Objective:
The programed death-1 (PD1)/programed death-ligand 1 (PD-L1) pathway plays a critical role in balancing immunity and host immunopathology. During chronic HIV/SIV infection, there is persistent immune activation accompanied by accumulation of virus-specific cells with terminally differentiated phenotypes and expression of regulatory receptors such as PD1. These observations led us to hypothesize that the PD1/PD-L1 pathway contributes to the functional dysregulation and ineffective viral control, and its blockade may be a potential immunotherapeutic target.
Methods:
Lymph node biopsies from HIV-infected patients (n = 23) were studied for expression of PD1 and PD-L1. In addition, we assessed the safety and biological activity of a human anti-PD-L1 antibody (Avelumab) in chronically SIV-infected rhesus macaques.
Results:
PD-L1 expression was observed in cells with myloid/macrophage morphology in HIV-infected lymph nodes. Administration of anti-PD-L1 was well tolerated, and no changes in body weights, hematologic, or chemistry parameters were observed during the study. Blockade of PD-L1 led to a trend of transient viral control after discontinuation of treatment.
Conclusion:
Administration of anti-PD-L1 in chronic SIV-infected rhesus macaques was well tolerated. Overall, these data warrant further investigation to assess the efficacy of anti-PD-L1 treatment on viral control in chronic SIV infection as a prelude to such therapy in humans.
Keywords: anti–programed death-ligand 1 immunotherapy, HIV, HIV immunotherapy, programed death-1, programed death-ligand 1
Introduction
The programed death-1 (PD1)/programed death-ligand 1 (PD-L1) pathway plays a critical role in regulating immune responses against pathogens by controlling the balance between immunity and limiting tissue damage. In chronic viral infections such as HIV/SIV, hepatitis B virus, and hepatitis C virus, failure to completely eliminate virus leads to a sustained inflammatory/activated environment accompanied by the accumulation of T cells with diminished in-vitro effector function, ‘exhausted T cells’ [1–3]. In HIV/SIV infections, chronic T-cell immune activation is reflected by increased expression of a variety of immunomodulatory receptors including, PD1, CTLA-4, LAG3, CD244/2B4, CD160, and others [4–11]. In-vitro and in-vivo studies of HIV/SIV infection have shown that blockade of PD1 is associated with enhanced virus-specific responses and improved viral control [12–15]. These observations support the hypothesis that PD1/PD-L1 interactions contribute to the functional dysregulation, exhaustion, and ineffective viral control [8,16–18]. This evidence suggests that the PD1/PD-L1 pathway may be a potential target for immune intervention in patients with HIV infection.
PD1 is expressed upon activation by T, B, and natural killer (NK) cells, dendritic cells, and activated monocytes, although its function in the latter is not well defined [19]. PD1 interacts with two ligands, PD-L1 and PD-L2. PD-L1 is mainly expressed by hematopoietic cells, such as T and B cells, dendritic cells, macrophages, and some epithelial cells (lung and vascular endothelium) [19–22]. In contrast, PD-L2 expression is more restricted and is inducible in dendritic cells, macrophages, bone marrow–derived mast cells, and some B cells [19,23].
PD1 interaction with its ligands regulates T cell receptor signaling by distinct mechanisms leading to diminished effector function, including cytokine secretion, proliferation, cytotoxicity, motility, and cell survival [24–27]. In addition, engagement of PD1 with its ligands induces reverse signaling on PD-L1/PD-L2 expressing cells [19,28,29].
Lymphoid organs are the primary targets of HIV/SIV infection [30–33]. In HIV/SIV-infected lymph nodes, the germinal center (GC) T follicular helper CD4+ T cells (CD4+ Tfh) exhibit higher expression of PD1 compared with extra follicular CD4+ and CD8+ T cells. PD-L1 expression has been observed inside and outside germinal centers [34,35].
To obtain further insights as to the potential role of PD-L1 blockade in the treatment of HIV infection, we analyzed the expression of PD1 and PD-L1 in human lymph node from 23 patients infected with HIV. In addition, we have evaluated the safety and the effect on SIV viral load of in-vivo PD1/PD-L1 blockade using a fully human anti-PD-L1 mAb (MSB0010718C, Avelumab, EMD-Serono, Inc., Rockland, Massachusetts, USA) in rhesus (Rh) macaques chronically infected with SIVmac239, that had previously received IL-15 [36].
