Objective:
Traditional cardiovascular risk factors do not fully account for ethnic differences in cardiovascular disease. We tested if arterial function indices, particularly augmentation index (AIx), and their determinants from childhood could underlie such ethnic variability among young British adults in the ‘DASH’ longitudinal study.
Methods:
DASH, at http://dash.sphsu.mrc.ac.uk/, includes representative samples of six main British ethnic groups. Pulse wave velocity (PWV) and AIx were recorded using the Arteriograph device at ages 21–23 years in a subsample (n = 666); psychosocial, anthropometric, and blood pressure (BP) measures were collected then and in two previous surveys at ages 11–13 years and 14–16 years. For n = 334, physical activity was measured over 5 days (ActivPal).
Results:
Unadjusted values and regression models for PWVs were similar or lower in ethnic minority than in White UK young adults, whereas AIx was higher – Caribbean (14.9, 95% confidence interval 12.3–17.0%), West African (15.3, 12.9–17.7%), Indian (15.1, 13.0–17.2%), and Pakistani/Bangladeshi (15.7, 13.7–17.7%), compared with White UK (11.9, 10.2–13.6%). In multivariate models, adjusted for sex, central SBP, height, and heart rate, Indian and Pakistani/Bangladeshi young adults had higher AIx (β = 3.35, 4.20, respectively, P < 0.01) than White UK with a similar trend for West Africans and Caribbeans but not statistically significant. Unlike PWV, physical activity, psychosocial or deprivation measures were not associated with AIx, with borderline associations from brachial BP but no other childhood variables.
Conclusion:
Early adult AIx, but not arterial stiffness, may be a useful tool for testing components of excess cardiovascular risk in some ethnic minority groups.
Keywords: augmentation index, cardiovascular risk, ethnicity, vascular stiffness
INTRODUCTION
Britain, like many Western countries, has a significant and rapidly growing ethnically diverse population, well prior to arrivals of refugees from Middle Eastern wars. Recent census data [1] show that the White European ethnic group accounted for 86.0% of the usual resident population in England and Wales in 2011, decreasing from 91.3% in 2001 and 94.1% in 1991. Meanwhile, over the last two decades, ethnic minority groups (South Asian, African-Caribbean, and Black African) continued to rise, and in metropolitan areas like London, proportions increased up to 40% of the total.
These population data may continue to have long-term influences on health profiles, particularly ethnic differences in cardiometabolic disease, noted over the last 40 years [2].
Cardiovascular disease (CVD) is the leading cause of death worldwide [3] with increasing rates globally, in part related to ageing. Mortality from coronary heart disease (CHD) and stroke in South Asian migrants to the United Kingdom is between 50 and 100% higher than the White British population [4]; conversely, people of black African and African Caribbean origin are still significantly protected from CHD, although mortality from stroke is even higher than in South Asians [4]. Higher cardiovascular risk in migrant populations is not just a British issue [5,6]; similar results were obtained recently in Norway where South Asian migrants had increased risks of myocardial infarction and stroke compared with the resident population, and stroke was more common in people from sub-Saharan Africa and Southeast Asia [7]. Very similar data were also published for migrants to the Netherlands [8,9]. These remarkable ethnic differences cannot be fully explained by traditional cardiovascular and metabolic risk factors, such as hypertension, dyslipidemia, central adiposity, or insulin resistance, measured in midlife [10].
As arterial stiffness has become a major intermediary outcome for cardiovascular events and mortality [11–13], it could be a useful tool to investigate ethnic patterns of CVD. Further, arterial function indices could be early targets for intervention at a stage of life when traditional cardiovascular risk factors only weakly predict later disease and mortality. Not only pulse wave velocity (PWV) but arterial wave reflections [14,15] have emerged as important markers of vascular health and predict cardiovascular risk independent of conventional risk factors, including blood pressure (BP); however, data regarding arterial indices in ethnic minorities are rare.
Here we examine how far central augmentation index (AIx), the most widely used index of wave reflections, may underlie ethnic differences in cardiovascular risk among a multiethnic British population cohort – the ‘DASH’ longitudinal study.
METHODS
Details of the DASH study can be found at http://dash.sphsu.mrc.ac.uk/ and in a published cohort profile [16]. The sample was recruited between 2002 and 2003, from 51 secondary schools in 10 London boroughs. A total of 6643 students, aged 11–13 years, took part in the baseline survey. In 2005–2006, 4782 (88% of children in 49 schools, 72% of the cohort, aged 14–16 years, took part in the first follow-up. A 10% subsample of (N = 666, 97% participation rate) took part in a pilot follow-up study, which was completed in March 2014. Response rates (≥90% of the invited pilot sample) were similar by ethnicity and sex. The subsample consisted of 107 White UK, 102 Black Caribbeans, 132 Black Africans, 99 Indian, 111 Pakistani/Bangladeshi, and 115 other (mainly mixed) ethnicities, and chosen to be representative by sex and socioeconomic status across the 10 London boroughs and 51 schools.
