Abstract
Tomato yellow leaf curl virus (TYLCV), a member of the genus Begomovirus, is one of the most important viruses of cultivated tomatoes worldwide, mainly causing yellowing and curling of leaves with stunting in plants. TYLCV causes severe problems in sub-tropical and tropical countries, as well as in Korea. However, the mechanism of TYLCV infection remains unclear, although the function of each viral component has been identified. TYLCV C4 codes for a small protein involved in various cellular functions, including symptom determination, gene silencing, viral movement, and induction of the plant defense response. In this study, through yeast-two hybrid screenings, we identified TYLCV C4-interacting host proteins from both healthy and symptom-exhibiting tomato tissues, to determine the role of TYLCV C4 proteins in the infection processes. Comparative analyses of 28 proteins from healthy tissues and 36 from infected tissues showing interactions with TYLCV C4 indicated that TYLCV C4 mainly interacts with host proteins involved in translation, ubiquitination, and plant defense, and most interacting proteins differed between the two tissues but belong to similar molecular functional categories. Four proteins—two ribosomal proteins, S-adenosyl-L-homocysteine hydrolase, and 14-3-3 family protein—were detected in both tissues. Furthermore, the identified proteins in symptom-exhibiting tissues showed greater involvement in plant defenses. Some are key regulators, such as receptor-like kinases and pathogenesis-related proteins, of plant defenses. Thus, TYLCV C4 may contribute to the suppression of host defense during TYLCV infection and be involved in ubiquitination for viral infection.
Keywords: defense genes, protein-protein interactions, tomato, Tomato yellow leaf curl virus, yeast-two hybrid
Tomato yellow leaf curl (TYLC) caused by Tomato yellow leaf curl virus (TYLCV) is one of the most significant viral diseases in cultivated tomatoes in tropical and subtropical regions. In the 1960s, the first case of TYLC was reported in Israel (Czosnek and Laterrot, 1997). TYLC has occurred since 1960s in other countries, including Europe (Kheyr-Pour et al., 1991), Asia (Rochester et al., 1990), South America, Africa (Harrison et al., 1991), and Australia (Dry et al., 1993). Recently, TYLC has been frequently observed in Korea because the climate is becoming warmer and changing to a subtropical climate (Lee et al., 2010). Symptoms of the disease include upward curling with stunting and flower abortion, as well as reduction and yellowing of young leaves (Moriones and Navas-Castillo, 2000). This may lead to huge economic losses of cultivated tomatoes. The infection mechanism of TYLCV remains unclear. Thus, it is important to determine the precise infection mechanisms.
TYLCV belongs to the geminiviruses, a unique family of circular, single-stranded DNA viruses, which have a small genome compared to other common viruses. Two genes, V1 and V2, are encoded by the genome; the coat protein is encoded by V1, whereas a movement protein is encoded by V2. In addition to these two genes, the complementary viral strand consists of four genes, C1–C4; C1 codes for a protein related to replication, C2 codes for a transcription activator, C3 codes for a replication enhancer, and C4 codes for a small protein that may act as a symptom determinant (Péréfarres et al., 2012). Among the viral proteins related to the infection mechanism, the most interesting is C4, which contributes to induction of the plant defense response (Van Wezel et al., 2002) and is associated with pathogenicity (Rigden et al., 1994), virus movement (Jupin et al., 1994), and virus-induced post-transcriptional gene silencing (Matić et al., 2016; Vanitharani et al., 2005).
Infection processes of TYLCV, similarly to other viruses, require protein-protein interactions (PPIs) either among viral components or between the virus and host. Some PPIs are known to be key in causing plant diseases (Bisaro, 2006). For example, DNA methylation was decreased or suppressed by interfering with the methyl cycle and post-transcriptional gene silencing. TYLCV also highly depends on host proteins because this virus is too small to contain all essential proteins, indicating that the interaction between TYLCV proteins and host proteins is essential for infection processes. This interaction was shown to be involved in DNA replication, transcription, intracellular communication, and virus pathogenicity. Thus, viral core proteins, including C4 protein, form PPI networks likely because most proteins belong to signal pathways as well as metabolic pathways (Van Wezel et al., 2002). These networks covering many information on all levels of cellular functions may be useful for predicting or identifying the functions of proteins involved in the cellular mechanism through PPIs (Brückner et al., 2009). At the genome-wide scale, the most effective method for evaluating PPIs is yeast two-hybrid (Y2H) screening, although this method shows a high rate of false-positives. The development of high-throughput Y2H screens has contributed to the establishment of large datasets at the proteome scale, which can be employed for unbiased investigation of potential PPIs (Cusick et al., 2005; Seo et al., 2011) and investigating gene functions as a biological network (Bang et al., 2013).
The defense mechanism of plants is highly complex, depending on the specific conditions. Thus, we hypothesized that the disease-causing protein C4 of TYLCV differs in its PPIs between healthy and infected tomatoes. After generation of the infected tomato plants, using Agrobacterium-mediated inoculation of TYLCV (Bang et al., 2014), we conducted high-throughput Y2H screening to identifying proteins that interact with C4 and compared the PPIs of C4-interacting proteins expressed in healthy tomato cDNA libraries to those in infected-tomato cDNA libraries of TYLCV. This approach was adopted to establish the infection mechanisms of TYLCV and plant defense pathways.
By this approach, we found that TYLCV C4 mainly interacts with host proteins involved in translation, ubiquitination, and plant defense. The identified proteins in symptom-developed tissues were involved in plant defenses, and include key components such as receptor-like kinases and pathogenesis-related proteins. Thus, TYLCV C4 may contribute to the suppression of the host defense during TYLCV infection, or be targeted by host defense systems, although TYLCV can overcome this via other mechanisms.
Materials and Methods
Generation of TYLCV-infected tomatoes
Two-week-old tomato plants were inoculated with the infectious TYLCV clone (pCAMBIA0390-TY-1.9mer) (Bang et al., 2014) and maintained in an insect-free containment growth chamber at 27°C with a 16-h photoperiod. Four weeks later, the symptoms including upward curling, yellowing of leaves, and stunting were observed on the leaves of tomato plants.
Construction of bait vector for Y2H screening
A gene encoding TYLCV C4 protein was introduced into a bait vector to identify the C4-interaction proteins in tomato. The gene encoding C4 protein consists of 294 nucleotide. This gene was amplified by PCR, using a specific primer set (Table 1). The amplified gene was confirmed by sequencing and cloned into pENTR/D-TOPO (Invitrogen, Carlsbad, CA, USA) for utilization with the Gateway system, known as pENTR-C4. The C4 gene from entry clones (pENTR-C4) was introduced into pGBKT7 (Clontech Laboratories Inc., Mountain View, CA, USA), which contains a GAL4 DNA-binding domain, Kanr for selection in Escherichia coli, and TRP1 nutritional marker for selection in yeast. As a result, the pGBKT7 vector including the gene coding for C4 protein was generated as bait in GAL4-based two-hybrid system, named as pGBKT7-C4.