Materials and methods
Patient samples
Patient characteristics are described in Supplementary material and methods and Table S1.
Study design
Six chronically SIV-infected Rh macaques received weekly doses of saline (n = 3) or a mAb anti-PD-L1 (Avelumab) at a dose of 20 mg/kg (n = 3). At week 24, all treatments were discontinued, and animals were followed for 10 additional weeks (Supplementary material and methods, Fig. S1 and Table S2).
Results
Expression of programed death-1/programed death-ligand 1 in human lymph nodes from patients with HIV infection
During HIV/SIV infection, there is an increase in PD1 expression on CD4+ and CD8+ T cells in peripheral blood [8,10]. To determine the distribution of PD1 and PD-L1 expression in lymphoid organs, we studied a cohort of HIV-infected patients (n = 23) that had previously undergone a lymph node biopsy. These patients had a median of 1 year [interquartile range: 0–4 years] of diagnosed infection (Supplementary Table S1 and Fig. 1a). For analysis, the patients were divided into three groups on the basis of viral loads, and the groups showed similar CD4+ and CD8+ T cell counts (Fig. 1a). Lymph nodes were stained with mAbs against PD1 and PD-L1 and blind-scored for expression of PD1 inside the germinal center and outside the germinal center (extra follicular). The score used for expression levels was negative (−), positive/negative (+/−), weakly positive (w+), and positive (+, ++, and +++). These scores correspond to 0, 0.5, 0.7, 1, 2, and 3, respectively in Fig. 1b. As reported by other groups [34,37], PD1high cells were found within the germinal center and presumably represent CD4+ Tfh cells. PD1+ lymphoid were also found scattered in the extra follicular region (Fig. 1b, b1). PD-L1 expression was extra follicular and largely restricted to cells with myloid/macrophage morphology using this scoring system (magnified black square, Fig. 1b, b1). There were no significant correlations between expression of PD1 or PD-L1 and the levels of viremia (Fig. 1b, b2). Examination of longitudinal specimens from these patients, obtained 2, 6, and more than 24 months after the first biopsy, showed no changes in PD1 or PD-L1 expression despite viral suppression (data not shown). These results suggest that during HIV infection circulating PD1-expressing CD4+ and CD8+ T cells will likely encounter PD-L1 expressing cells in lymphoid organs.
Fig. 1.
Expression of programed death-1 and programed death-ligand 1 in human lymph nodes from HIV-infected patients.
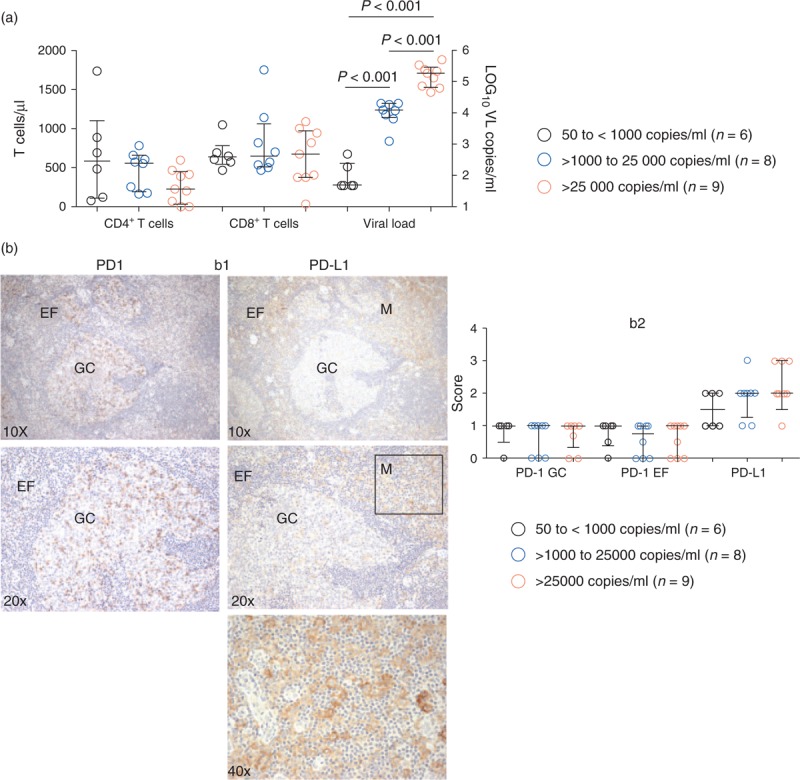
(a) HIV-infected patients (n = 23) were divided on the basis of viral loads at the time of the lymph nodes biopsy. CD4+ and CD8+ T-cell counts are shown on the left y-axis. Viral load is shown on the right y-axis. Nonparametric Mann–Whitney test was used for the group comparisons, P value less than 0.05 was considered significant. (b) Lymph nodes biopsies (n = 23) were blind scored for programed death-1 expression inside the germinal center, outside the germinal center (GC), and in extra follicular (EF) regions, and programed death-ligand 1 expression was assessed outside the germinal centers in myloid/macrophage (M) morphology cells: shown in panel b1. The score used for expression levels was negative (−), positive/negative (+/−), weakly positive (w+), and positive (+, ++, and +++). These scores correspond to 0, 0.5, 0.7, 1, 2, and 3, respectively: shown in panel b2.
