Abstract
To study the control of postharvest decay caused by Colletotrichum gloeosporioides and Penicillium expansum, gamma irradiation alone or in combination with fumigation was evaluated to extend the shelf life of apples in South Korea. An irradiation dose of 2.0 kGy resulted in the maximum inhibition of C. gloeosporioides and P. expansum spore germination. The gamma irradiation dose required to reduce the spore germination by 90% was 0.22 and 0.35 kGy for C. gloeosporioides and P. expansum, respectively. Microscopic observations revealed that when the fungal spores were treated with gamma irradiation (4.0 kGy), conidial germination was stopped completely resulting in no germ tube formation in C. gloeosporioides. Treatment with the eco-friendly fumigant ethanedinitrile had a greater antifungal activity against C. gloeosporioides and P. expansum in comparison with the non-treated control under in vitro conditions. The in vitro antifungal effects of the gamma irradiation and fumigation treatments allowed us to further study the effects of the combined treatments to control postharvest decay on stored apples. Interestingly, when apples were treated with gamma irradiation in combined with fumigation, disease inhibition increased more at lower (< 0.4 kGy) than at higher doses of irradiation, suggesting that combined treatments reduced the necessary irradiation dose in phytosanitary irradiation processing under storage conditions.
Keywords: Colletotrichum gloeosporioides, combination, ethanedinitrile, gamma irradiation, Penicillium expansum
Apple (Malus pumila Mill.) is one of the most economically important fruit crops in South Korea and is widely cultivated in temperate regions. Korea is considered one of the major apple-producing countries in Asia (Cheon et al., 2016; Mostafavi et al., 2012). Usually, apples are harvested for a limited period; therefore, it is necessary to store the fruits for long periods until the completion of marketing. The durability of various crops, including apples, is affected by a number of factors, including postharvest diseases that cause losses due to metabolic activity during storage (Jeong et al., 2015; Kazemi et al., 2011; Omaima and Karima, 2007). When fresh fruits and vegetables are exported, phytosanitary treatment is important to preserve quality during cold storage in order to prevent from rotting (Jung et al., 2014). Postharvest pathogens cause major losses in apple production and more than 90 fungal pathogen species have been reported as causal agents in apple decay during storage (Jones and Aldwinckle, 1990; Mostafavi et al., 2011).
Colletotrichum gloeosporioides causes anthracnose in many plants and is one of the most common Colletotrichum fungal plant pathogens. It also causes anthracnose in various crops, particularly perennials, in tropical regions (Onofre and Antoniazzi, 2014; Waller, 1992). However, apple cultivars are susceptible to diseases, including C. gloeosporioides, which can cause losses after harvesting. Penicillium expansum is a postharvest pathogen that infects several host plants, including apples; it causes blue mold on stored apples (Sutton et al., 2014). This plant pathogen can be isolated from a wide range of hosts, in addition to apples (Ashizawa and Lad, 2000; Morales et al., 2007). P. expansum is one of the most aggressive and commonly reported Penicillium spp. (Pianzzola et al., 2004); it produces a mycotoxin with neurotoxic functions on apple fruits (Brause et al., 1996). Thus, it is important to control postharvest diseases to increase the shelf life of apples.
Gamma irradiation, particularly from cobalt-60, is used as an effective non-chemical treatment to sterilize agricultural commodities in order to control postharvest losses caused by various diseases (Chu et al., 2015; Hallman, 2011; Mostafavi et al., 2010). During irradiation, high-energy photons are emitted from an isotope source (e.g., cobalt-60) throughout the object (Choi and Lim, 2015; Mostafavi et al., 2011); this process damages the cells at the molecular level, and the resulting DNA changes may cause death or the inability to reproduce. In addition to the irradiation, the fumigation method was used in our present study. In general, the fumigant methyl bromide is widely used to control pests and diseases because it has a wide spectrum of activity (Fields and White, 2002). However, due to its negative impacts on human health as well as environmental risks (UNEP, 1995), the use of methyl bromide has been banned in the world by the year 2005 (Klose et al., 2006). Therefore, many researchers have focused on eco-friendly postharvest technologies to control diseases. Numerous viable alternatives, such as heat, cold, and irradiation treatments have been used to replace methyl bromide (Diehl, 2002; Hallman, 2011). Ethanedinitrile (EDN) is also known as cyanogen and generally used as an eco-friendly fumigant to disinfect the soil in the nurseries, as an alternative to methyl bromide (Mattner et al., 2006). EDN is an environmentally-safe fumigant, a colorless gas with an almond-like odor. It is a gas at room temperature and easily soluble in water. EDN’s main route of decomposition is to derivatives of oxalic acid (Hooper et al., 2003). There is no information regarding the effectiveness of gamma irradiation combined with fumigation to control postharvest diseases in apples. Therefore, the objective of this study was to investigate the efficacy of gamma irradiation treatment in combination with EDN fumigation to control postharvest decay in apples, and to determine the optimal doses of gamma irradiation to control storage-related diseases in apples.