Table 1.
Primers for sequencing of pGBKT7 and pGADT7 and amplifying cDNA inserted in pGADT7 of putative interacting clones from healthy and TYLCV C4-infected tomatoes
| Purpose | Primer | Sequence (5′ to 3′) |
|---|---|---|
| Sequencing for bait (pGBKT7) and prey (pGADT7) | Matchmaker 5′ BD | TCATCGGAAGAGAGTAGTAAC |
| Matchmaker 3′ BD | GAGTCACTTTAAAATTTGTAT | |
| Matchmaker 5′ AD | CTATTCGATGATGAAGATAC | |
| Matchmaker 3′ AD | GTGAACTTGCGGGGTTTTTC | |
| Primer for first-strand cDNA synthesis | SMART III | AAGCAGTGGTATCAACGCAGAGTGGCCATTATGGCCGGG |
| CDS III | ATTCTAGAGGCCGAGGCGGCCGACATG-d(T)30VN | |
| cDNA amplification | 5′ PCR | TTCCACCCAAGCAGTGGTATCAACGCAGAGTGG |
| 3′ PCR | GTATCGATGCCCACCCTCTAGAGGCCGAGGCGGCCGACA |
TYLCV, Tomato yellow leaf curl virus.
Total RNA and mRNA extraction from healthy and TYLCV-infected tomato plants
Leaves of healthy and TYLCV-infected tomato plants were homogenized or ground in liquid nitrogen into fine powders. The fine powder was used as total RNA, up to 500 mg. Total RNA extraction was extracted using Trizol reagent according to the manufacturer’s instructions (Ambion, Carlsbad, CA, USA). The quality and concentration of total RNAs were confirmed using a Nanodrop 2000 spectrometer (Thermo Scientific, Waltham, MA, USA). To acquire pure mRNAs, extracted total RNAs from healthy and TYLCV-infected tomato leaves were purified using the oligotex mRNA spin column protocol (Qiagen, Hilden, Germany).
Synthesis of first-strand cDNAs and amplification of double-strand cDNAs
Extracted mRNAs from two healthy and TYLCV-infected tomato leaves were used to construct two sets of cDNA libraries. Each of the mRNAs from two distinguishable leaves was used to transcribe up to 2 μl of product (approximately 1.0 μg) to obtain each first-strand cDNA, using the Make Your Own Mate & Plate™ Library System (Clontech Laboratories Inc.). This system used two specific primers, SMART III Oligo and CDS III Primer (Clontech Laboratories Inc.) (Table 1), to synthesize each first-strand cDNA from mRNAs. To amplify highly pure cDNAs, RNase H was added to the first-strand cDNAs. Long-distance PCR proceeded to amplify cDNAs that were treated with RNase H, using Advantage 2 Polymerase Mix (Clontech Laboratories Inc.). The mixture containing 10 μl of 10× advantage 2 PCR buffer, 10 μl of 10× melting solution, 2 μl of 50× dNTP mix, 2 μl of 50× advantage 2 polymerase mix 2 μl, 2 μl of 5′-PCR primer, 2 μl of 3′-PCR primer, and 2 μl of first-strand cDNA were added in a reaction volume of 100 μl. PCR protocol was as follows: 95°C for 30 s, followed by 25 cycles considering the amount of mRNA, denaturation step at 95°C for 10 s, and elongation at 68°C for 6 min, increasing the extension time by 5 s with each successive cycle. Amplified double-strand cDNAs were successfully obtained by PCR.
Yeast transformation for PPI and Y2H screening, using nutrient deficient media
We modified the Frozen-EZ Yeast Transformation II kit (Zymo Research, Irvine, CA, USA) based on the Yeastmaker Yeast Transformation System 2 (Clontech Laboratories Inc.). Amplified cDNA libraries were added to competent yeast cells of with a yeastmaker DNA carrier in accordance with the Yeastmaker Yeast Transformation System 2, pGADT7-Rec vector, which is designed for the construction of GARL4 AD/cDNA libraries by homologous recombination in yeast. After co-transformation with pGBKT7-C4, yeast transformants were spread on an appropriate plate. We used SD/Leu−/Trp−/His− medium for cell growth (synthetic minimal medium lacking leucine, tryptophan, and histidine) (Clontech Laboratories Inc.). Yeast transformants were grown in an incubator for 4 days at 30°C.
Plasmid extraction from yeast AH109 containing pGADT7
After incubation, positive colonies were incubated in 5 ml of SD/Leu−/Trp− (Clontech Laboratories Inc.) broth at 30°C for 12 h with shaking. Each culture was spotted onto SD/Leu−/Trp−/His− plates to verify the surviving colonies. After spotting the culture, positive colonies exhibiting PPIs were collected. We extracted plasmid including pGADT7 from the collected patches of positive colonies using the Easy Yeast Plasmid isolation kit (Clontech Laboratories Inc.). Extracted plasmids were cloned into E. coli DH5a for amplification and sequencing.
Sequencing of cDNA libraries in pGADT7 vector
Using the specific primer Matchmaker 5′ AD, we sequenced the cDNA of pGADT7 prepared from mRNA containing information regarding PPIs with C4 protein in the pGBKT7 vector, using a 3730XL DNA analyzer (Macrogen, Seoul, Korea).
Validation of PPI through retransformation
To test the reproducibility of candidate interactions in retransformed yeast cells to reduce the false-positive interactions after propagation in E. coli, extracted plasmids were retransformed into yeasts and tranformants were spread on SD/Leu−/Trp−/His− or SD/Leu−/Trp−/His−/Ade− (Clontech Laboratories Inc.) plates to verify the PPI.
