and then provide feedback to the staff on the steering committee. The staff will make some modifications after personally verifying the related reasons. Fourth, details of withdrawals from the study will be recorded during the intervention and follow-up periods.
Sample size
Based on a mean difference between groups in the score of 1.0 point, with a SD of 1.2 in previous studies, we estimated using PASS V.11 software that each group should comprise 20 participants in order to obtain a statistical power of 80% at an α level of 5%. Then allowing for the possibility of 15% missing subjects, we calculated the final sample size of each group should be 23.
Statistical analysis
The variables will be analysed using SPSS V.18.0 software (SPSS, Chicago, Illinois, USA). Continuous variables with a normal distribution will be shown as the mean and SD and those with an abnormal distribution will be presented as the median and IQR. Categorical variables will be expressed as a percentage or constituent ratio. For baseline characteristics, a one-way analysis of variance (for normally distributed continuous data), a χ2 test (for categorical data) and an independent sample of the Kruskal–Wallis test (for abnormally distributed continuous data) will be used to compare the statistical difference between groups. Longitudinal changes between groups will be evaluated using the principle of intention-to-treat analysis, and missing data will be imputed by the last-observation-carried-forward approach. Mixed-model repeated-measures analysis of variance with adjustment for baseline characteristics will be performed for different measurement time points between groups, and interaction between groups and measurement time. All significance tests will be two-tailed, and p values of <0.05 will be assumed to be statistically significant.
Dissemination
Study participants, healthcare professionals and other relevant groups in China will be informed of the results of this study. The results will be published in peer-reviewed medical journals and presented at relevant academic conferences, whether the findings are in accordance with the expected results or not.
Discussion
There is insufficient evidence from clinical trials about the complex syndrome of sarcopenic obesity to guide the clinical care of this population. This study will provide insight into the independent and synergistic effects of nutrition and exercise on physical function and body composition in Chinese older adults with sarcopenic obesity. If our hypothesis proves to be correct, both nutrition and exercise interventions will be effective, and the combined interventions will lead to better physical performance than either intervention alone. This intervention programme will offer important clinical evidence for the efficacy of complex non-pharmaceutical interventions for sarcopenic obesity, and will conduce to preserving independent function and a high quality of life for older people, thereby helping to alleviate the burden of an ageing population to society.
The strength of this study is its single-blind, randomised, controlled design. Thus, the findings will be applicable in geriatric clinical practice. In addition, we adopt comprehensive and multicomponent interventions in order to ensure a high rate of acceptance and adherence to interventions. Moreover, objective and subjective measures of physical function will be assessed during the study, because we cannot ignore the fact that subjective cognition plays an important role in any intervention.
However, this study also has several potential limitations. First, it is confined to older adults with sarcopenic obesity, and thus the results ought to be valid only for this population. Second, the study population is old and frail with many chronic diseases, and as a result is vulnerable to adverse events such as fall, fracture and cardiocerebral vascular incident. Finally, this study has a relatively small sample, and therefore may limit broader inferences from the results. Further studies with a larger sample are needed to increase the power of the study.
To the best of our knowledge, this is the first randomised clinical trial targeting sarcopenic obesity in a Chinese older population that focuses on the effects of nutrition and exercise on physical function and body composition.
Acknowledgments
The authors thank all the other staff in the departments of geriatrics, nutrition and rehabilitation medicine of Zhejiang Hospital for their full cooperation and support.
Footnotes
Contributors: S-SS and X-JC devised and designed the study. J-JC, X-KZ, TH, L-YX and J-RL contributed to the study protocol. S-SS wrote the original manuscript, and all the authors participated in its revision and decision to submit the final manuscript.
Funding: This study was supported by innovation disciplines of Zhejiang Province to X-JC. This study was also funded in part by grants from Zhejiang Medical Science and Technology Project (2016ZDB001 and 2015ZDA001).
Competing interests: None declared.
Ethics approval: Medical ethical committee of Zhejiang Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci 2000;904:437–48. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner RN, Wayne SJ, Waters DL et al. . Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004. 10.1038/oby.2004.250 [DOI] [PubMed] [Google Scholar]
- 3.Chung JY, Kang HT, Lee DC et al. . Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr 2013;56:270–8. 10.1016/j.archger.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 4.Davison KK, Ford ES, Cogswell ME et al. . Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc 2002;50:1802–9. [DOI] [PubMed] [Google Scholar]
- 5.Meng P, Hu YX, Fan L et al. . Sarcopenia and sarcopenic obesity among men aged 80 years and older in Beijing: prevalence and its association with functional performance. Geriatr Gerontol Int 2014;14(Suppl 1):29–35. 10.1111/ggi.12211 [DOI] [PubMed] [Google Scholar]
- 6.Prado CM, Wells JC, Smith SR et al. . Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr 2012;31:583–601. 10.1016/j.clnu.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 7.National Bureau of Statistics of the People's Republic of China. China 2010 census data 2010. http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm (accessed 15 Dec 2001).
