Abstract
Background
In this study, we aimed to investigate the association between UCA1 and miR-27b in gastric cancer and further study their involvement in multi-drug resistance (MDR) of gastric cancer.
Material/Methods
The microarray data of dysregulated lncRNAs in gastric cancer tissues was retrieved in the GEO dataset. QRT-PCR analysis was performed to assess UCA1 expression based on 28 paired cancerous and peritumoral normal tissues. The human gastric cancer cell line SGC-7901, and SGC-7901 derived Adriamycin (doxorubicin) resistant SGC-7901/ADR, cisplatin resistant SGC-7901/DDP, and 5-FU resistant SGC-7901/FU cells were used as in vitro cell models to assess the effect of UCA1 and miR-27b on MDR.
Results
UCA1 was significantly upregulated in the cancerous tissues and its expression was negatively correlated with miR-27b expression level. Inhibition of UCA1 significantly restored miR-27b expression in MDR gastric cancer cells. UCA1 knockdown and miR-27b overexpression reduced IC50 of ADR, DDP, and 5-FU in SGC-7901/ADR cells and increased ADR induced cell apoptosis. UCA1 overexpression and miR-27b inhibition increased the IC50 of ADR, DDP, and 5-FU in SGC-7901 cells and reduced ADR induced cell apoptosis. Western blot analysis showed that UCA1 knockdown and miR-27b overexpression also decreased anti-apoptotic protein BCL-2 and increased apoptotic protein cleaved caspase-3.
Conclusions
UCA1 is negatively correlated with miR-27b expression in gastric cancer tissue. Knockdown of UCA1 restored miR-27b expression in gastric cancer cells. The UCA1-miR-27b axis was involved in regulation of chemosensitivity of gastric cancer cells.
MeSH Keywords: Genes, MDR; MicroRNAs; RNA, Long Noncoding; Stomach Neoplasms
Background
Although the use of chemotherapy during the past decades has significantly improved the survival of patients with gastric cancer, the development of multi-drug resistance (MDR) is still a crucial problem leading to therapeutic failure and cancer-related death [1–4]. However, the underlying molecular mechanisms have not been fully elucidated.
LncRNAs are non-protein coding RNAs, which are >200 bp and account for at least 80% of the transcripts of the entire genome [5]. Some recent studies suggest that lncRNAs are involved in regulation of MDR via regulating mRNA transcription or translation, or miRNA expression at transcriptional or post-transcriptional levels [6,7]. Overexpression of the lncRNA plasmacytoma variant translocation 1 (PVT1) in gastric cancer cells can increase the expression of MDR1, MRP, mTOR, and HIF-1α and promote the development of multi-drug resistance [8]. LncRNA MRUL can promote ABCB1 expression in MDR gastric cancer cell sublines and MRUL depletion can reduce ABCB1 mRNA levels and reverse the MDR phenotype of cells [9]. LncRNA NEAT1 may act as a miR-98-5p sponge to upregulate EGCG-induced CTR1 and promote cisplatin sensitivity in non-small cell lung cancer [10].
Urothelial Carcinoma Associated 1 (UCA1) is a long intergenic non-coding RNA first identified in bladder cancer [11]. A recent study reported that overexpression of UCA1 significantly increased bladder cancer cell viability after cisplatin (DDP) treatment [12], while UCA1 upregulation could also induce Adriamycin (ADR) resistance in breast cancer cells [13]. These results suggest that UCA1 might be an important lncRNA modulating chemosensitivity of cancer cells. UCA1 upregulation is also observed in gastric cancer and is considered a predictive biomarker [14,15]. One recent study found that knockdown of UCA1 inhibited malignant proliferation and also reduced the resistance to ADR of gastric cancer cells [16]. However, the molecular mechanism of UCA1 in MDR of gastric cancer is still not clear. MiR-27b has been reported as a tumor suppressor in gastric cancer, which is usually downregulated in cancer tissues [17,18]. In addition, it is also involved in regulation of MDR in gastric cancer cells [19]. However, the mechanism of its downregulation in gastric cancer is not fully elucidated.