Results
In situ Y2H screening, using as a TYLCV C4 bait both in healthy and TYLCV-infected tomatoes
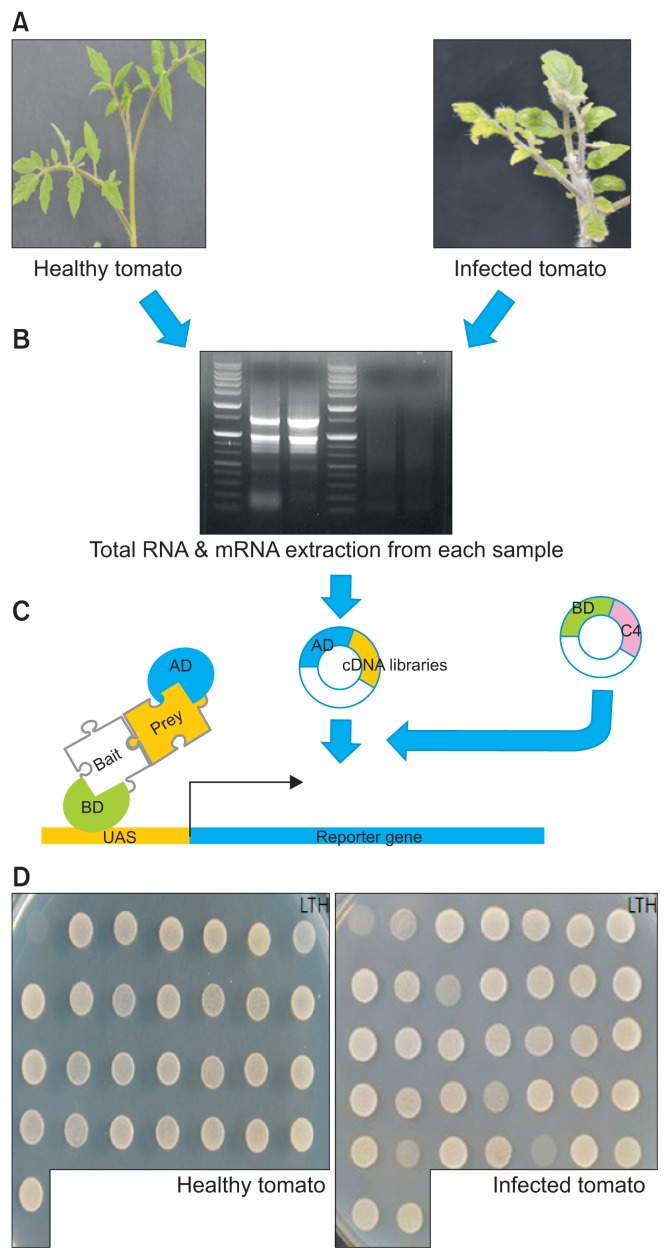
Two-week old tomatoes were inoculated using Agrobacterium with the infectious TYLCV clone and empty vector. Both healthy leaves from tomatoes treated with empty vector and symptom-exhibiting leaves from tomatoes treated with the infectious TYLCV clone were collected. After the generation of cDNA libraries from both leaves, we performed Y2H screening, using as a TYLCV C4 bait (pGBKT7-C4). The overall procedures for in situ Y2H screening is shown in Fig. 1.
Fig. 1.
Diagram of yeast two-hybrid (Y2H) screening using Tomato yellow leaf curl virus (TYLCV) C4 bait with cDNA libraries generated from healthy and TYLCV-infected tomatoes. (A) Preparation of healthy and TYLCV-infected tomatoes. (B) Extraction of total RNA and mRNA. (C) Construction of cDNA libraries. (D) Y2H method to identify C4-interacting proteins. TYLCV C4 and tomato protein interactions were confirmed by clone survival on SD/Leu−/Trp−/His− medium. AD, activation domain; BD, biding domain; UAS, upstream activating sequence; LTH, SD/Leu−/Trp−/His− medium.
Before Y2H screening, we tested the auto-activation of TYLCV C4 to verify the interaction between the bait and prey fusion proteins, using the expression of the reporter gene HIS3 and His3 auxotrophic yeast cells on histidine-deficient medium. When pGBKT7 including C4 was co-expressed with pGADT7-empty in histidine-deficient medium, yeast cell growth was not detected.
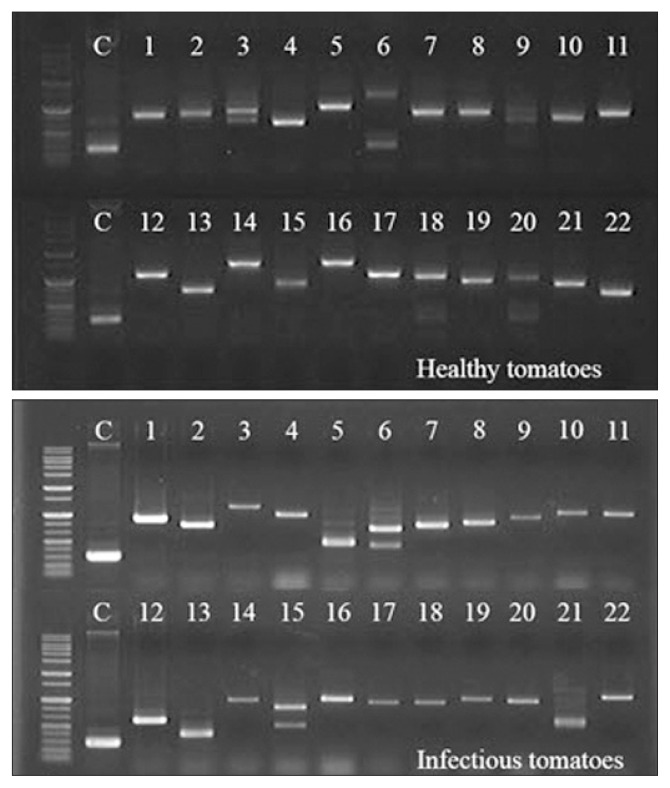
In the screening of clones on SD/Leu−/Trp−/His− medium, 75 and 76 clones were chosen as candidate clones from healthy and TYLCV-infected tomatoes, respectively (Table 2). The insert sizes of the acquired clones were determined by Y2H screening, using cDNA libraries prepared from leaves of healthy and symptom-exhibiting tomatoes, and evaluated by PCR (Table 1). Diverse bands indicated the sizes of inserted cDNA were observed by electrophoresis (Fig. 2).
Table 2.
Reproducibility of candidate interactions in retransformed yeast cells for validating the false positive interactions
| Healthy tomato | Infectious tomato | |
|---|---|---|
| Total survival clone at initial screens | 75 | 76 |
| Positive clones after retransformation | 28 | 36 |
Values are presented as clone numbers.
Fig. 2.
Amplified bands of cDNA inserts from positive C4-interacting clones. Diversity of C4 interaction partners in pGADT7 from healthy and Tomato yellow leaf curl virus C4-infected tomatoes was detected by PCR, using Matchmaker AD primers (Table 1). DNA ladder marker is 1-kb(+) ladder molecular size marker. Amplified empty pGADT7-Rec was used as a control (C).