The study was approved by the NHS Research Ethics Committees. Written informed consent was obtained from participants. Ethnicity in DASH was measured by self-reported ethnicity, checked against reported parental ethnicity and grandparents’ country of birth. Bangladeshis and Pakistanis were combined because of relatively small sample sizes.
Physical measures
Measurement protocols can be found at http://dash.sphsu.mrc.ac.uk and were taken from the World Health Organization manual.
SBP and DBP were measured using validated OMRON M5-I semiautomatic devices and appropriately sized cuffs, after the participant had sat quietly for a timed 5 min, with more than 1 min between three subsequent readings. The mean of the second and third readings was used in analysis, as previously reported [17–19] At 21–23 years, PWV, central AIx, central SBP (cSBP), and brachial BP were also measured using the Arteriograph 24-h device, previously calibrated and standardized [20]. The device records up to eight cardiac cycles, three separate times in one sitting. The aortic path length is measured with a long arm caliper, from suprasternal notch to pubic rami.
Physical activity was not measured in detail during adolescence. In the follow-up, a subsample of participants N = 334, 76% of those invited, wore a waterproofed ActivPal monitor continuously for 5 days. Worn on the front of the thigh, the monitor is valid for identifying sitting standing and walking [21]. The following were derived and reported per day: steps taken, upright time, time walking at more than 100 steps/min (equivalent to moderate–vigorous physical activity), sit-to-stand transitions, and proportion of daytime sitting (between 0900 and 2100 h) spent in prolonged (>20 min) bouts.
Social measures
A self-administered questionnaire measured other social factors, including health behaviors, racism, and socioeconomic circumstances (SECs). Reported racism was assessed using standardized questions on ‘unfair treatment ’on the grounds of race, skin color, country of birth, or religion in various locations (school, street, work, etc.) [22].
In adolescence, SEC was measured through parental employment plus the family affluence scale based on number of cars, computers, holidays, etc. [23]. In adulthood, SEC was measured through own education and employment.
Statistical method
The core model for AIx contained heart rate, height, cSBP (at 21–23y), sex, and ethnicity. We first examined the influence of current exposures at 21–23 years, and then tested the influence of similarly measured exposures at ages 11–13 years, by adding them to the core model. Each variable was tested in univariate models (added to the core model) before final multivariable linear regressions were conducted; all modeling were performed using Stata 13 (Stat Corp. LP, College Station, Texas, USA). Statistical significance was considered at P value <0.05.
RESULTS
As we previously reported [24], unadjusted PWVs in Black Caribbean and White UK young men were similar (mean + SD 7.9 + 0.3 vs. 7.6 + 0.4 m/s) and lower in other groups at similar SBPs (120 mmHg) and BMIs (24.6 kg/m2). Furthermore, in fully adjusted models all ethnic minorities had lower (or similar) values of PWV compared with White UK young people. Two physical activity measures (number of steps/day and time walking >10 000 steps/day), were also negatively associated with PWV [24].
Focusing on wave reflection, unadjusted AIx values were higher in women (mean, 95% confidence interval 16.2, 15–17.3%) compared with men (12.5, 11.3–13.6%; P < 0.001) and in all ethnic minority groups – Indian (15.1, 13.0–17.2%), Pakistani/Bangladeshi (15.7, 13.7–17.7%), Black Caribbean (14.9, 12.3–17.0%), and Black African (15.3, 12.9–17.7%) – compared with White UK (11.9, 10.2–13.6%), even if borderline significant (P = 0.07; Fig. 1). Univariate analysis showed significant correlations between the index of wave reflection and ethnic minorities (all P < 0.05); however, factors which are known to have an influence on AIx showed major differences too: South Asians (i.e. both Indian and Pakistani/Bangladeshi groups) were shorter and had lower SBP compared with White UK and other minorities, whereas BA and Black Caribbean tended to have lower heart rates compared with others (Table 1).
FIGURE 1.
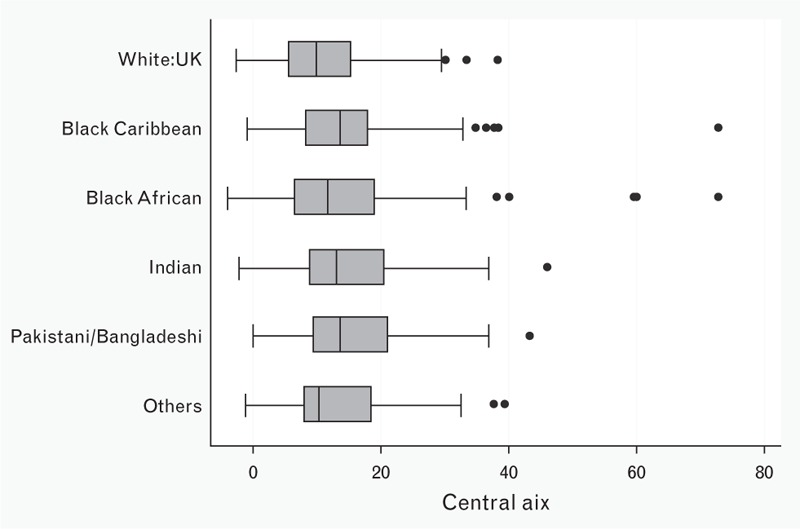
Augmentation index and ethnicity at 21–23 years. The determinants of adolescent, now young adults, social well-being and health study. Central AIx, augmentation index.