In this study, we investigated the association between UCA1 and miR-27b in gastric cancer and further studied their involvement in MDR of gastric cancer.
Material and Methods
Human tissue collection
Twenty-eight patients diagnosed with primary gastric cancer, who had not received previous chemotherapy or radiotherapy, were recruited from Qilu Hospital of Shandong University. This study was approved by the Ethics Committee of the Qilu Hospital. Informed consent was obtained from the patients before the study. Gastric cancer tissues and adjacent normal tissues were obtained during gastrectomy and were frozen in liquid nitrogen immediately after resection and stored at −80 C for future use.
Cell culture and transfection
The human gastric cancer cell line SGC-7901 was obtained from American Type Culture Collection (Manassas, VA, USA). The SGC-7901 derived Adriamycin resistant SGC-7901/ADR, cisplatin resistant SGC-7901/DDP, and 5-FU resistant SGC-7901/FU cells were generated by using a conventional stepwise method (described in a previous study [20]), which induces drug resistance by increasing concentrations of the drugs over eight months. The cancer cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 μg/mL penicillin, and 100 U/mL streptomycin. Two UCA1 siRNAs were chemically synthesized by Ribobio (Guangzhou, China) with following sequence: si-UCA1-1: 5′-GTTAATCCAGGAGACAAAGA-3′, si-UCA1-2: 5′-TCTTTGTCTCCTGGATTAAC-3′. SGC-7901/ADR, SGC-7901/DDP and SGC-7901/FU cells were transfected with 75 nM UCA1 siRNA or the scramble negative control (Ribobio), while SGC-7901/ADR cells were transfected with 100 nM miR-27b mimics or the scramble negative control (Ribobio) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Then 48 hours later, cells transfected with siRNA were harvested for quantitative real-time PCR (qRT-PCR) to determine the inhibiting efficiency. The siRNA with higher inhibitive efficacy was used for following studies.
The complementary DNA encoding UCA1 was PCR-amplified and inserted into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA) between the BamhI/XhoI sites. SGC-7901 cells were transfected with pcDNA3.1-UCA1 expression vector or 50 nM miR-27b inhibitor (Ribobio) using Lipofectamine 2000 reagent (Invitrogen).
Bioinformatics analysis
The microarray data of lncRNA profiles in gastric cancer tissues and paired peritumoral tissues were retrieved in NCBI GEO Datasets (http://www.ncbi.nlm.nih.gov/gds, No. GSE53137). The binding sites between UCA1 and miR-27b were predicted using DIANA tools (http://carolina.imis.athena-innovation.gr/diana_tools/web).
QRT-PCR analysis
Total RNAs in the tissue and cell samples were extracted using the TRIzol reagent (Invitrogen). Then, reverse transcription was performed to get the first strand cDNA by using the PrimeScript® RT reagent kit (TaKaRa, Dalian, Liaoning, China). UCA1 expression was then detected by qRT-PCR with following primers: F, 5′-CTCTCCATTGGGTTCACCATTC-3′; R, 5′-GCGGCAGGTCTTAAGAGATGAG-3′, and SYBR® Premix DimerEraser Kit (TaKaRa). To detect mature miR-27b expression, miRNA specific cDNA was first synthesized using the stem-loop primers and the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Then, qRT-PCR analysis was performed using TaqMan MicroRNA Assay Kit (Applied Biosystems). All qRT-PCR reactions were performed using an ABI Prism 7500 (Applied Biosystems). The results were calculated using the 2−ΔΔCT methods. The correlation between UCA1 expression and miR-27b expression was assessed using linear regression analysis.