Validation of positive interactions through retransformation
To test the reproducibility of the 75 and 76 candidate proteins in retransformed yeast cells, the extracted plasmids, after propagation in E. coli, were re-transformed into yeast and co-expressed with TYLCV C4. Yeast transformants were spread onto SD/Leu−/Trp−/His− plates and positive interactions were determined based on survival. Twenty-eight of seventy-five clones from healthy tomatoes and 36 of 76 from healthy tomatoes survived on SD/Leu−/Trp−/His− plates (Table 2). These positive 28 and 36 clones were re-spread onto SD/Leu−/Trp−/His−/Ade− plates to verify the strong interactions. Finally, all 28 positive clones from healthy tomatoes and 33 of 36 clones from healthy tomatoes showed strong C4-interacting proteins.
Identification of C4 interaction proteins encoded by pGADT7
Prey vectors, which were pGADT7 with cDNA inserts, from 28 and 36 positive clones were extracted for sequencing of the cDNA inserts in pGADT7. The sequences of cDNA inserted into pGADT7 during amplification in E. coli DH5a were obtained and cDNA inserts were identified using the National Center for Biotechnology Information BLASTn, which searches based on sequence similarity (Table 3, 4).
Table 3.
Identification of C4-interacting partners from healthy tomatoes by NCBI using sequence similarity based on BLASTn
| NCBI accession No. | Gene BLASTn |
|---|---|
| C4 interaction partners in healthy tomatoes | |
| XM_004233057.2 | PREDICTED: Solanum lycopersicum 3-oxoacyl-[acyl-carrier-protein] synthase I, chloroplastic (LOC101267959), mRNA |
| XM_004252922.2 | PREDICTED: Solanum lycopersicum DEAD-box ATP-dependent RNA helicase 42 (LOC101254580), transcript variant X3, mRNA |
| XM_015209601.1 | PREDICTED: Solanum pennellii ribulose bisphosphate carboxylase small chain 2C, chloroplastic (LOC107010325), mRNA |
| NM_001247083.2 | Solanum lycopersicum S-adenosyl-L-homocysteine hydrolase (SAHH), mRNA |
| NM_001247257.2 | Solanum lycopersicum mRNA for catalase 2 (CAT2 gene) |
| NM_001308944.1 | Solanum lycopersicum ribulose bisphosphate carboxylase small chain 2A, chloroplastic (LOC543974), mRNA |
| NM_001309210.1 | Solanum lycopersicum ribulose bisphosphate carboxylase small chain 3B, chloroplastic-like (LOC101268337), mRNA |
| XM_004235218.2 | PREDICTED: Solanum lycopersicum uncharacterized LOC101268660 (LOC101268660), mRNA |
| XM_004239679.2 | PREDICTED: Solanum lycopersicum probable small nuclear ribonucleoprotein G (LOC101245652), mRNA |
| NM_001247487.1 | Solanum lycopersicum beta-glucosidase 08 (LOC100191128), mRNA |
| XM_004239073.2 | PREDICTED: Solanum lycopersicum glutamate—glyoxylate aminotransferase 2 (LOC101265236), mRNA |
| XM_004236967.2 | PREDICTED: Solanum lycopersicum probable ADP-ribosylation factor GTPase-activating protein AGD11 (LOC101263994), mRNA |
| NM_001287365.1 | Solanum lycopersicum ubiquitin-conjugating enzyme E2 variant 1B-like (Suv), mRNA |
| NM_001247178.2 | Solanum lycopersicum 14-3-3 family protein (LOC543565), mRNA |
| XM_004251778.2 | PREDICTED: Solanum lycopersicum chlorophyll a–b binding protein 8, chloroplastic-like (LOC101265617), mRNA |
| NM_001308943.1 | Solanum lycopersicum ribulose bisphosphate carboxylase small chain 1, chloroplastic (LOC543973), mRNA |
| XM_004242475.2 | PREDICTED: Solanum lycopersicum probable pyridoxal biosynthesis protein PDX1 (LOC101257782), mRNA |
| XM_004247514.2 | PREDICTED: Solanum lycopersicum cysteine synthase (LOC101244415), mRNA |
| XM_004253046.2 | PREDICTED: Solanum lycopersicum eukaryotic initiation factor 4A-2-like (LOC101266405), mRNA |
| XM_004234267.2 | PREDICTED: Solanum lycopersicum 40S ribosomal protein SA-like (LOC101250574), mRNA |
| XM_004251632.2 | PREDICTED: Solanum lycopersicum ubiquitin-conjugating enzyme E2 2 (LOC101250809), mRNA |
| XM_004251632.2 | PREDICTED: Solanum lycopersicum WD repeat-containing protein 61-like (LOC101246693), mRNA |
| XM_004230521.2 | PREDICTED: Solanum lycopersicum nitronate monooxygenase (LOC101251627), mRNA |
| NM_001247570.2 | Solanum lycopersicum eukaryotic translation initiation factor 5A-1 (eIF-5A1), mRNA |
| NM_001246901.1 | Solanum lycopersicum CONSTANS interacting protein 4 (CIP4), mRNA |
| XM_004252320.2 | PREDICTED: Solanum lycopersicum 60S ribosomal protein L28-1 (LOC101250778), mRNA |
| XM_004237653.2 | PREDICTED: Solanum lycopersicum probable methyltransferase PMT24 (LOC101252680), mRNA |
| NM_001247128.2 | Solanum lycopersicum glycine-rich protein (LOC544061), transcript variant 1, mRNA |
NCBI, National Center for Biotechnology Information.
Table 4.