TABLE 1.
Descriptive profile of the sample (95% confidence interval) by ethnic groups. The determinants of adolescent, now young adults, social well-being and health study
| Ethnicity | ||||||
| Variable | White UK | Black Caribbean | Black African | Indian | Pakistani/Bangladeshi | Others |
| AIx (%) | 11.9 (10.2–13.6) | 15.3 (12.9–17.7)* | 14.7 (12.4–17.0)* | 15.1 (13.0–17.2)* | 15.7 (13.7–17.7)* | 13.2 (11.5–14.9) |
| Heart rate (bpm) | 70.1 (68.1–72.2) | 66.8 (65.0–68.6)* | 67.2 (65.2–69.2)* | 70.1 (68.0–72.3) | 71.3 (69.3–70.2) | 68.4 (66.7–70.2) |
| Height (cm) | 172.3 (170.7–174.1) | 167.1 (165.3–168.9) | 166.5 (163.5–169.5)** | 161.9 (160.0–164)** | 163.3 (161.6–165)** | 166.1 (163.7–168.4)** |
| BMI (kg/m2) | 23.8 (23–24.7) | 26.1 (25–27.2)** | 25.9 (25–26.8)** | 23.8 (22.8–24.9) | 24.3 (23.5–25.2) | 24.5 (23.7–25.3) |
| SBP (mmHg) | 120.5 (117.7–123.2) | 119.7 (117.2–122.3) | 118.7 (116.5–120.9) | 115.9 (113.5–118.3)** | 115 (113–117.2)** | 115.2 (113.2–117.1)** |
| DBP (mmHg) | 71 (69.2–72.8) | 70.9 (68.9–72.7) | 70.1 (68.5–71.7) | 67.8 (66.1–69.5)** | 67.5 (66.1–68.9)** | 68.1 (66.6–68.9)* |
| cSBP (mmHg) | 108.9 (106.4–111.4) | 109.1 (106–112.1) | 107.6 (105.2–110) | 104.8 (102.2–107.3)* | 105.2 (102.9–107.5)* | 104.4 (102.3–106.7)** |
| MAP (mmHg) | 87.5 (85.4–89.5) | 87.1 (85.1–89.1) | 86.3 (84.6–88) | 83.8 (82–85.6)** | 83.3 (82–85.6)** | 83.7 (82.2–85.2)** |
AIx, augmentation index; cSBP, central SBP; MAP, mean arterial pressure.
P value derived from Anova test.
*P < 0.05 compared with White UK.
**P < 0.01 compared with White UK.
Therefore, all these variables were included in the core model where sex, heart rate, height, and cSBP were strongly associated with AIx (all P < 0.001). Focusing on ethnicity, Indian and Pakistani/Bangladeshi continued to have significantly higher AIx values compared with other groups after fully adjusting for confounding factors (respective β = 3.35 and 4.20, both P < 0.005; Table 2). AIx was also borderline significantly higher in Black African (β = 2.65, P = 0.047), including adjustments for childhood variables, and only in Black Caribbean it remained marginally but not significantly higher than in White UK peers.
TABLE 2.
Augmentation index at 21–23 years: regression models for the influence of blood pressure, height, heart rate, sex and social exposures from early adolescence. The Determinants of Adolescent, now young Adults, Social well-being and Health study
| Core modela | + Socioeconomical variablesb | +Childhood variablesc | |||||||
| Variables | Coeff. | 95% CI | P value | Coeff. | 95% CI | P value | Coeff. | 95% CI | P value |
| Sex (Male – Ref) | |||||||||
| Female | 3.53 | (1.67, −5.39) | <0.001 | 3.73 | (1.80–5.70) | <0.001 | 5.37 | (2.80–8.00) | <0.001 |
| Pulse | −0.28 | (−0.35, −0.22) | <0.001 | −0.31 | (−0.40, –0.20) | <0.001 | −0.31 | (−0.40, –0.20) | <0.001 |
| Central SBP | 0.43 | (0.38, 0.49) | <0.001 | 0.43 | (0.40, 0.50) | <0.001 | 0.41 | (0.30, 0.30) | <0.001 |
| Height | −0.36 | (−0.45, −0.26) | <0.001 | −0.39 | (−0.50, –0.30) | <0.001 | −0.23 | (−0.40, –0.90) | 0.002 |
| Ethnicity (White UK – Ref) | |||||||||
| Black Caribbean | 1.32 | (−0.99, 3.63) | 0.260 | 0.91 | (−1.50, 3.30) | 0.460 | 0.87 | (−1.70, 3.50) | 0.507 |
| Black African | 1.73 | (−0.39, −3.85) | 0.110 | 1.36 | (−0.90, 3.60) | 0.240 | 2.65 | (0.03–5.30) | 0.047 |
| Indian | 3.35 | (1.02, 5.67) | 0.005 | 3.00 | (0.60, 5.40) | 0.015 | 3.97 | (1.40, 6.60) | 0.003 |
| Pakistani/Bangladeshi | 4.20 | (1.89, 6.52) | <0.001 | 4.60 | (2.10, 7.10) | <0.001 | 4.34 | (1.60, 7.10) | 0.002 |
| Others | 1.17 | (−1.03, 3.37) | 0.590 | 0.96 | (−1.70, 3.30) | 0.420 | 1.29 | (−1.30, 3.90) | 0.324 |
| Reported racism (No – Ref) | |||||||||
| Yes | 0.89 | (−0.80, 2.60) | 0.315 | ||||||
| Own employment (Yes – Ref) | |||||||||
| No | −0.50 | (−1.90, 0.90) | 0.470 | ||||||
| Education (No – Ref) | |||||||||
| Yes | –0.92 | (–2.3, 0.50) | 0.194 | ||||||
| MAP (11–13 years) | −0.11 | (−0.20, –0.01) | 0.055 | ||||||
| Height (11–13 years) | −0.09 | (−0.20, −0.30) | 0.130 | ||||||
| Reported racism (No – Ref) | |||||||||