RNA fluorescence in situ hybridization (FISH)
Biotin-labeled UCA1-Locked Nucleic Acid (LNA) probe, miR-27b-LNA probe, and the corresponding control oligo for in situ hybridization were purchased from Exiqon (Vedbaek, Denmark). SGC-7901 and SGC-7901/ADR cells were grown on cover slips and the cells were fixed when reaching 60–70% confluence. Hybridization was performed according to the methods described in a previous study [21]. The signals were detected using anti-Biotin-Cy3 (C5585, Sigma-Aldrich) at 37°C for 30 minutes. Nuclei were counterstained with DAPI, then the immunofluorescence were detected under FV1000 fluorescence microscope (Olympus, Tokyo, Japan).
IC50 measurement
SGC-7901/ADR cells were transfected with UCA1 siRNA or miR-27b mimics, while SGC-7901 cells were transfected with UCA1 expression vector or miR-27b inhibitors.
24 hours after transfection, the cells were seeded in a 96-well plate. Then 24 hours later, the cells were treated with varying concentrations of ADR, DDP, or 5-FU for 48 hours. Cell viability was measured using a conventional MTT (Sigma Aldrich) assay. Absorbance was recorded at 490 nm using a microplate reader. The IC50 value was determined by creating dose-response curves.
Flow cytometric analysis of cell apoptosis
Cell apoptosis was detected by using Annexin V-FITC Apoptosis Detection Kit (ab14085, Abcam, Cambridge, UK) and the apoptosis rates were measured by using a flow cytometer (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
In brief, samples containing 30 μg of total protein were loaded per lane and then were separated by 10% SDS-PAGE. After that, the protein samples were electrophoretically transferred onto nitrocellulose membranes. The membranes were first blocked, washed, and then incubated with primary antibodies against BCL-2 (ab32124, Abcam) and cleaved caspase-3 (#9661, Cell Signaling, Danvers, MA, USA) and β-actin (ab8227, Abcam) overnight. After washing, the membranes were then incubated with HRP conjugated secondary antibodies. Protein bands were visualized by super ECL detection reagent (Applygen, Beijing, China).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0. The difference between groups was evaluated by unpaired, two-tailed Student t-test; p<0.05 indicated statistical significance.
Results
UCA1 was negatively correlated with miR-27b in gastric cancer
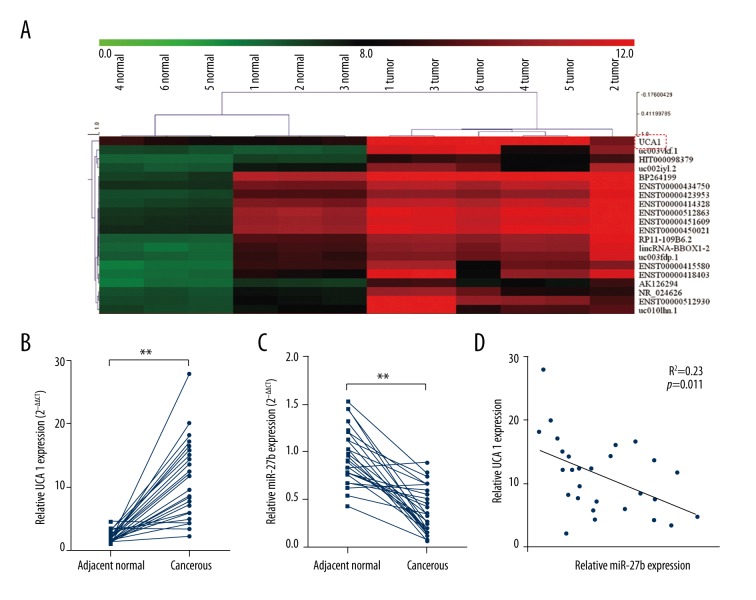
In this study, we first studied the dysregulated lncRNAs in gastric cancer tissues via retrieving the microarray data in the GEO dataset. A recent study analyzed the lncRNA profile based on six gastric cancer tissues and six paired peritumoral tissues [14]. By reviewing their microarray data (accession No. GSE53137), we observed that UCA1 was one of the most upregulated lncRNAs in gastric cancer tissues (Figure 1A). To further verify the dysregulation, we further performed qRT-PCR analysis based on 28 paired cancerous and peritumoral normal tissues. The results showed that UCA1 was significantly upregulated in cancerous tissues (Figure 1B). Interestingly, our qRT-PCR results showed that miR-27b, a miRNA with suppressive effect on multidrug resistance in gastric cancer cells, was markedly reduced in cancerous tissues (Figure 1C). By performing linear regression analysis, we further identified a negative correlation between UCA1 and miR-27b (R2=0.23, p=0.01) (Figure 1D). Therefore, we decided to further investigate whether there was any association between them and their involvement in MDR of gastric cancer cells.