Materials and Methods
Raw material preparation
Apple (cultivar Fuji) fruits of uniform shape and size, with firm texture and at proper maturity, were procured from the Agricultural Products Processing Center (APPC), Andong, Gyeongbuk Province, South Korea from October to November, 2014. Fruits were pre-cooled at 2°C for 24 h in a cold storage chamber. The pre-cooled fruits were manually graded in order to ensure uniformity. The fruits were surface-disinfected in 2% sodium hypochlorite for 3 min and rinsed with sterile distilled water (SDW); then they were air-dried and packed in cardboard boxes. Eighteen fruits were arranged on cardboard trays and placed in cardboard boxes. One box was used for each treatment and therefore each treatment consisted of 18 replicates.
Fungal strains, culture conditions, and spore suspension preparation
The two pathogenic fungi (C. gloeosporioides and P. expansum) used in this study were originally isolated from ‘Fuji’ apples in Korea, and isolated fungi were identified by PCR (White et al., 1990). The fungi were maintained on potato dextrose agar (PDA; Difco, Franklin Lakes, NJ, USA) at 28°C. To prepare spore suspensions, symptomatic tissues were cut from the apple fruits and were subjected to surface sterilization using 1% sodium hypochlorite (NaOCl) solution for 1 min and 70% ethanol for 30 s, and were then rinsed twice in SDW. After sterilization, the tissues were dried on sterile filter paper, transferred to PDA plates, and incubated at 25°C for 7 days. Conidia suspensions of pathogenic fungi were prepared by suspending mycelia with SDW scraped from 7-day-old cultures onto PDA plates. The resulting suspensions were filtered through a double-layered cheesecloth, and their concentrations were adjusted to 105 conidia/ml using a hemocytometer (Eckert and Brown, 1986).
Effects of gamma radiation on spore germination of C. gloeosporioides and P. expansum
For irradiation treatments, the spore suspensions were spread onto PDA plates for culturing. The plates were exposed to gamma rays at doses of 0.2–2.0 kGy using a cobalt-60 source at a dose of 0.6 kGy/h from the Korea Atomic Energy Research Institute, Jeongeup, Korea. A cobalt-60 gamma irradiator (150 TBq capacity, ACEL; MDS Nordion, Ottawa, ON, Canada) was used for irradiation. All absorbed doses were calibrated using an alanine dosimeter with a diameter of 5 mm (Bruker Instruments, Rheinstetten, Germany); an EMS 104 EPR analyzer (Bruker Instruments) was used to determine the free radical signals. The dose uniformity ratio, or the ratio of the maximal and minimal dose absorbed in the irradiated material (Dmax/Dmin), was ~1. Spore germination was recorded at 12, 24, and 48 h after incubating the plates at 25°C, and spore viability was obtained by serial dilution on PDA plates. Later, these preparations were observed with a light microscope (BX400; Olympus, Tokyo, Japan) at a magnification of 400×. The spore germination percentage was determined using a hemocytometer for the different doses of irradiation after spore suspensions incubated for 3 days at 25°C. Germ tube length was recorded using ProgRes Capture Pro v2.8.8 (JENOPTIK, Jena, Germany). Three replicates were used for each treatment. The viability of irradiated and non-irradiated spores was determined using a dilution plating method in three subsets (up to 10−4 or 10−5 dilution with SDW) for 72 h on PDA plates (Lacey et al., 1980). The survival curves were constructed by plotting the survival spores/ml against the actual irradiation dose.
In vitro antifungal activity against C. gloeosporioides and P. expansum by fumigation
The fungi C. gloeosporioides and P. expansum were cultured on PDA plates at 25°C in the dark. EDN was applied as a fumigant using a vacuum desiccator. For fumigation, the spore suspensions were cultured by spreading on PDA plates. The plates were exposed to fumigation at various doses (1.0, 1.5, and 2.0 g/m3) for 2 h at 4°C in the vacuum desiccator. After incubation at 25°C in the dark for 3 days, mycelial growth of C. gloeosporioides and P. expansum was measured as the average colony radius. The experiment was repeated three times.
Evaluation of combined gamma irradiation and fumigation to control C. gloeosporioides and P. expansum in apples under storage conditions
A 20-μl conidial suspension (2 × 104 spores ml−1) was placed on surface-sterilized apple fruits that had been wounded by piercing to a depth of 1 to 2 mm with a pin. The apple fruits were fumigated with EDN at doses of 1.0 and 1.5 g/m3 using a vacuum desiccator under commercial conditions. The treated samples and untreated controls were placed in a storage container packed in cardboard boxes. Irradiation of apple fruits was performed after fumigation. The pre-cooled fumigated fruits were irradiated using doses of 0.2 and 0.4 kGy at a minimum dose rate of 0.6 kGy/h with a PANBIT irradiator (ACEL; MDS Nordion, Ottawa, ON, Canada) and cobalt-60 as the gamma irradiation source in 2014 and 2015. After irradiation, fruits were kept at 25 ± 2°C and 4°C storage conditions for 20 days and 30 days, respectively, after which decay was estimated as a percentage. Each treatment contained 18 replicates (fruits), and the experiment was repeated at least two times.