| Yes | 0.69 | (−0.90, 2.40) | 0.430 | ||||||
| Family affluence scale 5(≥3 – Ref) | |||||||||
| 1–2 | 0.25 | (−1.40, 1.90) | 0.760 | ||||||
| 1–3 | 2.82 | (−0.90, 7.50) | 0.340 | ||||||
| Parental employment (Yes – Ref) | |||||||||
| No | −0.47 | (−3.01, 1.30) | 0.429 | ||||||
CI, confidence interval; MAP, mean arterial pressure.
aCore model: AIx adjusted for age, heart rate, height, central BP at 21–23 years, sex, and ethnicity.
bCore model + socioeconomic circumstances at 21–23 years: reported racism, own employment, and education.
cCore model + childhood variables (11–13 years): MAP, height, reported racism at 11–13 years, family affluent scale at 11–13 years, per annum.
No influence was detectable for body composition parameters or ‘fat’ such as BMI (P = 0.5), waist-to-height ratio (P = 0.4) or overweight status (P = 0.2) so these were not included in the core model. Regarding BP, DBP (P = 0.001), mean arterial pressure (MAP; P = 0.025), cSBP (P < 0.001) but not brachial SBP (P = 0.679) were positively related to AIx; using MAP instead of cSBP in the core model (not shown) did not change any correlations. Psychosocial or deprivation measures also were not correlated with AIx (and their inclusion did not change any previous associations); further adjustments of the core model for 11–13 years variables only found a borderline (negative) association with adolescent MAP (P = 0.055).
Of the subsample (n = 415) with objective ActivPal measures, both men and women spent some 70% of waking hours sedentary so overall only about 36 min/day in moderate–vigorous activity. No variable associated with physical activity emerged as related to AIx after inclusion in the core model (not shown).
DISCUSSION
The present study showed clear ethnic differences in AIx, considered an index of wave reflection, in a multiethnic cohort living in similar areas, addressing an issue on which little is published.
Previous small studies in adults showed conflicting results regarding ‘White’ and ‘Black’ young adults in the United States and Europe, respectively [25,26]. Another found that differences in AIx between 94 East Asian and 47 age-matched White peers disappeared after adjusting for height [27]. In large population-based studies throughout the world (10 550 adults), after adjustment for age, heart rate, MAP, and body size, black Africans had markedly higher AIx than British Whites [28]. In 2057 adults aged 21–90 years with type 2 diabetes in the multiethnic state of Singapore, AIx was significantly higher in Indians (28.1 ± 10.8%) than Chinese (26.1 ± 10.7%, P < 0.002) [29]. Epidemiological data in the same area found that CHD and mortality was higher in the Indian population compared with the Chinese one [30,31] supporting the idea that arterial stiffness and wave reflection may, at least partially, underlie the differences in such adverse outcome.
In our multi ethnic cohort living in the London area, PWV was similar or lower in ethnic minorities compared with White UK [24], whereas AIx was significantly higher in Indian and Bangladeshi/Pakistani than White UK even after adjustment for confounding factors (sex, heart rate, height, and cSBP, – Table 2). These results may suggest that the parameter of wave reflection, particularly in young South Asian individuals, could indicate important ethnic differences in central hemodynamics, which cannot be fully assessed with conventional sphygmomanometry.
Previously, the general view has been that brachial BP outcomes were ‘more severe’, or ‘worse’ in black populations, even at given levels of BP. However, there remains some doubt whether this is and was so in unbiased genuinely representative population samples (now difficult to find) or after full adjustment for increased hypertension prevalence and other, including social, factors. That view, long considered here [32,33], was substantiated in the long-term follow-up of US studies [34], and recently in the long-term UK ‘SABRE’ study [35]. In the latter three-dimensional echo study, cardiac remodeling rather than hypertrophy was found to be the main issue.