Figure 1.
UCA1 is negatively correlated with miR-27b in gastric cancer. (A) Microarray data of the most upregulated lncRNAs in six gastric cancer tissues and six paired peritumoral tissues. Data was retrieved from GEO dataset, with the accession No. GSE53137. (B, C) QRT-PCR analysis of UCA1 expression (B) and miR-27b (C) expression in 28 gastric cancer tissues and paired peritumoral tissues. (D) Linear regression analysis of the correlation between UCA1 and miR-27b.
Knockdown of UCA1 restored miR-27b expression in the MDR gastric cancer cells
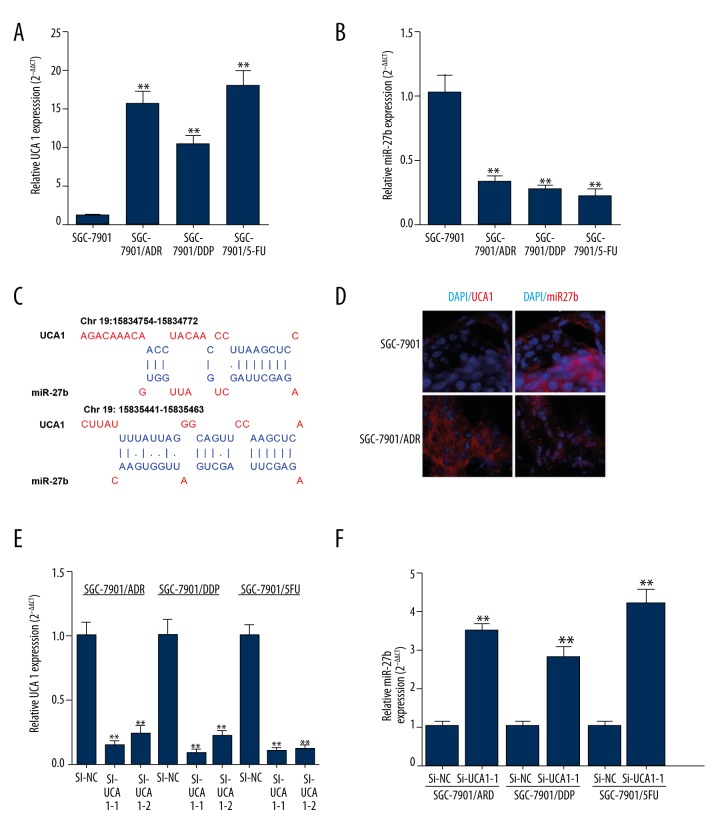
By performing qRT-PCR, we found that the MDR gastric cancer cells, including SGC-7901/ADR, SGC-7901/DDP, and SGC-7901/5-FU cells, had further increased UCA1 expression (Figure 2A) and decreased miR-27b expression (Figure 2B). Notably, the bioinformatics analysis showed that UCA1 had two possible binding sites of miR-27b (Figure 2C). Therefore, we decided to investigate whether UCA1 had a regulative effect on miR-27b expression. The results of FISH assay showed that UCA1 and miR-27b were distributed in both nucleus and cytoplasm in SGC7901 and SGC-7901/ADR cells (Figure 2D). The expression of UCA1 was significantly higher than in SGC-7901/ADR cells, while miR-27b expression was higher in SGC7901 cells (Figure 2D). Then, SGC-7901/ADR, SGC-7901/DDP, and SGC-7901/5-FU cells were transfected with UCA siRNA (Figure 2E). Si-UCA1-1, with a relatively higher inhibitive effect, was used in our studies. QRT-PCR results showed that inhibition of UCA1 significantly restored miR-27b expression in the MDR gastric cancer cells (Figure 2F).