Identification of C4-interacting partners from TYLCV C4-infected tomatoes by NCBI using sequence similarity based on BLASTn
| NCBI accession | No. Gene BLASTn |
|---|---|
| C4 interaction partners in infectious tomatoes | |
| AK323048.1 | Solanum lycopersicum metallothionein-like protein (LOC778298), mRNA |
| KF225312.1 | Tomato yellow leaf curl virus isolate Korea, complete genome, coat protein |
| XM_004228902.1 | PREDICTED: Solanum lycopersicum 2-oxoisovalerate dehydrogenase subunit beta, mitochondrial-like (LOC101254327), mRNA |
| NM_001247385.1 | Solanum lycopersicum PR protein (PR1b1), mRNA |
| XM_004234267.1 | PREDICTED: Solanum lycopersicum 40S ribosomal protein SA-like (LOC101250574), mRNA |
| XM_004238417.1 | PREDICTED: Solanum lycopersicum AT-rich interactive domain-containing protein 5-like (LOC101244579), mRNA |
| NM_001247708.1 | Solanum lycopersicum 14-3-3 family protein (TFT7), mRNA |
| XM_004234280.1 | PREDICTED: Solanum lycopersicum asparagine--tRNA ligase, chloroplastic/mitochondrial-like (LOC101254276), mRNA |
| XM_004236228.1 | PREDICTED: Solanum lycopersicum d-3-phosphoglycerate dehydrogenase, chloroplastic-like (LOC101246616), mRNA |
| NM_001246911.1 | Solanum lycopersicum flavonoid biosynthesis oxidoreductase protein (LOC100736526), mRNA |
| NM_001246909.1 | Solanum lycopersicum carotenoid cleavage dioxygenase 1-2 (CCD1-2), mRNA |
| KC184125.1 | PREDICTED: Solanum lycopersicum 3-dehydroquinate synthase-like (LOC101252666), mRNA |
| XM_004241576.1 | PREDICTED: Solanum lycopersicum aspartic proteinase Asp1-like (LOC101266949), mRNA |
| XM_004251107.1 | PREDICTED: Solanum lycopersicum elongation factor 1-alpha-like, transcript variant 2 (LOC101264700), mRNA |
| XM_004249574.1 | PREDICTED: Solanum lycopersicum histone deacetylase HDT1-like (LOC101262270), mRNA |
| BT012911.1 | PREDICTED: Solanum lycopersicum RING-H2 finger protein ATL7-like (LOC101251996), mRNA |
| XM_004251815.1 | PREDICTED: Solanum lycopersicum early nodulin-like protein 2-like (LOC101251653), mRNA |
| BT012693.1 | Solanum lycopersicum glyceraldehyde-3-phosphate dehydrogenase (GAPDH), mRNA |
| XM_004242857.1 | PREDICTED: Solanum lycopersicum adagio protein 1-like (LOC101262932), mRNA |
| XM_004231896.1 | PREDICTED: Solanum lycopersicum phytosulfokines 3-like (LOC101268443), mRNA |
| AK246300.1 | Solanum lycopersicum eukaryotic translation initiation factor 5A-3 (eIF-5A3), mRNA |
| XM_004249363.1 | PREDICTED: Solanum lycopersicum putative syntaxin-24-like (LOC101249973), mRNA |
| XM_004246288.1 | PREDICTED: Solanum lycopersicum GDP-mannose-dependent alpha-mannosyltransferase-like (LOC101252422), mRNA |
| XM_004246540.1 | PREDICTED: Solanum lycopersicum E3 ubiquitin-protein ligase UPL7-like (LOC101250834), mRNA |
| CU326399.2 | PREDICTED: Solanum lycopersicum probable LRR receptor-like serine/threonine-protein kinase At4g08850-like (LOC101265539), mRNA |
| XM_004246326.1 | PREDICTED: Solanum lycopersicum 18.5 kDa class I heat shock protein-like, transcript variant 2 (LOC101263239), mRNA |
| NM_001247083.2 | Solanum lycopersicum S-adenosyl-L-homocysteine hydrolase (SAHH), mRNA |
| AK320830.1 | PREDICTED: Solanum lycopersicum importin subunit alpha-1a-like (LOC101257502), mRNA |
| XM_004232986.1 | PREDICTED: Solanum lycopersicum U-box domain-containing protein 17-like (LOC101245319), mRNA |
| XM_004232030.1 | PREDICTED: Solanum lycopersicum oxygen-evolving enhancer protein 1, chloroplastic-like, transcript variant 2 (LOC101258321), mRNA |
| BT013330.1 | PREDICTED: Solanum lycopersicum triose phosphate/phosphate translocator, chloroplastic-like, transcript variant 2 (LOC101249306), mRNA |
| XM_004231069.1 | PREDICTED: Solanum lycopersicum putative late blight resistance protein homolog R1A-10-like (LOC101263647), mRNA |
| NM_001247791.1 | Solanum lycopersicum metallothionein II-like protein (MTA), mRNA |
| XM_004252320.2 | PREDICTED: Solanum lycopersicum 60S ribosomal protein L28-1 (LOC101250778), mRNA |
| XM_004230999.1 | PREDICTED: Solanum lycopersicum hydroxypyruvate reductase (HPR), mRNA |
| XM_004251063.1 | PREDICTED: Solanum lycopersicum ABC transporter F family member 1-like (LOC101250771), mRNA |
TYLCV, Tomato yellow leaf curl virus; NCBI, National Center for Biotechnology Information.
Functional grouping of C4-interacting proteins based on gene ontology (GO)
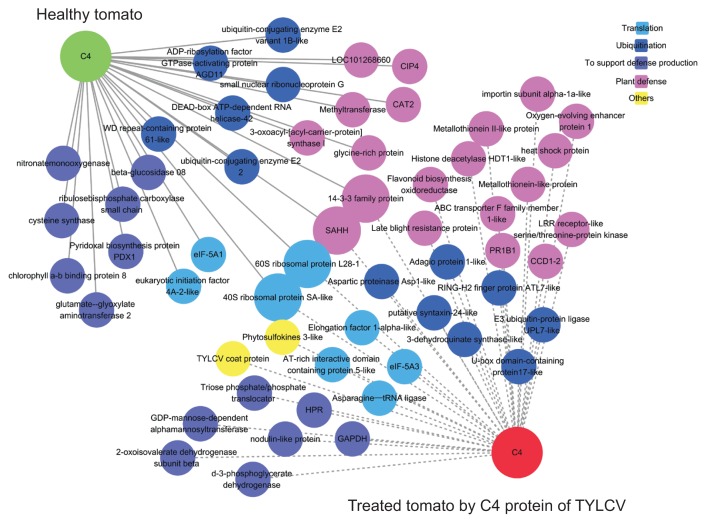
C4-interacting proteins were categorized into five groups, including translation, ubiquitination, support of defense production, plant defense, and others, based on open source of GO (http://www.uniprot.org/) (Fig. 3, Table 5).
Fig. 3.
Construction of protein-protein interaction (PPI) map between Tomato yellow leaf curl virus (TYLCV) C4 and putative interacting proteins based on gene ontology analysis. C4-interacting partners in healthy and TYLCV-infected tomatoes were divided into five groups: translation (blue node), ubiquitination (dark blue node), support of defense production (purple node), plant defense (pink node), and others (yellow node). A green color node represents C4 as a bait with cDNA library generated from healthy tomatoes and red represents C4 as a bait with cDNA library generated from TYLCV-infected tomatoes.