Statistical analysis
The data were subjected to analysis of variance using SAS JMP software (SAS Institute, 1995). Differences among treatment means were assessed using the least significant difference test and significance was established at P < 0.05. All the experiments were performed at least two times. For each experiment, the data were analyzed separately, and the results of one representative experiment are presented.
Results and Discussion
Effect of gamma irradiation on conidial germination of C. gloeosporioides and P. expansum
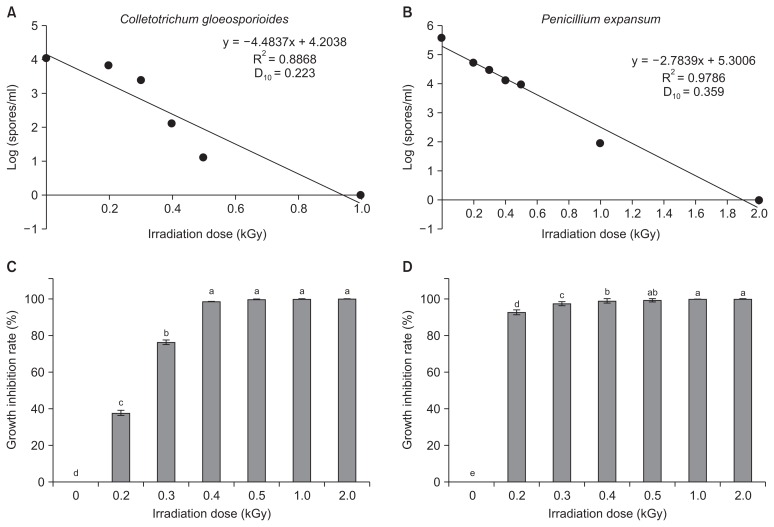
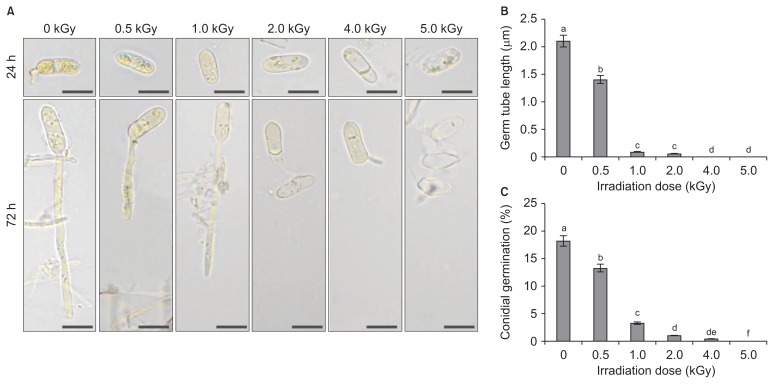
When the conidial spores of C. gloeosporioides and P. expansum were exposed to irradiation at doses ranging from 0.2 to 2.0 kGy under in vitro conditions, different degrees of impairment of conidial germination and germ tube lengths were observed, and these were confirmed by comparison with a non-treated control. In an analysis of survival with respect to irradiation dose, the gamma irradiation doses required to reduce the conidial population by 90%, the dosimetry values were 0.22 and 0.35 kGy for C. gloeosporioides (Fig. 1A) and P. expansum (Fig. 1B), respectively. Irradiation doses ranging from 0.4 to 2.0 kGy resulted in an increased percentage of conidial germination inhibition for C. gloeosporioides to a greater level compared with the non-treated control. However, the doses 0.2 kGy caused 37.6% and 92.6% inhibition for C. gloeosporioides (Fig. 1C) and P. expansum (Fig. 1D). The microscopic observations of C. gloeosporioides at a magnification of 400× showed that the germ tube length decreased gradually when spores were treated with increasing doses of radiation. An irradiation dose at 5.0 kGy caused no germ tube formation in C. gloeosporioides at 72 h after incubation at 25°C, while germ tube formation was observed at 0.5 kGy dose (Fig. 2A, B). In the spore germination analysis using a hemocytometer, the greatest percentage of spore inhibition was observed at 5.0 kGy dose, and spore germination was arrested completely at 4.0 kGy (Fig. 2C). However, a lower level of germination was observed for a dose of 1.0 kGy in comparison with the non-treated control. Doses of > 2.0 kGy are necessary to control some postharvest pathogens, but Fuji apples and Niitaka pears irradiated at doses greater than 0.8 kGy show alterations in the physiological progress of firmness (Jung et al., 2014). A previous study by Cia et al. (2007) reported that a post-harvest anthracnose disease caused by C. gloeosporioides in papaya was controlled by the combined application of ultraviolet-C and gamma irradiation. Similarly, prestorage heat treatment improves the quality of mango fruits by reducing postharvest decay caused by C. gloeosporioides following cold storage at 4°C (Kesta et al., 2000).
Fig. 1.