Figure 2.
Knockdown of UCA1 restores miR-27b expression in the MDR gastric cancer cells. (A, B) QRT-PCR analysis of UCA1 expression (A) and miR-27b (B) expression in SGC-7901 cells, and SGC-7901 derived SGC-7901/ADR, SGC-7901/DDP, and SGC-7901/5-FU cells. (C) Predicted binding sites between UCA1 and miR-27b. (D) Fluorescence in situ hybridization experiments show that UCA1 and miR-27b (red) display opposite expression levels in SGC-7901 and SGC-7901/ADR cells. (E, F) QRT-PCR analysis of UCA1 expression (E) and miR-27b (F) expression in SGC-7901/ADR, SGC-7901/DDP, and SGC-7901/5-FU cells with or without the transfection of UCA1 siRNA. ** p<0.01.
UCA1 knockdown and miR-27b overexpression sensitized MDR gastric cancer cells to chemotherapeutic agents
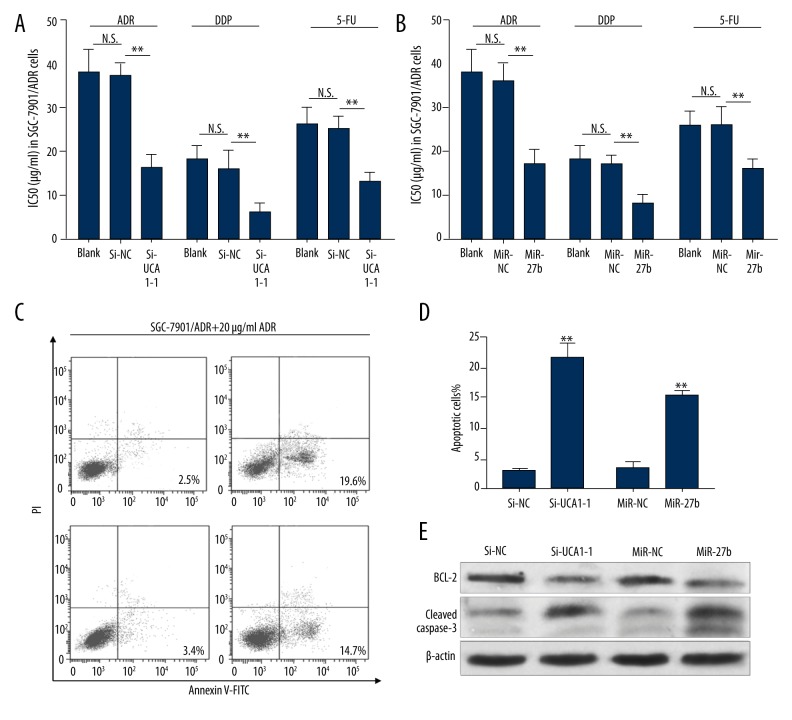
To further investigate the role of UCA1 and miR-27b in MDR of gastric cancer cells, MTT assays were performed to detect ADR, DDP, and 5-FU IC50 in SGC-7901/ADR cells after transfection of UCA1 siRNA (Figure 3A) or miR-27b mimics (Figure 3B). The results showed that both UCA1 knockdown and miR-27b overexpression decreased the IC50 of ADR, DDP, and 5-FU in SGC-7901/ADR cells (Figure 3A, 3B). Then, we performed flow cytometric analysis to detect ADR-induced apoptosis in SGC-7901/ADR cells after treatment with 20 μg/mL ADR. The results showed that the cells with UCA1 knockdown and miR-27b overexpression had significantly increased apoptosis after ADR treatment (Figure 3C, 3D). To further verify ADR-induced cell apoptosis, we performed Western blot analysis to detect the change of anti-apoptotic protein BCL-2 and apoptotic protein cleaved caspase-3. The results showed that UCA1 knockdown and miR-27b overexpression significantly reduced the expression of BCL-2 and increased the expression of cleaved caspase-3 in SGC-7901/ADR cells after treatment with 20 μg/mL ADR (Figure 3E). These results suggest that UCA1 knockdown and miR-27b overexpression sensitizes gastric cancer cell to chemotherapeutic agents.