- 8.Lim S, Kim JH, Yoon JW et al. . Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652–4. 10.2337/dc10-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkins JL, Whincup PH, Morris RW et al. . Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc 2014;62:253–60. 10.1111/jgs.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamboni M, Mazzali G, Fantin F et al. . Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 2008;18:388–95. 10.1016/j.numecd.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Cauley JA. An overview of sarcopenic obesity. J Clin Densitom 2015;18:499–505. 10.1016/j.jocd.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 12.Rantanen T, Harris T, Leveille SG et al. . Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 2000;55:M168–73. [DOI] [PubMed] [Google Scholar]
- 13.de Souza Vasconcelos KS, Dias JM, de Araujo MC et al. . Land-based versus aquatic resistance therapeutic exercises for older women with sarcopenic obesity: study protocol for a randomised controlled trial. Trials 2013;14:296 10.1186/1745-6215-14-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balachandran A, Krawczyk SN, Potiaumpai M et al. . High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: a randomized controlled trial. Exp Gerontol 2014;60:64–71. 10.1016/j.exger.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 15.Villareal DT, Chode S, Parimi N et al. . Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Miller GD, Messier SP et al. . Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci 2007;62:866–71. [DOI] [PubMed] [Google Scholar]
- 17.Chomentowski P, Dube JJ, Amati F et al. . Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci 2009;64:575–80. 10.1093/gerona/glp007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landi F, Calvani R, Cesari M et al. . Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med 2015;31:367–74. 10.1016/j.cger.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 19.Tieland M, van de Rest O, Dirks ML et al. . Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720–6. 10.1016/j.jamda.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Paddon-Jones D, Short KR, Campbell WW et al. . Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 2008;87:1562S–6S. [DOI] [PubMed] [Google Scholar]
- 21.Rolland Y, Dupuy C, Abellan van Kan G et al. . Treatment strategies for sarcopenia and frailty. Med Clin North Am 2011;95:427–38. 10.1016/j.mcna.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Tieland M, Dirks ML, van der Zwaluw N et al. . Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713–19. 10.1016/j.jamda.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 23.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc 2008;40:1213–19. 10.1249/MSS.0b013e31816a85ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert CP, Wright NR, Finck BN et al. . Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 2008;105:473–8. 10.1152/japplphysiol.00006.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadelha AB, Paiva FM, Gauche R et al. . Effects of resistance training on sarcopenic obesity index in older women: a randomized controlled trial. Arch Gerontol Geriatr 2016;65:168–73. 10.1016/j.archger.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 26.Goisser S, Kemmler W, Porzel S et al. . Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—a narrative review. Clin Interv Aging 2015;10:1267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent HK, Raiser SN, Vincent KR. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev 2012;11:361–73. 10.1016/j.arr.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes 2013;20:412–19. 10.1097/01.med.0000433071.11466.7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner L, Shamseer L, Altman DG et al. . Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev 2012;(11):MR000030 10.1002/14651858.MR000030.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen LK, Liu LK, Woo J et al. . Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 31.Deutz NE, Bauer JM, Barazzoni R et al. . Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929–36. 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson RJ, Noble BJ. Perception of physical exertion: methods, mediators, and applications. Exerc Sport Sci Rev 1997;25:407–52. [PubMed] [Google Scholar]
- 33.Guralnik JM, Simonsick EM, Ferrucci L et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 34.Zhao D, Li B, Yu K et al. . Digit ratio (2D:4D) and handgrip strength in subjects of Han ethnicity: impact of sex and age. Am J Phys Anthropol 2012;149:266–71. 10.1002/ajpa.22130 [DOI] [PubMed] [Google Scholar]
- 35.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 36.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- 37.Karelis AD, Chamberland G, Aubertin-Leheudre M et al. . Validation of a portable bioelectrical impedance analyzer for the assessment of body composition. Appl Physiol Nutr Metab 2013;38:27–32. 10.1139/apnm-2012-0129 [DOI] [PubMed] [Google Scholar]