Effect of different doses of gamma irradiation on the conidial germination of Colletotrichum gloeosporioides and Penicillium expansum. Spores per milliliter of C. gloeosporioides (A) and P. expansum (B) and inhibition rate (%) of conidial germination of C. gloeosporioides (C) and P. expansum (D) at different doses of irradiation treatment compared with the non-treated control. Surviving viable microorganisms were plotted against irradiation dose. The experiment was repeated at least two times with three replicates per treatment. Bars with the same letters indicate no statistical difference between the treated and non-treated control, according to the least significant difference test (P < 0.05).
Fig. 2.
(A) Microscopic observations of the fungal spore germination after gamma radiation treatment on Colletotrichum gloeosporioides during 24 h and 72 h. Scale bar = 10 μm. (B) Germ tube length was drastically reduced upon the increasing of irradiation dose. (C) The percent conidial germination rate was reduced at higher doses of irradiation. The germination counting was done using a hemocytometer. The experiment was repeated three times with three replicates per treatment. Bars with the same letters indicate no statistical difference between the treated and non-treated control, according to the least significant difference test (P < 0.05).
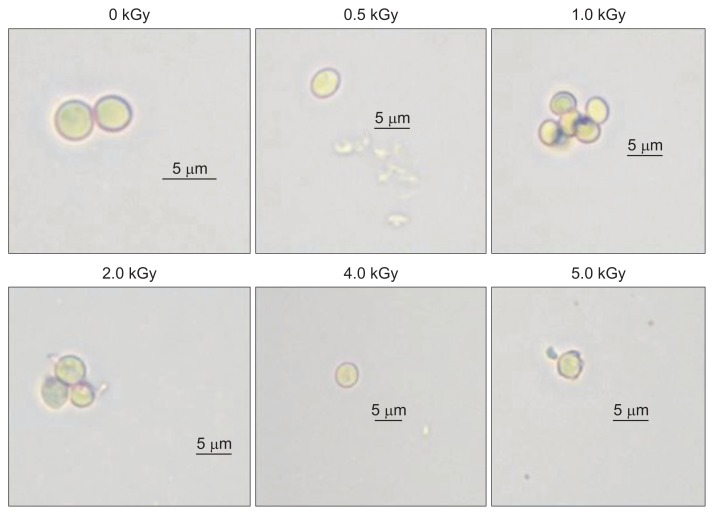
In the case of P. expansum, there was no germ tube formation or spore germination at any dose of irradiation, even 3 days after incubation (Fig. 3). Previous studies have shown that irradiation treatment inhibits mycelial growth in P. expansum at doses > 3.0 kGy (Mostafavi et al., 2011). A dose of approximately 600 Gy frequently killed P. expansum spores. Our results are consistent with those of Jitareerat et al. (2005), who showed that gamma irradiation controls spore germination and mycelial growth in P. expansum. In addition, the complete inhibition of fungal growth has been reported as a result of radiation-induced DNA damage, which results in non-functioning cells and cell wounding (Monk et al., 1995; Smith and Pillai, 2004). Gamma irradiation has been shown to successfully inactivate fungi from various agricultural commodities (De Silva et al., 2006). Interestingly, a recent study by Jeong et al. (2015) has shown that three fungal pathogens have a greater sensitivity to the e-beam treatment than to gamma or X-ray irradiations. However, a few previous studies have shown that plant pathogenic fungi differ with respect to radiosensitivity to irradiation (Saleh et al., 1988; Tiryaki, 1990). Accordingly, gamma irradiation could be an alternative method to reduce fungicide use (Cia et al., 2007).
Fig. 3.
Microscopic observations of the spore germination of Penicillium expansum at 72 h after gamma irradiation treatment in comparison with the non-treated control. There was no formation of germ tube. The spore damage occurred by the irradiation treatment at the doses > 1.0 kGy.