Table 5.
Functional groups of C4-interacting proteins based on gene ontology
| Classification | Control tomato | Treated tomato |
|---|---|---|
| Translation | 40S ribosomal protein SA-like 60S ribosomal protein L28-1 Eukaryotic translation initiation factor 5A-1 Eukaryotic initiation factor 4A-2-like |
40S ribosomal protein SA-like AT-rich interactive domain containing protein 5-like Asparagine—tRNA ligase Elongation factor 1-alpha-like Eukaryotic translation initiation factor 5A-3 60S ribosomal protein L28-1 |
| Ubiquitination | Ubiquitin-conjugating enzyme E2 variant 1B-like Ubiquitin-conjugating enzyme E2 2 WD repeat-containing protein 61-like DEAD-box ATP-dependent RNA helicase-42 Small nuclear ribonucleoprotein G ADP-ribosylation factor GTPase-activating protein (AGD11) |
3-dehydroquinate synthase-like RING-H2 finger protein ATL7-like Adagio protein 1-like Putative syntaxin-24-like E3 ubiquitin-protein ligase UPL7-like U-box domain-containing protein17-like Aspartic proteinase Asp1-like |
| To support defense production | Nitronatemonooxygenase Chlorophyll a–b binding protein 8 Beta-glucosidase 08 Glutamate–glyoxylate aminotransferase 2 Ribulosebisphosphate carboxylase small chain 2A Ribulosebisphosphate carboxylase small chain 1 Ribulosebisphosphate carboxylase small chain 2C Ribulosebisphosphate carboxylase small chain 3B Pyridoxal biosynthesis protein PDX1 Cysteine synthase |
Triose phosphate/phosphate translocator Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Hydroxypyruvatereductase (HPR) Early nodulin-like protein 2-like (LOC101251653) D-3-phosphoglycerate dehydrogenase 2-oxoisovalerate dehydrogenase subunit beta GDP-mannose-dependent alphamannosyltransferase |
| Plant defense | LOC101268660 (uncharacteristic) catalase 2 (CAT2 gene) CONSTANS interacting protein 4 (CIP4) 3-oxoacyl-[acyl-carrier-protein] synthase I Methyltransferase S-adenosyl-L-homocysteine hydrolase (SAHH) 14-3-3 family protein Glycine-rich protein |
ABC transporter F family member 1-like Metallothionein-like protein Metallothionein II-like protein (MTA) 14-3-3 family protein 18.5 kDa class I heat shock protein-like Late blight resistance protein homolog R1A-10-like S-adenosyl-L-homocysteine hydrolase (SAHH) Histone deacetylase HDT1-like LRR receptor-like serine/threonine-protein kinase Flavonoid biosynthesis oxidoreductase Carotenoid cleavage dioxygenase 1-2 (CCD1-2) Pathogenesis-related leaf protein 6 (PR1B1) Oxygen-evolving enhancer protein1 Importin subunit alpha-1a-like |
| Others | TYLCV coat protein Phytosulfokines 3-like |
Within the translation group, all four proteins were ribosomal proteins and eukaryotic translation factors in healthy tomatoes, whereas two additional proteins, AT-rich interactive domain containing protein 5 and asparagine-tRNA ligase, were observed in TYLCV-infected tomatoes. Two proteins, 40S ribosomal protein SA and 60S ribosomal protein L28-1, were identified both in healthy and TYLCV-infected tomatoes. Six and seven proteins related to the ubiquitination group were identified in healthy and TYLCV-infected tomatoes, respectively. Regarding the support of defense production, most proteins were ribulose bisphosphate carboxylase small chains in healthy tomatoes, whereas many in TYLCV-infected tomatoes were related to dehydrogenase function (Table 5).
Notably, a 2-fold greater number of proteins related to plant defense were observed in TYLCV-infected tomatoes than in healthy tomatoes. In addition, some in TYLCV-infected tomatoes are well-known regulators of plant defense, including late blight resistance protein homolog R1A-10-like, leucine-rich repeat receptor-like serine/threonine-protein kinase, and pathogenesis-related leaf protein 6. Two plant defense-related proteins, S-adenosyl-L-homocysteine hydrolase (SAHH) and 14-3-3 family protein, were found to be C4-interacting proteins both in healthy and TYLCV-infected tomatoes.
Within the others group, TYLCV coat protein and phytosulfokines 3-like, plant peptide hormones belonging to the growth factor family were identified only in TYLCV-infected tomatoes. Interactions of TYLCV C4 with TYLCV coat protein and phytosulfokines 3-like suggest that TYLCV C4 is involved in other unknown functions such as the proliferation of tomato cells.
Discussion
We conducted the Y2H screening using cDNA libraries prepared from healthy and TYLCV-infected tomatoes, and identified C4-interacting proteins in two different tomato leaves. These C4-interacting proteins were divided into five major groups based on GO analyses, as shown in Table 5. Although the identified C4-interacting proteins between healthy and TYLCV-infected tomato leaves were different, except for four proteins such as two ribosomal proteins, SAHH, and 14-3-3 family protein, these proteins mostly belonged to the same GO categories (Fig. 3). C4-interacting partners related to plant defense systems were much more frequently observed in the cDNA libraries of infected tomato leaves than in those of healthy tomato leaves. This suggests that host regulators involved in the infection mechanisms of TYLCV or defense mechanism against TYLCV are more prevalent in TYLCV-infected plants than in healthy plants because the cellular mechanisms between healthy and infected plants differ during virus infection. Among these putative C4-interacting partners were the plant defense proteins SAHH and 14-3-3 family protein, which were identified both Y2H screenings. These two putative C4 interaction partners can be considered key factors related to the defense mechanism of plants against infection with TYLCV.