Figure 3.
UCA1 knockdown and miR-27b overexpression sensitizes MDR gastric cancer cells to chemotherapeutic agents. (A, B) ADR, DDP and 5-FU IC50 in SGC-7901/ADR cells after transfection of UCA1 siRNA (A) or miR-27b mimics (B). (C, D) Representative images (C) and quantitation (D) of flow cytometric analysis of apoptotic SGC-7901/ADR cells with the transfection of UCA1 siRNA or miR-27b mimics and then treated with 20 μg/mL ADR for 48 hours. (E) Western blot analysis BCL-2 and cleaved caspase-3 expression in SGC-7901/ADR cells with the transfection of UCA1 siRNA or miR-27b mimics and then treated with 20 μg/mL ADR for 48 hours. ** p<0.01.
UCA1 overexpression and miR-27b knockdown confered MDR to gastric cancer cells
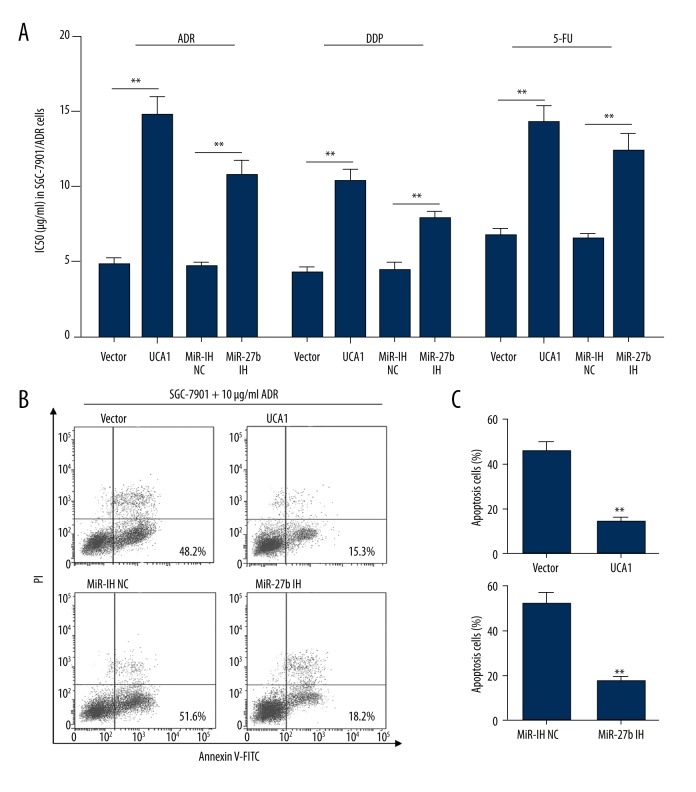
To further investigate the regulative effect of UCA1 and miR-27b on MDR of gastric cancer cells, SGC-7901 cells were first transfected with UCA1 expression vector or miR-27b inhibitors. MTT assays confirmed that both UCA1 overexpression and miR-27b knockdown increased the IC50 of ADR, DDP, and 5-FU in SGC-7901 cells (Figure 4A). The flow cytometric study showed that both UCA1 overexpression and miR-27b knockdown significantly reduced ADR (10 μg/mL) induced apoptosis (Figure 4B, 4C).
Figure 4.
UCA1 overexpression and miR-27b knockdown confers MDR to gastric cancer cells. (A) ADR, DDP, and 5-FU IC50 in SGC-7901 cells after transfection of UCA1 expression vector or miR-27b inhibitors. (B, C) Representative images (B) and quantitation (C) of flow cytometric analysis of apoptotic SGC-7901 cells with the transfection of UCA1 expression vector or miR-27b inhibitors and then treated with 10 μg/mL ADR for 48 hours. ** p<0.01.