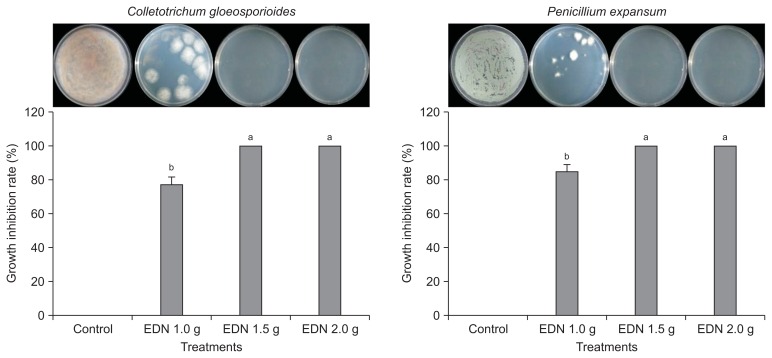
In vitro antifungal activity against C. gloeosporioides and P. expansum in response to fumigation
The degree of antifungal activity is presented as the rate of inhibition in comparison with the non-treated control. The EDN fumigation treatment at 1.5 g and 2.0 g doses caused 100% growth inhibition rate in both postharvest pathogens C. gloeosporioides and P. expansum; while fumigation treatment at doses below 1.5 g resulted in 77.5% and 85.7% inhibition of fungal growth for C. gloeosporioides and P. expansum, respectively (Fig. 4). An EDN dose > 1.5 g caused complete growth inhibition for the two pathogens. Previous reports (Sholberg and Randall, 2007; Song et al., 1996) have suggested that the vapors of hexanal, a C-6 carbon aldehyde, are effective for the control of both blue and gray molds on matured apples. Many previous studies support our results. Lee et al. (2009) reported that the volatile organic compounds (VOCs) from Oxyporus latemarginatus EF069 inhibit several pathogenic fungi, including C. gloeosporioides. The purified VOC 5-pentyl-2-furaldehyde inhibits the mycelial growth of Rhizoctonia solani in a dose-dependent manner. The application of higher concentrations of chemicals to control anthracnose disease increases the risk of high levels of toxic residues in papaya (Hernandez-Albiter et al., 2007). The mycelial growth of C. gloeosporioides and C. acutatum was inhibited by VOCs from yeast, and the exposure of pathogenic fungi to VOCs induces an increase in peroxidation levels, indicating the occurrence of oxidative stress (Rezende et al., 2015). A previous report revealed that the volatile oil of Illicium verum strongly inhibits the mycelial growth of the postharvest pathogens Botrytis cinerea and C. gloeosporioides by > 90% as a natural fumigant (Lee et al., 2007), suggesting that natural eco-friendly fumigants are highly effective for the suppression of fungal growth.
Fig. 4.
Antifungal activity of an eco-friendly fumigant ethanedinitrile (EDN) against Colletotrichum gloeosporioides and Penicillium expansum in comparison with the non-treated control under in vitro conditions. The experiment was repeated at least once with three replicates per treatment. Bars with the same letters indicate no statistical difference between the treated and non-treated control, according to the least significant difference test (P < 0.05). The photographx were taken 7 days after incubation at 25°C.
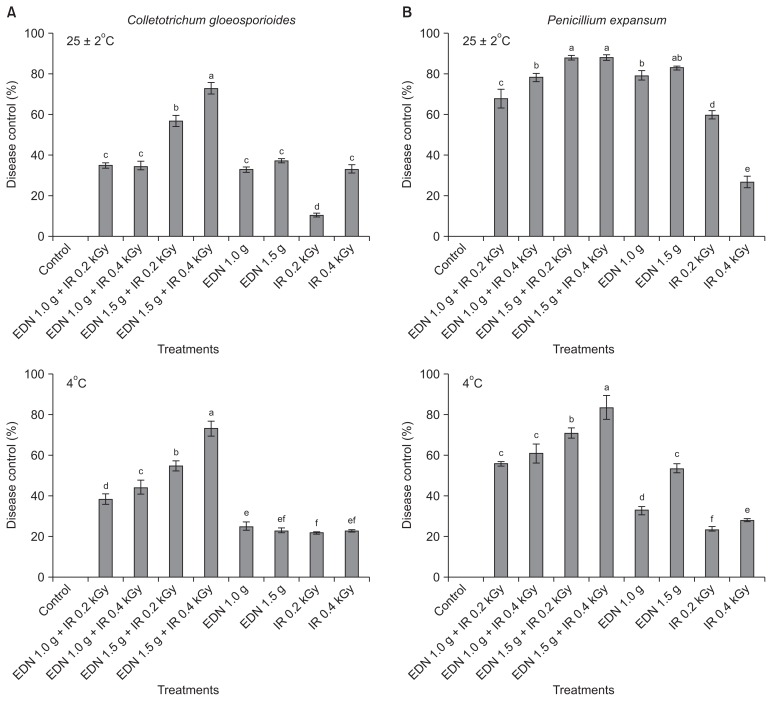
Effect of combined gamma irradiation and fumigation to control postharvest diseases caused by C. gloeosporioides and P. expansum in apples under storage conditions
The combined treatment of gamma irradiation and EDN resulted in greater control of both diseases under both ambient (25 ± 2°C) and cold (4°C) temperatures as compared with either treatment alone. The degree of disease reduction was assessed as the inhibition percentage. The combined treatment with 1.5 g EDN and 0.4 kGy of irradiation caused 72.8% inhibition of C. gloeosporioides, which was the highest disease control rate among all the treatments with respect to apple anthracnose (Fig. 5A). There was no difference in the percentage of disease control of C. gloeosporioides between temperature conditions of 25 ± 2°C and 4°C for a storage period of 20 days and 30 days, respectively. The maximum inhibition of apple anthracnose caused by C. gloeosporioides was observed for the combined treatment with EDN (1.5 g) + irradiation (0.4 kGy), followed by the combined treatment with EDN (1.5 g) + irradiation (0.2 kGy) under both temperature conditions. Our results are also consistent with those of Jung et al. (2014), who also described the significance of the combined treatment with irradiation.
Fig. 5.