Administration of a human anti–programed death-ligand 1 antibody to chronically SIV-infected rhesus macaques leads to a trend of transient virologic control
To test the hypothesis that blockade of the PD1/PD-L1 pathway may facilitate virologic control during HIV/SIV infection, we studied SIV-infected Rh macaques. We first examined spleens from Rh macaques infected with SIVmac239 for tissue distribution of PD1, PD-L1, and SIV-RNA. As previously described [34,37] and similar to observations in human lymph nodes (Fig. 1a), PD1high cells were localized within the germinal center overlaying with CD4+ staining. Outside the germinal center (extra follicular), PD1 expression was dimmer and scattered. In contrast, PD-L1 expression was scattered within the germinal center and largely localized to cells with myeloid/macrophage morphology outside the germinal center (Fig. 2a).
Fig. 2.
Delay in the viral rebound after cART discontinuation in animals treated with anti–programed death-ligand 1.

(a) Spleens from chronically SIVmac239-infected rhesus macaques were stained for CD4+, CD8+, programed death-1, and programed death-ligand 1 expression. Magnification images (a1: 10×; a2: 20×). SIVmac239 was detected by RNAscope (a2). (b) Measurements of CD4+ and CD8+ T-cell counts and viral load during study. The control group (n = 3) is represented by black symbols, and the anti–programed death-ligand 1 treatment group (n = 3) is represented by red symbols. The x-axis represents days of the study, the left side: Days –30 to 161, and right side: Days 161–250. Day 161 indicates interruption of anti–programed death-ligand 1 and cART.
We next investigated the effects of in-vivo blockade of PD1/PD-L1 using a fully human anti-PD-L1 mAb in SIVmac239-infected Rh macaques with combination antiretroviral therapy (cART)-suppressed viremia. The animals received 24 weekly doses by intravenous infusion of saline for the control group (n = 3) or 20 mg/kg of anti-PD-L1 (n = 3) for the treatment group (Supplementary Fig. S1). After administration of the 24th dose (Day 161), both cART and anti-PD-L1 were discontinued, and viral loads, CD4+, and CD8+ T cell counts were monitored for an additional 10 weeks (Fig. 2b and Supplementary Table S2). At Day 169, animals had viral loads of 1.8, 2.9, and 1.2 copies/ml in the treatment group compared with 3.7, 3.0, and 1.8 copies/ml in the control group. Following an additional 2 weeks, SIV levels were 4.4, 4.4, and 4.6 copies/ml in the treatment group compared with 6.0, 6.4, and 5.2 copies/ml in the control group (Supplementary Table S2). The comparison of the area under the curve between the groups after treatment discontinuation (Days 161–240) did not reach significance (Fig. 2b, P = 0.14). We found no significant changes in CD4+ or CD8+ T cell counts between the treatment groups (Fig. 2b). In addition, no changes were observed in the expression of PD1 or PD-L1 by T, B, NK cells, and monocytes nor in T-cell cytokine secretion (Supplementary Figs. S2–S5). Expression of PD-L1 in splenic cells was higher than that observed in cells from peripheral blood mononuclear cells (Fig. S5). These results support the design of larger studies with greater power to assess the efficacy of anti-PD-L1 on SIV viral control to determine its potential use in HIV-infected patients.