It seems possible that wave reflections, here as AIx, may contribute to the development of left ventricular hypertrophy or ’remodeling’ and could explain the higher rates of hypertensive target organ damage in some ethnic minorities, as was found in African-Americans and in African-Caribbeans in Britain [36–38]. Left ventricular hypertrophy in nonathletic people reflects subclinical organ damage and is very likely an early indicator of cardiovascular, especially hypertensive heart disease.
Taking these considerations together, it seems that in ethnic minorities, and particularly in South Asians, increased central wave reflection from early adulthood could be a determinant of target organ damage before the onset of other well established risk factors.
In such context, discrepancy between the results of PWV and AIx is not surprising as AIx is not a surrogate marker of PWV [39]. Rather, as a measure of arterial wave reflection, it probably depends both on the speed of the pressure wave and on anatomical and mechanical characteristics of the arterial tree. These latter characteristics may be influenced by the vascular tone of the small muscular arteries and arterioles rather than by the elastic properties of the aorta [40].
In our analysis, SBP was not correlated with AIx, likely in part explained by characteristic aortic pressure amplification in young people. In the elderly, the reflected waves return to the aorta during systole, thereby increasing SBP and pulse pressure; in contrast, in younger people, reflected pulse waves return during diastole, resulting in an increase in mean DBP [41].
Thus, in our final model we used only cSBP and MAP. Having outlined these concepts, some traditional some not, we should say that the origins of the ‘augmentation’ are by no means certain. Both amplified Windkessel-like effects and excess (aortic) ‘reservoir’ pressure may be additional, or even replacement, causes of these apparent wave reflections [42,43]. Recently, in patients undergoing cardiac catheterization the reduction in augmentation pressure after nitroglycerin administration was, at least in part, dependent on ventricular contraction/relaxation dynamics rather than reflection effects [44].
In the core model, no ‘fat parameters’ were added because of lack of significant correlation in univariate analysis. Although arterial stiffness and vascular remodeling are generally greater in obese compared with nonobese individuals [45], this may be because of co-occurrence of cardiovascular risk factors rather than obesity per se. DeVallance et al.[46] found in a small population (102 adults) without type 2 diabetes and CVD that AIx increased in men but decreased in women with increasing BMI; contrarily, in a diabetic cohort, no correlation was found between BMI and augmentation pressure [47].
Psychosocial or deprivation measures did not show any correlation with AIx (and their inclusion in the model did not change any previous associations); further adjustments of the core model for 11–13 years variables only underlined a borderline (negative) association with adolescent MAP (P = 0.055).
As previously described in DASH [24], SBP became higher in men than women at 14–16 years and there was a marked increase in this sex difference at 21–23 years with mean SBP hardly changing in women. However, at the age of 11–13 years, SBP was similar between Black African, Black Caribbean, and White UK and lower for Pakistani/Bangladeshi and Indian (P < 0.005), with no ethnic differences for DBP, suggesting that growth acceleration maybe a critical period in which a ‘delayed maturation’ of the cardiovascular system may, potentially, be a risk factor for development of CVD. Clearly, the influence of childhood BP on AIx in later life needs further investigation, considering that in our populations no data on cSBP and AIx were collected at the age of 11–13 years. Finally, we found no association between AIx and parameters of physical activity in contrast with our previous data on PWV [24].
Beneficial effects of physical activity on arterial stiffness are found, and across various populations [48–50], probably related to arterial stiffening resulting from both passive (increased intima–media thickness) and active (smooth vascular muscle tone) components, both of which can be altered by physical activity [51]. However, our results for AIx are in line with the Framingham analysis (2376 participants, mean age 47 years) [52] which did not show any effect of physical activity, measured continuously for 8 days, on AIx nor on flow-mediated dilatation. Those data suggest that physical activity could be associated with attenuated age-related increases in intima–media thickness, with very small effects on the active components of vascular muscle tone and endothelial dysfunction. These suggestions again need further investigation in younger people, free from CVD.
In conclusion, a multiethnic cohort of young adults living in the same area both PWV and AIx showed ethnic differences; however, only AIx was higher in ethnic minorities compared with White UK. These data suggest that parameters of wave reflection, rather than arterial stiffness, may underlie ethnic differences at this age and could be a useful tool for testing components of excess cardiovascular risk.
Limitations
Limitations include a relatively small sample size (about 100 per ethnic group, balanced by sex, and smaller numbers with physical activity measured) and its cross-sectional nature so far.
Another is how far AIx is a measure of reflection as there may be components of arterial compliance and reservoir function as well as or rather than wave reflection per se[53]. A study comparing the relationship between carotid AIx and wave reflection indices from wave intensity and wave separation analysis across adult ages in 65 healthy people suggested AIx gave misleading result, especially for its negative values [54]. However, this analysis was a relatively small sample (n = 65) done on carotid arteries.