Evaluation of combined application of irradiation with fumigation at various doses in control of anthracnose and blue mold caused by Colletotrichum gloeosporioides (A) and Penicillium expansum (B), respectively, at two different storage temperatures (25 ± 2°C and 4°C) on postharvest apple fruits. The experiment was repeated at least once with 18 replicates (fruits) per treatment. Bars with the same letters indicate no statistical difference between the treated and non-treated control, according to the least significant difference test (P < 0.05). EDN, ethanedinitrile; IR, irradiation.
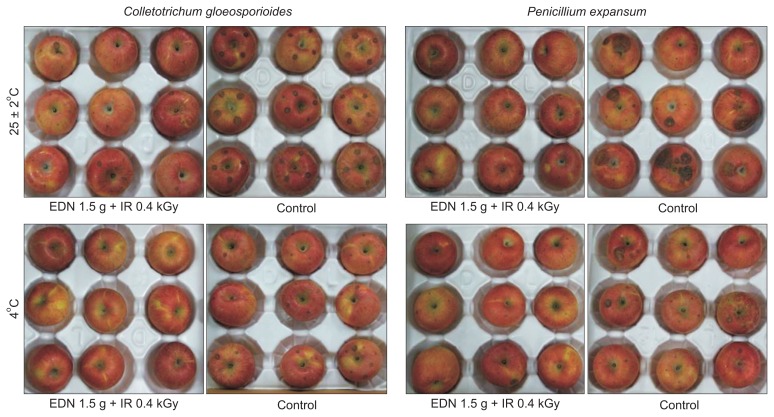
In the case of blue mold disease control caused by P. expansum, the maximum levels of disease control were 88.4% and 87.7% for the combined treatments of EDN (1.5 g) + irradiation (0.4 kGy) and EDN (1.5 g) + irradiation (0.2 kGy), respectively, for a storage duration of 20 days at an ambient temperature (25 ± 2°C). However, interestingly, the fumigant treatment of 1.5 g of EDN alone was effective for disease control and the effects were nearly equal to those observed in the combined treatment of EDN (2.0 g) + irradiation (0.4 kGy) (Fig. 5B). The combined treatment EDN (1.5 g) + irradiation (0.4 kGy) showed the maximum disease control of 83.4% for the storage period of 30 days at 4°C, and the next highest disease control was 70.5% for the combined treatment of EDN (1.5 g) + irradiation (0.2 kGy). Our results suggest that gamma irradiation can contribute to a reduction in postharvest diseases by reducing the use of fungicides. Fig. 6 shows disease control during postharvest decay by C. gloeosporioides and P. expansum for the combined treatment of EDN (1.5 g) + irradiation (0.4 kGy) in comparison with the untreated control under two different temperature conditions 25 ± 2°C and 4°C for 20 days and 30 days, respectively. Among the development of ionizing radiation technologies, gamma ray irradiation has been widely investigated for various agricultural commodities to protect against postharvest fungal pathogens, including C. gloeosporioides (Cia et al., 2007) and P. expansum (Mostafavi et al., 2011). These studies generally show postharvest disease control at low doses than at high doses, suggesting that gamma irradiation at < 0.75 kGy inhibits conidial germination when combined with fumigation (Cia et al., 2007). These results are in agreement with our results. In conclusion, low doses of gamma irradiation produced a stimulatory effect on disease control in combination with fumigation. The lethal doses recorded were 0.2 kGy and 0.4 kGy for radiosensitive species, and these results were attributed the high suppression of disease development when the treatments were combined with fumigation.
Fig. 6.
Disease control of postharvest pathogens, anthracnose and blue mold caused by Colletotrichum gloeosporioides and Penicillium expansum, respectively, in stored apples after the combined treatment of fumigation and irradiation in comparison with the non-treated control. The photographs were taken 20 days and 30 days after storage at 25 ± 2°C and 4°C, respectively. EDN, ethanedinitrile; IR, irradiation.
Acknowledgments
This work was supported by the Export Promotion Technology Development Program, Minstry of Agriculture, Food and Rural Affairs. Additionally, we thank the Korea Atomic Energy Research Institute for helping us to perform the gamma irradiation experiment.
Footnotes
Articles can be freely viewed online at www.ppjonline.org.