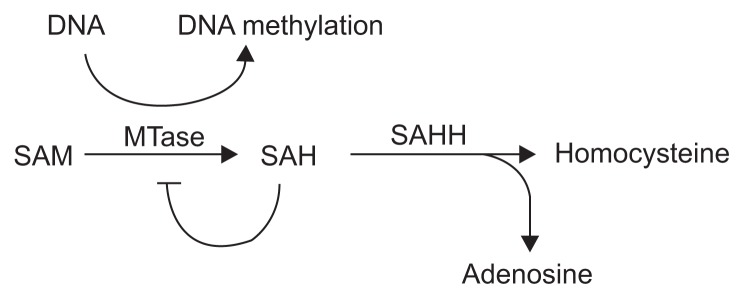
According to previous studies (Li et al., 2015; Pereira et al., 2007), SAHH is a key enzyme that regulates the methyl cycle. This methyl cycle begins with S-adenosyl-methionine (SAM), which donates a methyl group. S-Adenosylhomocysteine (SAH) is produced from SAM via methyl group transfer. Since SAH is a powerful inhibitor of SAM-dependent methyltransferase, SAH should be quickly hydrolyzed by SAHH. Thus, SAM-dependent methyltransferase relies on SAHH for its function in the methyl cycle (Fig. 4; Cañizares et al., 2013). For example, knock-down of SAHH in tobacco plants, which mediates the methylation of the 5′ end of some viral RNAs and is a prerequisite for the replication of such viruses, enhanced plant resistance to viruses such as Potato virus X, Cucumber mosaic virus, and Tobacco mosaic virus (Both et al., 1975; Choi et al., 2011). In contrast, the βC1 protein of betasatellite of Tomato yellow leaf curl China virus (TYCCNB) inhibits SAHH activity and suppresses methylation-mediated transcriptional gene silencing (Yang et al., 2011). Hence, TYLCV C4 can inhibit either the methyl cycle, which mediates transcriptional gene silencing in plants, or replication of viral RNAs to induce successful infection (Fig. 4).
Fig. 4.
Diagram of methylation cycle. DNA methylation is regulated by S-adenosyl-L-homocysteine hydrolase (SAHH) hydrolyzing S-adenosylhomocysteine (SAH), a strong inhibitor of MTase. SAM, S-adenosylmethionine.
We expect that another responsive regulator against TYLCV is a 14-3-3 family protein because this well-known protein contributes to signal transduction cascades. The 14-3-3 proteins act as sensors of the status of specific phosphorylation sites with multiple functions and bind diverse signaling proteins. Their general functional mechanisms include the blocking of binding to other proteins interacting with target proteins, alter localization in a target protein, cross-link two proteins, transition of catalytic activity at a target protein, and protect a target protein from post-translational regulation (Lozano-Durán and Robatzek, 2015). Among the functions of 14-3-3 proteins related to plant defense, the HopM1 effector of Pseudomonas syringae suppresses pathogen-associated molecular patterns-triggered response oxidative stress by binding Arabidopsis 14-3-3 protein (Lozano-Durán et al., 2014; Nomura et al., 2006). The regulation of programmed cell death in tomato is associated with the 14-3-3 protein TFT7 against several pathogen virulence proteins (Oh et al., 2010). Although 14-3-3 family proteins play an important role in plant-pathogen interactions, it remains unclear how 14-3-3 family protein acts in defense responses against virus infection or how the virus escapes 14-3-3 protein-mediated defense.
In addition to the above two putative C4 interaction partners, there are two other common partners: 60S ribosomal protein L28-1 and 40S ribosomal protein SA-like. These two proteins may be involved in either translation of viral mRNA or translation of host proteins, which contributes to creating favorable conditions for TYLCV infection.
As described above, C4 showed four interacting partners in both cDNA libraries. Some C4-interacting proteins among the plant defense group in TYLCV-infected tomatoes are well-known regulators of plant defense responses. For example, leucine-rich repeat receptor-like serine/threonine-protein kinase and late blight resistance protein homolog R1A-10-like are likely involved in signaling and disease resistance (Goring and Walker, 2004). Pathogenesis-related leaf protein 6 is activated by pathogen attack (Goyal et al., 2016). The importin subunit alpha-1a-like plays a key role in plant defense signaling (Wirthmueller et al., 2013) and oxygen-evolving enhancer protein 1 is thought to be involved in the defense response to pathogens, such as reactive oxygen species. The identification of these proteins suggests that diverse immune systems occur during TYLCV infection processes.
In conclusion, we conducted Y2H screening to verify the differences of TYLCV C4-interacting proteins between healthy and infected tomato leaves. By this approach, we identified putative key regulators, including SAHH, 14-3-3 proteins, leucine-rich repeat-receptor like kinase, and PR1R1 related to responsive proteins against infection of TYLCV, and found that C4-interacting proteins from infected plants were more frequently associated with defense or disease responses than those in healthy plants. Our results regarding C4-interacting proteins agree with known TYLCV C4-mediated cellular functions, induction of plant defense response, pathogenicity, and virus-induced post transcriptional gene silencing. Our results also suggest putative host regulators in these functions.
Acknowledgments
This work was supported by a 2-year Research Grant of Pusan National University (Y.-S.S.).
Footnotes
Articles can be freely viewed online at www.ppjonline.org.
References
- Bang B, Lee J, Kim S, Park J, Nguyen TT, Seo YS. A rapid and efficient method for construction of an infectious clone of Tomato yellow leaf curl virus. Plant Pathol J. 2014;30:310–315. doi: 10.5423/PPJ.NT.03.2014.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang B, Park J, Jeon JS, Seo YS. Establishment and application of the yeast two-hybrid (Y2H)-based plant interactome for investigation of gene functions. J Plant Biol. 2013;56:367–374. doi: 10.1007/s12374-013-0389-7. [DOI] [Google Scholar]
- Bisaro DM. Silencing suppression by geminivirus proteins. Virology. 2006;344:158–168. doi: 10.1016/j.virol.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Both GW, Banerjee AK, Shatkin AJ. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975;72:1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner A, Polge C, Lentze N, Auerbach D, Schlattner U. Yeast two-hybrid, a powerful tool for systems biology. Int J Mol Sci. 2009;10:2763–2788. doi: 10.3390/ijms10062763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañizares MC, Lozano-Durán R, Canto T, Bejarano ER, Bisaro DM, Navas-Castillo J, Moriones E. Effects of the crinivirus coat protein-interacting plant protein SAHH on post-transcriptional RNA silencing and its suppression. Mol Plant-Microbe Interact. 2013;26:1004–1015. doi: 10.1094/MPMI-02-13-0037-R. [DOI] [PubMed] [Google Scholar]