Discussion
In the present study, we describe the expression and spatial distribution of PD1 and PD-L1 in human lymph nodes from HIV-infected patients. In addition, we have assessed the safety and effect of a human anti-PD-L1 mAb on viral load in chronically SIV-infected Rh macaques. Administration of anti-PD-L1 was well tolerated, and no changes in body weights, hematologic, or chemistry parameters were observed during the study. We found that administration of anti-PD-L1 led to a trend of transient viral control after discontinuation of antiretroviral therapy. These data support larger studies to better assess the efficacy of anti-PD-L1 administration on viral control to evaluate its potential use in HIV-infected patients.
In chronic viral infections such as HIV/SIV, antigen persistence drives immune activation leading to an accumulation of terminally differentiated virus-specific cells in association with failure to achieve virologic control. Among the main characteristics of these virus-specific cells are increased expression of regulatory markers (PD1, CTLA-4, Tim3, and CD160) and diminished in-vitro effector function in terms of polyfunctional cytokine secretion, cytotoxicity, and proliferative capacity [8,10,16,17,38–40]. The PD1/PD-L1 pathway plays a central role in host defense, balancing pathogen elimination and limiting host immunopathology. Even in the setting of suppressed viral replication by cART, the immune systems of HIV-infected patients are unable to eliminate residual virus. This is associated with ongoing chronic immune activation as reflected by increased expression of PD-1 and PD-L1 [17,41,42]. It is postulated that modulation of this pathway may lead to better virologic control by promoting CD8+ T-cell expansion.
In the lymphoid organs, the primary targets of HIV infection, PD1 and PD-L1 are differentially expressed with specific spatial distribution and cell expression. PD1high cells, corresponding mainly to CD4+ Tfh cells, are localized within the germinal center where they presumably interact with PD-L1 expressing B cells [34,37]. In contrast, circulating PD1+ T cells will interact with extra follicular PD-L1 expressing cells, such as myloid cells. In addition, our finding of a trend in virologic control associated with administration of anti-PD-L1 is consistent with previous observations in which administration of anti-PD1 to chronically SIV-infected nonhuman primates enhanced proliferation and function of virus-specific CD8+ T cells [12]. The enhanced anti-SIV response by anti-PD1 administration led to better virologic control in animals at an early phase of infection despite high levels of viremia. This effect was transient in those animals at later stages of chronic infection [12]. In addition, anti-PD1 treatment was reported to enhance memory B cell survival and humoral responses against SIV and to reduce immune activation [12,13,15]. Similar to the present study, administration of anti-PD-L1 (BMS-936559) to Rh macaque chronically infected with SIVmac239 led to a delay in viral rebound after discontinuation of cART, whereas the precise mechanisms are unclear [43]. In the present study, two of the animals in each group previously received infusions of IL-15 (Table S2), and the potential impact of this prior treatment on the present results will require further investigation. A recent study of a single-dose administration of anti-PD-L1 mAb (BMS-936559, 0.3 mg/kg) in 6 HIV-infected participants on cART and HIV-1 RNA of less than 40 and at least 0.4 copies/ml by single-copy assay (SCA) showed that treatment was well tolerated, and there was a trend toward an increased proportion of Gag-specific CD8+ T cells in two individuals. No changes were noted in the SCA over the 28 days postinfusion. This study was discontinued because of retinal toxicity in the animal studies [44].