Relatively little data on prognostic value of PWV and AIx measured with Arteriograph device are available. Only a few follow-up studies evaluated prediction by the Arteriograph's stiffness and wave reflection parameters on cardiovascular events or mortality with conflicting results. Akkus et al.[55] reported that arterial stiffness and wave reflection measured by it can predict further cardiovascular events in patients after myocardial infarction and the same group [56] showed that AIx and PWV predicted mortality independent of other variables in a small population of advanced heart failure patients. A follow-up study in CKD found no prognostic value for Arteriograph PWV and AIx on cardiovascular mortality in hemodialysis [57].
The prognostic value of PWV and AIx measured with oscillometric devices in a wider range of clinical conditions and in young populations free from CVD are needed.
ACKNOWLEDGEMENTS
We acknowledge the invaluable support of participants and their parents, the Participant Advisory Group, schools, civic leaders, local GP surgeries and community pharmacies, the Clinical Research Centre at Queen Mary University of London, the Clinical Research Facility at University College Hospital, the survey assistants and nurses involved with data collection, the Primary Care Research Network, and Professors Sanders and Cruickshank at the Diabetes and Nutritional Sciences Division at Kings College London for hosting the feasibility study. S.H. is the principal investigator of DASH. All authors contributed to study design, analyses, and writing of the article. The study was funded by the Medical Research Council (MC_U130015185/MC_UU_12017/1/MC_UU_12017–13.), Chief Scientist Office (SPHSU13) and North Central London Research Consortium and the Primary Care Research Network.
The study was funded by the MRC (MC_U130015185/MC_UU_12017/1-13), Chief Scientist Office (SPHSU13), North Central London Research Consortium and the Primary Care Research Network.
Conflicts of interest
There are no conflicts of interest.
Reviewers’ Summary Evaluations
Referee 1
Strengths: 1. Population-based sample participating in the Determinants of Adolescent Social well being and Health (DASH); 2. The study assesses central SBP and augmentation index as potential precursors of greater risk in minority populations vs. the white majority; 3. The authors identify clear differences in augmentation index between South Asian populations and White UK youth.
Weaknesses: 1. Relatively limited number of youth in each group studied (∼100); 2. Absence of target organ data, e.g. LVH, carotid intimal–media thickness. Clinical events would not be expected in this young population.
Footnotes
Abbreviations: AIx, Augmentation index; B, Bangladeshi; BA, Black African; BC, Black Caribbean; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; Coeff., coefficient; cSBP, central systolic blood pressure; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; FAS, family affluence scale; I, Indian; LVH, left ventricular hypertrophy; MAP, mean arterial pressure; NHS, national health service; P, Pakistani; PA, physical activity; PWV, pulse wave velocity; Ref., reference; SBP, systolic blood pressure; SEC, socio-economic circumstances; WU, White UK
REFERENCES
- 1.Part of 2011 Census, Key Statistics for Local Authorities in England and Wales. Release. Office for National Statistics, 11 December 2012. [Google Scholar]
- 2.Cruickshank JK, Beevers DG, Osbourne VL, Haynes RA, Corlett JC, Selby S. Heart attack, stroke, diabetes, and hypertension in West Indians, Asians, and whites in Birmingham, England. BMJ 1980; 281:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015; 27:132. [DOI] [PubMed] [Google Scholar]
- 4.Wild SH, Fischbacher C, Brock A, Griffiths C, Bhopal R. Mortality from all causes and circulatory disease by country of birth in England and Wales 2001–2003. J Public Health (Oxf) 2007; 29:191–198. [DOI] [PubMed] [Google Scholar]
- 5.Hedlund E, Lange A, Hammar N. Acute myocardial infarction incidence in immigrants to Sweden. Country of birth, time since immigration, and time trends over 20 years. Eur J Epidemiol 2007; 22:493–503. [DOI] [PubMed] [Google Scholar]
- 6.McKeigue PM, Miller GJ, Marmot MG. Coronary heart disease in south Asians overseas: a review. J Clin Epidemiol 1989; 42:597–609. [DOI] [PubMed] [Google Scholar]
- 7.Rabanal KS, Selmer RM, Igland J, Tell GS, Meyer HE. Ethnic inequalities in acute myocardial infarction and stroke rates in Norway 1994–2009: a nationwide cohort study (CVDNOR). BMC Public Health 2015; 15:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oeffelen A, Agyemang C, Koopman C, Stronks K, Bots M, Vaartjes I. Downward trends in acute myocardial infarction incidence: how do migrants fare with the majority population? Results from a nationwide study. Eur J Prev Cardiol 2014; 21:1493–1500. [DOI] [PubMed] [Google Scholar]