- Choi J, Choi D, Lee S, Ryu CM, Hwang I. Cytokinins and plant immunity: old foes or new friends? Trends Plant Sci. 2011;16:388–394. doi: 10.1016/j.tplants.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Cusick ME, Klitgord N, Vidal M, Hill DE. Interactome: gateway into systems biology. Hum Mol Genet. 2005;14:R171–R181. doi: 10.1093/hmg/ddi335. [DOI] [PubMed] [Google Scholar]
- Czosnek H, Laterrot H. A worldwide survey of Tomato yellow leaf curl viruses. Arch Virol. 1997;142:1391–1406. doi: 10.1007/s007050050168. [DOI] [PubMed] [Google Scholar]
- Dry IB, Rigden JE, Krake LR, Mullineaux PM, Rezaian MA. Nucleotide sequence and genome organization of Tomato leaf curl geminivirus. J Gen Virol. 1993;74:147–151. doi: 10.1099/0022-1317-74-1-147. [DOI] [PubMed] [Google Scholar]
- Goring DR, Walker JC. Self-rejection: a new kinase connection. Science. 2004;303:1474–1475. doi: 10.1126/science.1095764. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Fatima T, Topuz M, Bernadec A, Sicher R, Handa AK, Mattoo AK. Pathogenesis-related protein 1b1 (PR1b1) is a major tomato fruit protein responsive to chilling temperature and upregulated in high polyamine transgenic genotypes. Front Plant Sci. 2016;7:901. doi: 10.3389/fpls.2016.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BD, Muniyappa V, Swanson MM, Roberts IM, Robinson DJ. Recognition and differentiation of seven whitefly-transmitted geminiviruses from India, and their relationships to African cassava mosaic and Thailand mung bean yellow mosaic viruses. Ann Appl Biol. 1991;118:299–308. doi: 10.1111/j.1744-7348.1991.tb05630.x. [DOI] [Google Scholar]
- Jupin I, De Kouchkovsky F, Jouanneau F, Gronenborn B. Movement of Tomato yellow leaf curl geminivirus (TYLCV): involvement of the protein encoded by ORF C4. Virology. 1994;204:82–90. doi: 10.1006/viro.1994.1512. [DOI] [PubMed] [Google Scholar]
- Kheyr-Pour A, Bendahmane M, Matzeit V, Accotto GP, Crespi S, Gronenborn B. Tomato yellow leaf curl virus from Sardinia is a whitefly-transmitted monopartite geminivirus. Nucleic Acids Res. 1991;19:6763–6769. doi: 10.1093/nar/19.24.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Song W, Kwak HR, Kim JD, Park J, Auh CK, Kim DH, Lee KY, Lee S, Choi HS. Phylogenetic analysis and inflow route of Tomato yellow leaf curl virus (TYLCV) and Bemisia tabaci in Korea. Mol Cells. 2010;30:467–476. doi: 10.1007/s10059-010-0143-7. [DOI] [PubMed] [Google Scholar]
- Li X, Huang L, Hong Y, Zhang Y, Liu S, Li D, Zhang H, Song F. Co-silencing of tomato S-adenosylhomo-cysteine hydrolase genes confers increased immunity against Pseudomonas syringae pv. tomato DC3000 and enhanced tolerance to drought stress. Front Plant Sci. 2015;6:717. doi: 10.3389/fpls.2015.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Durán R, Bourdais G, He SY, Robatzek S. The bacterial effector HopM1 suppresses PAMP-triggered oxidative burst and stomatal immunity. New Phytol. 2014;202:259–269. doi: 10.1111/nph.12651. [DOI] [PubMed] [Google Scholar]
- Lozano-Durán R, Robatzek S. 14-3-3 proteins in plant-pathogen interactions. Mol Plant-Microbe Interact. 2015;28:511–518. doi: 10.1094/MPMI-10-14-0322-CR. [DOI] [PubMed] [Google Scholar]
- Matić S, Pegoraro M, Noris E. The C2 protein of Tomato yellow leaf curl Sardinia virus acts as a pathogenicity determinant and a 16-amino acid domain is responsible for inducing a hypersensitive response in plants. Virus Res. 2016;215:12–19. doi: 10.1016/j.virusres.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Moriones E, Navas-Castillo J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000;71:123–134. doi: 10.1016/S0168-1702(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science. 2006;313:220–223. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- Oh CS, Pedley KF, Martin GB. Tomato 14-3-3 protein 7 positively regulates immunity-associated programmed cell death by enhancing protein abundance and signaling ability of MAPKKK {alpha} Plant Cell. 2010;22:260–272. doi: 10.1105/tpc.109.070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péréfarres F, Thierry M, Becker N, Lefeuvre P, Reynaud B, Delatte H, Lett JM. Biological invasions of geminiviruses: case study of TYLCV and Bemisia tabaci in Reunion Island. Viruses. 2012;4:3665–3688. doi: 10.3390/v4123665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LA, Todorova M, Cai X, Makaroff CA, Emery RJ, Moffatt BA. Methyl recycling activities are co-ordinately regulated during plant development. J Exp Bot. 2007;58:1083–1098. doi: 10.1093/jxb/erl275. [DOI] [PubMed] [Google Scholar]
- Rigden JE, Krake LR, Rezaian MA, Dry IB. ORF C4 of Tomato leaf curl geminivirus is a determinant of symptom severity. Virology. 1994;204:847–850. doi: 10.1006/viro.1994.1606. [DOI] [PubMed] [Google Scholar]
- Rochester DE, Kositratana W, Beachy RN. Systemic movement and symptom production following agroinoculation with a single DNA of Tomato yellow leaf curl geminivirus (Thailand) Virology. 1990;178:520–526. doi: 10.1016/0042-6822(90)90349-V. [DOI] [PubMed] [Google Scholar]
- Seo YS, Chern M, Bartley LE, Han M, Jung KH, Lee I, Walia H, Richter T, Xu X, Cao P, Bai W, Ramanan R, Amonpant F, Arul L, Canlas PE, Ruan R, Park CJ, Chen X, Hwang S, Jeon JS, Ronald PC. Towards establishment of a rice stress response interactome. PLoS Genet. 2011;7:e1002020. doi: 10.1371/journal.pgen.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitharani R, Chellappan P, Fauquet CM. Gemi-niviruses and RNA silencing. Trends Plant Sci. 2005;10:144–151. doi: 10.1016/j.tplants.2005.01.005. [DOI] [PubMed] [Google Scholar]
- van Wezel R, Dong X, Blake P, Stanley J, Hong Y. Differential roles of geminivirus Rep and AC4 (C4) in the induction of necrosis in Nicotiana benthamiana. Mol Plant Pathol. 2002;3:461–471. doi: 10.1046/j.1364-3703.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- Wirthmueller L, Roth C, Banfield MJ, Wiermer M. Hop-on hop-off: importin-α-guided tours to the nucleus in innate immune signaling. Front Plant Sci. 2013;4:149. doi: 10.3389/fpls.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xie Y, Raja P, Li S, Wolf JN, Shen Q, Bisaro DM, Zhou X. Suppression of methylation-mediated transcriptional gene silencing by βC1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog. 2011;7:e1002329. doi: 10.1371/journal.ppat.1002329. [DOI] [PMC free article] [PubMed] [Google Scholar]