Discussion
Emerging studies suggest that lncRNAs may play an important role in the development of MDR in gastric cancer. For example, knockdown of lncRNA MRUL in SGC7901/ADR and SGC7901/VCR cells resulted in increased rates of apoptosis, increased drug accumulation, and reduced drug release in the presence of ADR or vincristine [9]. The lncRNA PVT1 exhibited higher expression in both gastric cancer tissues and the SGC7901 paclitaxel-resistant cell line [22]. Overexpression of lncRNA PVT1 in gastric cancer cells significantly induced higher expression of MDR1, MRP, mTOR, and HIF-1α and subsequent development of MDR [8]. Another study reported that the expression of lncRNA ANRIL was positively correlated with the expression of MDR1 and MRP1, while ANRIL inhibition in gastric cancer cells can decrease the development of MDR [23].
UCA1 has been reported as an oncogenic lncRNA in gastric cancer that is usually upregulated in gastric cancer [6]. UCA1 high expression has been positively correlated with some malignant pathological characteristics of gastric cancer, such as higher grade and poor differentiation [16]. A recent study found that there was a significant negative association between UCA1 levels and overall survival (OS) and progression-free survival (PFS) time in gastric cancer patients [15]. Functionally, the silence of UCA1 could significantly inhibit cell proliferation of gastric cancer BGC-823 and SGC7901 cells and also reduce chemoresistance of SGC7901/ADR cells to ADR [16]. In this study, we decided to further investigate the regulative effect of UCA1 on MDR of gastric cancer cells. By retrieving previous microarray data in combination with our qRT-PCR analysis, we confirmed that UCA1 is significantly upregulated in the cancerous tissues.
In this study, we further identified a negative correlation between UCA1 and miR-27b in gastric cancer tissues. MiR-27b has been reported in other studies to be a tumor suppressor in gastric cancer, and is usually downregulated in the cancer tissues and cells [17,18]. Functionally, miR-27b can inhibit angiogenesis by downregulating vascular endothelial growth factor C expression in gastric cancer [18]. It can also suppress Helicobacter pylori-induced gastric tumorigenesis through negatively regulating Frizzled-7 [17]. In addition, miR-27b is also involved in regulation of MDR in gastric cancer cells via the miR-27b/CCNG1/P53/miR-508-5p axis [19]. Therefore, miR-27b might also play an important role in modulating MDR. In our study, we further explored the association between UCA1 and miR-27b and their involvement in MDR of gastric cancer cells. Our results showed that knockdown of UCA1 restored miR-27b expression in MDR gastric cancer cells. Functionally, UCA1 knockdown and miR-27b overexpression reduced the IC50 of ADR, DDP, and 5-FU in SGC-7901/ADR cells and increased ADR-induced cell apoptosis. UCA1 overexpression and miR-27b inhibition increased the IC50 of ADR, DDP, and 5-FU in SGC-7901 cells and reduced ADR-induced cell apoptosis. Our Western blot analysis showed that UCA1 knockdown and miR-27b overexpression also decreased levels of anti-apoptotic protein BCL-2 and increased levels of apoptotic protein cleaved caspase-3. Therefore, we inferred that the UCA1-miR-27b axis was involved in regulation of chemosensitivity of gastric cancer cells.
Conclusions
UCA1 is negatively correlated with miR-27b expression in gastric cancer. Knockdown of UCA1 restored miR-27b expression in cancer cells. The UCA1-miR-27b axis was involved in regulation of chemosensitivity of gastric cancer cells.