References
- Ashizawa EC, Lad PJ. Fungistatic composition and a fungistatic method utilizing the composition. EP19950107836 E.U. Patent. 2000
- Brause AR, Trucksess MW, Thomas FS, Page SW. Determination of patulin in apple juice by liquid chromatography: collaborative study. J AOAC Int. 1996;79:451–455. [PubMed] [Google Scholar]
- Cheon W, Lee SG, Jeon Y. First report on fruit spots caused by Colletotrichum gloeosporioides in apple (Malus pumila Mill.) in Korea. Plant Dis. 2016;100:210. doi: 10.1094/PDIS-11-14-1165-PDN. [DOI] [Google Scholar]
- Choi JI, Lim SY. Inactivation of fungal contaminants on Korean traditional cashbox by gamma irradiation. Radiat Phys Chem. 2015;118:70–74. doi: 10.1016/j.radphyschem.2015.05.009. [DOI] [Google Scholar]
- Chu EH, Shin EJ, Park HJ, Jeong RD. Effect of gamma irradiation and its convergent treatment for control of postharvest Botrytis cinerea of cut roses. Radiat Phys Chem. 2015;115:22–29. doi: 10.1016/j.radphyschem.2015.05.042. [DOI] [Google Scholar]
- Cia P, Pascholati SF, Benato EA, Camili EC, Santos CA. Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biol Technol. 2007;43:366–373. doi: 10.1016/j.postharvbio.2006.10.004. [DOI] [Google Scholar]
- De Silva M, Moraes AML, Nishikwa MM, Gatti MJA, Vallim da Alencar MA, Brandão LE, Nóbrega A. Inactivation of fungi from deteriorated paper materials by radiation. Int Biodeter Biodegr. 2006;57:163–167. doi: 10.1016/j.ibiod.2006.02.003. [DOI] [Google Scholar]
- Diehl JF. Food irradiation: past, present and future. Radiat Phys Chem. 2002;63:211–215. doi: 10.1016/S0969-806X(01)00622-3. [DOI] [Google Scholar]
- Eckert JW, Brown GE. Evaluation of postharvest treatments for citrus fruits. In: Hickey KD, editor. Methods for evaluating pesticides for control of plant pathogens. American Phytopathological Society; St. Paul, MN, USA: 1986. pp. 92–97. [Google Scholar]
- Fields PG, White ND. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Annu Rev Entomol. 2002;47:331–359. doi: 10.1146/annurev.ento.47.091201.145217. [DOI] [PubMed] [Google Scholar]
- Hallman GJ. Phytosanitary applications of irradiation. Compr Rev Food Sci Food Saf. 2011;10:143–151. doi: 10.1111/j.1541-4337.2010.00144.x. [DOI] [Google Scholar]
- Hernandez-Albiter RC, Barrera-Necha LL, Bautista-Banos S, Bravo-Luna L. Antifungal potential of crude plant extracts on conidial germination of two isolates of Colletotrichum gloeosporioides (Penz.) Penz. and Sacc. Mexican J Phytopathol. 2007;25:180–185. [Google Scholar]
- Hooper JL, Desmarchelier JM, Ren Y, Allen SE. Toxicity of cyanogen to insects of stored grain. Pest Manag Sci. 2003;59:353–357. doi: 10.1002/ps.648. [DOI] [PubMed] [Google Scholar]
- Jeong RD, Shin EJ, Chu EH, Park HJ. Effects of ionizing radiation on postharvest fungal pathogens. Plant Pathol J. 2015;31:176–180. doi: 10.5423/PPJ.NT.03.2015.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitareerat P, Kriratikron W, Phochanachai S, Uthiratanakij A. Effects of gamma irradiation on fungal growths and their pathogenesis on banana cv. ‘Kluai Kai’. International Symposium “New Frontier of Irradiated Food and Non-Food Products”; September 22–23, 2005; Bangkok, Thailand: King Mongkut’s University of Technology Thonburi (KMUTT); 2005. [Google Scholar]
- Jones AL, Aldwinckle HS. Compendium of apple and pear diseases. American Phytopathological Society Press; St. Paul, MN, USA: 1990. [Google Scholar]
- Jung K, Yoon MC, Park HJ, Lee KY, Jeong RD, Song BS, Lee JW. Application of combined treatment for control of Botrytis cinerea in phytosanitary irradiation processing. Radiat Phys Chem. 2014;99:12–17. doi: 10.1016/j.radphyschem.2014.01.025. [DOI] [Google Scholar]
- Kazemi M, Aran M, Zamani S. Effect of salicylic acid treatments on quality characteristics of apple fruits during storage. Am J Plant Physiol. 2011;6:113–119. doi: 10.3923/ajpp.2011.113.119. [DOI] [Google Scholar]
- Kesta S, Chidtragool S, Lurie S. Preharvest heat treatment and poststorage quality of mango fruit. Hort-Science. 2000;35:247–249. [Google Scholar]
- Klose S, Acosta-Martınez V, Ajwa HA. Microbial community composition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides. Soil Biol Biochem. 2006;38:1243–1254. doi: 10.1016/j.soilbio.2005.09.025. [DOI] [Google Scholar]
- Lacey J, Hill ST, Edwards MA. Microorganism in stored grains: their enumeration and significance. Trop Stored Prod Inf. 1980;39:19–33. [Google Scholar]