- 9.Agyemang C, van Oeffelen AA, Norredam M, Kappelle LJ, Klijn CJ, Bots ML, et al. Ethnic disparities in ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage incidence in the Netherlands. Stroke 2014; 45:3236–3242. [DOI] [PubMed] [Google Scholar]
- 10.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, et al. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans SABRE (Southall and Brent Revisited) a prospective population-based study. J Am Coll Cardiol 2013; 61:1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faconti L, Bruno RM, Ghiadoni L, Taddei S, Virdis A1. Ventricular and vascular stiffening in aging and hypertension. Curr Hypertens Rev 2015; 11:100–109. [DOI] [PubMed] [Google Scholar]
- 14.Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 15.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (multiethnic study of atherosclerosis). J Am Coll Cardiol 2012; 60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding S, Whitrow M, Maynard MJ, Teyhan A. Cohort profile: The DASH (Determinants of Adolescent Social well-being and Health) Study, an ethnically diverse cohort. Int J Epidemiol 2007; 36:512–517. [DOI] [PubMed] [Google Scholar]
- 17.Harding S, Maynard M, Cruickshank JK, Gray L. Anthropometry and blood pressure differences in Black Caribbean, African, South Asian and White adolescents: The MRC DASH study. J Hypertens 2006; 24:1507–1514. [DOI] [PubMed] [Google Scholar]
- 18.Harding S, Teyhan A, Maynard M, Cruickshank JK. Ethnic differences in overweight and obesity in early adolescence in the MRC DASH study: the role of adolescent and parental lifestyle. Int J Epidemiol 2008; 37:162–172. [DOI] [PubMed] [Google Scholar]
- 19.Harding S, Whitrow M, Lenguerrand E, Maynard M, Teyhan A, Cruickshank JK, Der G. Emergence of ethnic differences in blood pressure in adolescence: the determinants of adolescent social well-being and health study. Hypertension 2010; 55:1063–1069. [DOI] [PubMed] [Google Scholar]
- 20.Rezai MR, Wallace AM, Sattar N, Finn JD, Wu FC, Cruickshank JK. Ethnic differences in aortic pulse wave velocity occur in the descending aorta and may be related to vitamin D. Hypertension 2011; 58:247–253. [DOI] [PubMed] [Google Scholar]
- 21.Dowd KP, Harrington DM, Donnelly AE. Criterion and concurrent validity of the activPAL professional physical activity monitor in adolescent females. PLoS One 2012; 7:e47633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young Black and White adults. Am J Public Health 1996; 86:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currie C, Molcho M, Boyce W, Holstein B, Torsheim T, Richter M. Researching health inequalities in adolescents: the development of the Health Behaviour in School-aged Children (HBSC) family affluence scale. Soc Sci Med 2008; 66:1429–1436. [DOI] [PubMed] [Google Scholar]
- 24.Cruickshank JK, Silva M, Molaodi O, Enayat Z, Cassidy A, Karamanos A, et al. Ethnic differences in and childhood influences on early adult PWV: the DASH longitudinal study. Hypertension 2016; 67:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol 2008; 295:H2380–H2387. [DOI] [PubMed] [Google Scholar]
- 26.Lemogoum D, Van Bortel L, Leeman M, Degaute JP, van de Borne P. Ethnic differences in arterial stiffness and wave reflections after cigarette smoking. J Hypertens 2006; 24:683–689. [DOI] [PubMed] [Google Scholar]
- 27.Sugawara J, Komine H, Yoshiwaza M, Tarumi T, Maeda S, Tanaka H. Racial differences in relation between carotid and radial augmentation index. Artery Res 2010; 4:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirinos JA, Kips JK, Roman JM, Medina-Lezama J, Li Y, Woodiwiss AJ, et al. Ethnic differences in arterial wave reflections and normative equations for augmentation index. Hypertension 2011; 57:1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Liu JJ, Sum CF, Ying YL, Tavintharan S, Ng XW, et al. Ethnic disparity in central arterial stiffness and its determinants among Asians with type 2 diabetes. Atherosclerosis 2015; 242:22–28. [DOI] [PubMed] [Google Scholar]
- 30.Dalan R, Jong M, Choo R, Chew DE, Leow MK. Predictors of cardiovascular complication in patients with diabetes mellitus: a 5-year follow-up study in a multiethnic population of Singapore: CREDENCE II study. Int J Cardiol 2013; 169:e67–e69. [DOI] [PubMed] [Google Scholar]
- 31.Ma S, Cutter J, Tan CE, Chew SK, Tai ES. Associations of diabetes mellitus and ethnicity with mortality in a multiethnic Asian population: data from the 1992 Singapore national health survey. Am J Epidemiol 2003; 158:543–552. [DOI] [PubMed] [Google Scholar]
- 32.Cruickshank JK, Beevers DG. Is blood pressure really ‘worse’ in Black people? Lancet 1980; 2:371–372. [DOI] [PubMed] [Google Scholar]
- 33.Cruickshank JK. Cruickshank JR, Beevers DG. The natural history of BP and black populations in the West Indies, West Africa and the UK: a comparison with the USA. Ethnic factors in health and disease. London, UK:Butterworth-Heinemann; 1989. 268–279. [Google Scholar]