The PD1/PD-L1 pathway plays a critical role in balancing immunity against pathogens and host immunopathology. During HIV/SIV infection, T cells and virus-specific cells express PD1 even in the context of suppressed viremia. The present data support further consideration of the blockade of this pathway as immunotherapy for chronic HIV infection.
Acknowledgements
We thank the patients of the National Institute of Allergy and Infectious Diseases HIV Clinic for their participation. The SIVmac239 Gag Peptide Pool was obtained through the NIH-AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH. Some mAbs were provided by the NIH-Nonhuman Primate Reagent Resource. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. Leidos Biomedical Research, Inc. has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med 2000; 191:1499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 2002; 195:1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hersperger AR, Migueles SA, Betts MR, Connors M. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV AIDS 2011; 6:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 2010; 16:1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015; 36:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pombo C, Wherry EJ, Gostick E, Price DA, Betts MR. Elevated expression of CD160 and 2B4 defines a cytolytic HIV-specific CD8+ T-cell population in elite controllers. J Infect Dis 2015; 212:1376–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 2007; 110:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006; 203:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuchroo VK, Anderson AC, Petrovas C. Coinhibitory receptors and CD8 T cell exhaustion in chronic infections. Curr Opin HIV AIDS 2014; 9:439–445. [DOI] [PubMed] [Google Scholar]
- 10.Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet JP, Bordi R, et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS Pathog 2012; 8:e1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Far M, Halwani R, Said E, Trautmann L, Doroudchi M, Janbazian L, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep 2008; 5:13–19. [DOI] [PubMed] [Google Scholar]
- 12.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009; 458:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Titanji K, Velu V, Chennareddi L, Vijay-Kumar M, Gewirtz AT, Freeman GJ, et al. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest 2010; 120:3878–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol 2009; 182:980–987. [DOI] [PubMed] [Google Scholar]
- 15.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest 2012; 122:1712–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443:350–354. [DOI] [PubMed] [Google Scholar]
- 17.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12:1198–1202. [DOI] [PubMed] [Google Scholar]
- 18.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, et al. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood 2003; 101:2514–2520. [DOI] [PubMed] [Google Scholar]
- 19.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 2002; 9:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, et al. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol 2004; 155:172–182. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 2006; 580:755–762. [DOI] [PubMed] [Google Scholar]
- 23.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A 2003; 100:5336–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 2004; 574:37–41. [DOI] [PubMed] [Google Scholar]
- 25.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004; 173:945–954. [DOI] [PubMed] [Google Scholar]
- 26.Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009; 229:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med 2013; 210:757–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest 2003; 111:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993; 362:355–358. [DOI] [PubMed] [Google Scholar]
- 31.Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 1993; 362:359–362. [DOI] [PubMed] [Google Scholar]
- 32.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 2007; 178:6975–6983. [DOI] [PubMed] [Google Scholar]
- 33.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 2012; 18:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 2013; 19:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 2013; 210:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood 2011; 118:6845–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest 2012; 122:3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 2011; 117:4805–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demers KR, Reuter MA, Betts MR. CD8(+) T-cell effector function and transcriptional regulation during HIV pathogenesis. Immunol Rev 2013; 254:190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 2008; 29:1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosignoli G, Cranage A, Burton C, Nelson M, Steel A, Gazzard B, et al. Expression of PD-L1, a marker of disease status, is not reduced by HAART in aviraemic patients. AIDS 2007; 21:1379–1381. [DOI] [PubMed] [Google Scholar]
- 42.Cockerham LR, Jain V, Sinclair E, Glidden DV, Hartogenesis W, Hatano H, et al. Programmed death-1 expression on CD4(+) and CD8(+) T cells in treated and untreated HIV disease. AIDS 2014; 28:1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CROI 2014, Mason SW, Sanisetty S, Osuna-Gutierrez C, Lim S-Y, Chaniewski S, Campellone S, et al. Viral suppression was induced by anti-PD-L1 following ARV-interruption in SIV-infected monkeys. 2014. [Google Scholar]
- 44.CROI 2016, Eron JJ, Gay C, Bosch R, Ritz J, Hataye JM, Hwang C, et al. Safety, immunologic and virologic activity of anti-PD-L1 in HIV-1 participants on ART. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