- Lee SO, Kim HY, Choi GJ, Lee HB, Jang KS, Choi YH, Kim JC. Mycofumigation with Oxyporus latemarginatus EF069 for control of postharvest apple decay and Rhizoctonia root rot on moth orchid. J Appl Microbiol. 2009;106:1213–1219. doi: 10.1111/j.1365-2672.2008.04087.x. [DOI] [PubMed] [Google Scholar]
- Lee SO, Park IK, Choi GJ, Lim HK, Jang KS, Cho KY, Shin SC, Kim JC. Fumigant activity of essential oils and components of Illicium verum and Schizonepeta tenuifolia against Botrytis cinerea and Colletotrichum gloeosporioides. J Microbiol Biotechnol. 2007;17:1568–1572. [PubMed] [Google Scholar]
- Mattner SW, Gounder RK, Mann RC, Porter IJ, Mattiessen JN, Ren YL, Sarwar M. Ethanedinitrile (C2N2): a novel soil fumigant for strawberry production. Acta Hortic. 2006;708:197–203. doi: 10.17660/ActaHortic.2006.708.32. [DOI] [Google Scholar]
- Monk JD, Beuchat LR, Doyle MP. Irradiation inactivation of food-borne microorganisms. J Food Protec. 1995;2:128–222. doi: 10.4315/0362-028X-58.2.197. [DOI] [PubMed] [Google Scholar]
- Morales H, Marín S, Rovira A, Ramos AJ, Sanchis V. Patulin accumulation in apples by Penicillium expansum during postharvest stages. Lett Appl Microbiol. 2007;44:30–35. doi: 10.1111/j.1472-765X.2006.02035.x. [DOI] [PubMed] [Google Scholar]
- Mostafavi HA, Fathollahi H, Motamedi F, Mirmajlessi SM. Food irradiation: applications, public acceptance and global trade. Afr J Biotechnol. 2010;9:2826–2833. [Google Scholar]
- Mostafavi HA, Mirmajlessi SM, Fathollahi H, Minassyan V, Mirjalili SM. Evaluation of gamma irradiation effect and Pseudomonas flourescens against Penicillium expansum. Afr J Biotechnol. 2011;10:11290–11293. doi: 10.5897/AJB11.1006. [DOI] [Google Scholar]
- Mostafavi HA, Mirmajlessi SM, Mirjalili SM, Fathollahi H, Askari H. Gamma radiation effects on physicochemical parameters of apple fruit during commercial post-harvest preservation. Radiat Phys Chem. 2012;81:666–671. doi: 10.1016/j.radphyschem.2012.02.015. [DOI] [Google Scholar]
- Omaima MH, Karima HEH. Quality improvement and storability of apple cv. anna by pre-harvest applications of boric acid and calcium chloride. Res J Agric Boil Sci. 2007;3:176–183. [Google Scholar]
- Onofre SB, Antoniazzi D. Behavior of the fungus Colletotrichum gloeosporioides (Penz & Sacc.), which causes bitter rot in apples after harvesting. Adv Microbiol. 2014;4:202–206. doi: 10.4236/aim.2014.44026. [DOI] [Google Scholar]
- Pianzzola MJ, Moscatelli M, Vero S. Characterization of Penicillium isolates associated with blue mold on apple in Uruguay. Plant Dis. 2004;88:23–28. doi: 10.1094/PDIS.2004.88.1.23. [DOI] [PubMed] [Google Scholar]
- Rezende DC, Fialho MB, Brand SC, Blumer S, Pascholati SF. Antimicrobial activity of volatile organic compounds and their effect on lipid peroxidation and electrolyte loss in Colletotrichum gloeosporioides and Colletotrichum acutatum mycelia. Afr J Microbiol Res. 2015;2:1527–1535. [Google Scholar]
- Saleh YG, Mayo MS, Ahearn DG. Resistance of some common fungi to gamma irradiation. Appl Environ Microbiol. 1988;54:2134–2135. doi: 10.1128/aem.54.8.2134-2135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. JMP statistics and graphics guide: version 3.1 of JMP. SAS Institute; Cary, NC, USA: 1995. pp. 65–95. [Google Scholar]
- Sholberg PL, Randall P. Fumigation of stored pome fruit with hexanal reduces blue and gray mold decay. Hort-Science. 2007;42:611–616. [Google Scholar]
- Smith JS, Pillai S. Irradiation and food safety. Food Technol. 2004;58:48–55. [Google Scholar]
- Song J, Leepipattanawit R, Deng W, Beaudry RM. Hexanal vapor is a natural, metabolizable fungicide: Inhibition of fungal activity and enhancement of aroma biosynthesis in apple slices. J Am Soc Hortic Sci. 1996;121:937–942. [Google Scholar]
- Sutton TB, Aldwinkle HS, Agnello AM, Walgenbach JF. Compendium of apple and pear disease and pests. 2nd ed. APS Press; St. Paul, MN, USA: 2014. pp. 20–75. [Google Scholar]
- Tiryaki O. Inhibition of Penicillium expansum, Botrytis cinerea, Rhizopus stolonifer and Alternaria tenuissima, which were isolated from Ankara pears by gamma irradiation. J Turk Phytopathol. 1990;19:133–140. [Google Scholar]
- [UNEP] United Nations Environment Programme. 1994 Report of the Methyl Bromide Technical Options Committee, Nairobi, Kenya. 1995. Montreal protocol on substances that deplete the ozone layer. [Google Scholar]
- Waller JM. Colletotrichum diseases of perennial and other cash crops. In: Bailey JA, Jeger MJ, editors. Colletotrichum: biology, pathology, and control. CAB International; Wallingford, CT, USA: 1992. pp. 167–185. [Google Scholar]
- White TM, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–321. [Google Scholar]