- 34.Davey Smith G, Neaton JD, Wentworth D, Stamler R, Stamler J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Lancet 1998; 351:934–939. [DOI] [PubMed] [Google Scholar]
- 35.Park CM, March K, Ghosh AK, Jones S, Coady E, Tuson C, et al. Left-ventricular structure in the Southall And Brent REvisited (SABRE) study: explaining ethnic differences. Hypertension 2013; 61:1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network Study. Hypertension 2004; 43:1182–1188. [DOI] [PubMed] [Google Scholar]
- 37.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129. [DOI] [PubMed] [Google Scholar]
- 38.Chaturvedi N, Athanassopoulos G, McKeigue PM, Marmot MG, Nihoyannopoulos P. Echocardiographic measures of left ventricular structure and their relation with rest and ambulatory blood pressure in blacks and whites in the United Kingdom. J Am Coll Cardiol 1994; 24:1499–1505. [DOI] [PubMed] [Google Scholar]
- 39.Cecelja M, Jiang B, McNeill K, Kato B, Ritter J, Spector T, Chowienczyk P. Increased wave reflection rather than central arterial stiffness is the main determinant of raised pulse pressure in women and relates to mismatch in arterial dimensions: a twin study. J Am Coll Cardiol 2009; 54:695–703. [DOI] [PubMed] [Google Scholar]
- 40.Nichols WW, O’Rourke MF. McDonald's blood flow in arteries. Theoretical, experimental and clinical principles. 5th ed.Oxford, UK:Oxford University Press; 2005. [Google Scholar]
- 41.Nürnberger J, Dammer S, Opazo Saez A, Philipp T, Schäfers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens 2003; 17:153–158. [DOI] [PubMed] [Google Scholar]
- 42.Davies JE, Lacy P, Tillin T, Collier D, Cruickshank JK, Francis DP, et al. Excess pressure integral predicts cardiovascular events independent of other risk factors in the conduit artery functional evaluation sub-study of Anglo-Scandinavian Cardiac Outcomes Trial. Hypertension 2014; 64:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowienczyk P. Pulse wave analysis: what do the numbers mean? Hypertension 2014; 63:1050–1055. [DOI] [PubMed] [Google Scholar]
- 44.Fok H, Guilcher A, Li Y, Brett S, Shah A, Clapp B, Chowienczyk P. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: novel mechanism of reduction of pulse pressure by nitrates. Hypertension 2011; 57:1051–1052. [DOI] [PubMed] [Google Scholar]
- 45.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 2005; 23:1839–1846. [DOI] [PubMed] [Google Scholar]
- 46.DeVallance E, Fournier SB, Donley DA, Bonner DE, Lee K, Frisbee JC, Chantler PD. Is obesity predictive of cardiovascular dysfunction independent of cardiovascular risk factors? Int J Obes (Lond) 2015; 39:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wykretowicz A, Adamska K, Guzik P, Krauze T, Wysocki H. Indices of vascular stiffness and wave reflection in relation to body mass index or body fat in healthy subjects. Clin Exp Pharmacol Physiol 2007; 34:1005–1009. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000; 102:1270–1275. [DOI] [PubMed] [Google Scholar]
- 49.Jennersjo P, Ludvigsson J, Lanne T, Nystrom FH, Ernerudh J, Ostgren J. Pedometer-determined physical activity is linked to low systemic inflammation and low arterial stiffness in type 2 diabetes. Diabet Med 2012; 29:1119–1125. [DOI] [PubMed] [Google Scholar]
- 50.Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011; 18:980–984. [DOI] [PubMed] [Google Scholar]
- 51.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25:932–943. [DOI] [PubMed] [Google Scholar]
- 52.Andersson C, Lyass A, Larson MG, Spartano NL, Vita JA, Benjamin EJ, et al. Physical activity measures and its associations with cardiac structure and vascular function in young and middle-aged adults. J Am Heart Assoc 2015; 4:e001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verbeke F, Marechal C, Van LS, Van BW, Devuyst O. Aortic stiffness and central wave reflections predict outcome in renal transplant recipients. Hypertension 2011; 58:833–838. [DOI] [PubMed] [Google Scholar]
- 54.Hughes AD, Park C, Davies J, Francis D, McG Thom SA, Mayet J, Parker KH. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One 2013; 8:e59371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akkus O, Sahin DY, Bozkurt A, Nas K, Ozcan KS, Illyés M, et al. Evaluation of arterial stiffness for predicting future cardiovascular events in patients with ST segment elevation and non-ST segment elevation myocardial infarction. ScientificWorldJournal 2013; 2013:792693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demir S, Akpınar O, Akkus O, Nas K, Unal I, Molnar F, et al. The prognostic value of arterial stiffness in systolic heart failure. Cardiol J 2013; 20:665–671. [DOI] [PubMed] [Google Scholar]
- 57.Nemcsik J, Egresits J, El Hadj Othmane T, Fekete BC, Fodor E, Szabó T, et al. Validation of arteriograph: a new oscillometric device to measure arterial stiffness in patients on maintenance hemodialysis. Kidney Blood Press Res 2009; 32:223–229. [DOI] [PubMed] [Google Scholar]