Footnotes
Source of support: Departmental sources
References
- 1.Wu WD, Wang M, Ding HH, Qiu ZJ. FBXL5 attenuates RhoGDI2-induced cisplatin resistance in gastric cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:2551–57. [PubMed] [Google Scholar]
- 2.Wang LL, Zhang XH, Zhang X, Chu JK. MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT) Eur Rev Med Pharmacol Sci. 2016;20:1733–39. [PubMed] [Google Scholar]
- 3.Wang S, Liu F, Zhu J, et al. DNA Repair genes ERCC1 and BRCA1 expression in non-small cell lung cancer chemotherapy drug resistance. Med Sci Monit. 2016;22:1999–2005. doi: 10.12659/MSM.896606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu X, Li JY, Guo J, et al. Influence of miR-451 on drug resistances of paclitaxel-resistant breast cancer cell line. Med Sci Monit. 2015;21:3291–97. doi: 10.12659/MSM.894475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung T, Chang HY. Long noncoding RNA in genome regulation: Prospects and mechanisms. RNA Biol. 2010;7:582–85. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang N, Yang GQ, Shao XM, Wei L. GAS5 modulated autophagy is a mechanism modulating cisplatin sensitivity in NSCLC cells. Eur Rev Med Pharmacol Sci. 2016;20:2271–77. [PubMed] [Google Scholar]
- 7.Li DS, Ainiwaer JL, Sheyhiding I, et al. Identification of key long non-coding RNAs as competing endogenous RNAs for miRNA-mRNA in lung adenocarcinoma. Eur Rev Med Pharmacol Sci. 2016;20:2285–95. [PubMed] [Google Scholar]
- 8.Zhang XW, Bu P, Liu L, et al. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophys Res Commun. 2015;462:227–32. doi: 10.1016/j.bbrc.2015.04.121. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang D, Wu K, et al. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182–93. doi: 10.1128/MCB.01580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang P, Wu X, Wang X, et al. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016 doi: 10.18632/oncotarget.9712. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XS, Zhang Z, Wang HC, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–58. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281:1750–58. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, Huang O, Xie Z, et al. A novel long non-coding RNA-ARA: Adriamycin resistance-associated. Biochem Pharmacol. 2014;87:254–83. doi: 10.1016/j.bcp.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Gao T, Sun Y, et al. LncRNA expression profile reveals the potential role of lncRNAs in gastric carcinogenesis. Cancer Biomark. 2015;15:249–58. doi: 10.3233/CBM-150460. [DOI] [PubMed] [Google Scholar]
- 15.Hong HH, Hou LK, Pan X, et al. Long non-coding RNA UCA1 is a predictive biomarker of cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.10142. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang C, Guo Y, Zhang J, Huang B. Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer. Cancer Chemother Pharmacol. 2016;77:1061–67. doi: 10.1007/s00280-016-3029-3. [DOI] [PubMed] [Google Scholar]
- 17.Geng Y, Lu X, Wu X, et al. MicroRNA-27b suppresses Helicobacter pylori-induced gastric tumorigenesis through negatively regulating Frizzled7. Oncol Rep. 2016;35:2441–50. doi: 10.3892/or.2016.4572. [DOI] [PubMed] [Google Scholar]
- 18.Liu HT, Xing AY, Chen X, et al. MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget. 2015;6:37458–70. doi: 10.18632/oncotarget.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang Y, Feng B, Zhou L, et al. The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget. 2016;7:538–49. doi: 10.18632/oncotarget.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traverso N, Ricciarelli R, Nitti M, et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev. 2013;2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Mo YY. Detection of microRNAs in cultured cells and paraffin-embedded tissue specimens by in situ hybridization. Methods Mol Biol. 2011;676:73–83. doi: 10.1007/978-1-60761-863-8_6. [DOI] [PubMed] [Google Scholar]
- 22.Ding J, Li D, Gong M, et al. Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther. 2014;7:1625–30. doi: 10.2147/OTT.S68854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan WG, Xu DH, Xu C, et al. Silencing of long non-coding RNA ANRIL inhibits the development of multidrug resistance in gastric cancer cells. Oncol Rep. 2016;36:263–70. doi: 10.3892/or.2016.4771. [DOI] [PubMed] [Google Scholar]