Summary
Early relapsed or refractory follicular lymphoma (FL) warrants consolidation with transplantation, though graft source modality remains controversial. We analysed the outcomes of 44 patients transplanted with either autologous or allogeneic graft sources in the post-rituximab era. No difference in event-free (EFS) or overall survival (OS) was observed between allogeneic (81% and 81%) and autologous transplantation (64% and 70%) at 3 years. There was a significant difference in EFS between allogeneic and autologous transplantation patients with previous remission duration of ≤12 months (80% and 42% at 3 years, p<0.015). Very early relapsed FL may warrant consideration of allogeneic over autologous transplantation in the appropriate setting.
Keywords: follicular lymphoma, stem cell transplantation, non-Hodgkin lymphoma
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma, characterized by high overall response rates and prolonged remission duration(s) following initial therapy with a median overall survival (OS) exceeding 15 years. While the majority of patients experience initial remission duration to immunochemotherapy well in excess of 3 years, ~15–20% of patients will have an aggressive clinical course noted by shortened survival secondary to refractory disease or very short remission durations.(Hiddemann et al, 2005) A recent report from the LymphoCare database demonstrates a dramatically inferior OS of FL patients relapsing < 2 years post-initial induction therapy compared to those that experience remissions > 2 years post-induction with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), with a validation early progression cohort experiencing OS of only 34% at 5 years.(Casulo et al., 2015)
The relapsed/refractory (rel/ref) FL arena is rapidly evolving. Standard immunochemotherapy approaches and rapidly emerging novel non-chemotherapeutic targeted approaches are actively competing. There are few prospective randomized data to guide consolidation strategies with intent to improve OS. In the pre-rituximab era the prospective, randomized CUP (conventional Chemotherapy, Unpurged autograft, Purged autograft) trial suggested OS benefit to consolidative high-dose therapy and autologous stem cell transplantation (HDT-ASCT) in early rel/ref FL patients.(Schouten et al., 2003) Recently, data with reduced-intensity (RIC)/non-myeloablative (NMA) allogeneic haematopoietic cell transplantation (allo-HCT) as definitive therapy with curative intent in early select rel/ref FL patients (Khouri et al., 2008; Avivi et al., 2009) has stoked controversy around the appropriate consolidation modality, prompting an, unfortunately, under-accrued prospective study.(Tomblyn et al., 2011) Therefore, we conducted a retrospective analysis to determine clinical features of early rel/ref FL patients undergoing first consolidative transplantation with either HDT-ASCT or allo-HCT in second or greater remission that may prove prognostic.
Methods
Patients
All patients with rel/ref FL that proceeded to HDT-ASCT or NMA allo-SCT at the Memorial Sloan Kettering Cancer Center between 2006 and 2012 were retrospectively reviewed, with data cut-off of December 2014. Data from the NMA allo-HCT programme have been previously published.(Sauter et al., 2014a; Sauter et al., 2014b) The diagnosis of FL was confirmed in all patients based upon the World Health Organization 2008 classification by either excisional or core needle biopsy. Patients with pathological evidence of transformation to diffuse large B-cell lymphoma were excluded. Confirmation of FL at the time of rel/ref disease was at the discretion of the treating physician. Patients who had undergone prior HDT-ASCT or allo-HCT were excluded from this retrospective analysis. Chemosensitive disease was defined as a complete response (CR) or partial response (PR) to the last treatment regimen prior to consolidative transplantation by computerized tomography (CT) scans per International Working Group (IWG) criteria.(Cheson et al., 1999) Of the 39/44 that underwent pre-transplantation fluorine-18-deoxyglucose positron emission tomography, international harmonization project (IHP) criteria were independently utilized.(Cheson et al., 2007) All patients who underwent HDT-ASCT underwent BEAM (carmustine, etoposide, cytarabine, melphalan) conditioning. All patients who underwent NMA allo-HCT were conditioned with cyclophosphamide, fludarabine and 200 cGy of total-body irradiation (TBI) as previously described.(Sauter et al., 2014a)
Statistics
Events were defined as progression of FL or death from any cause. EFS and OS were estimated using the Kaplan-Meier method, with differences in EFS and OS across patient and pre-transplant characteristics accessed via the logrank test. A p-value < 0.05 was considered statistically significant.
Results and Discussion
We identified 44 patients with early rel/ref FL who had undergone HDT-ASCT (N=22) or allo-HCT (N=22), as first transplant, and exposed to at least two previous lines of therapy. All patients were previously exposed to rituximab. The allo-HCT group was exposed to a median of 3 previous lines of therapy (range: 2–5) while HDT-ASCT patients had a median of 2 previous lines (range: 2–4). The previous median remission durations prior to transplantation consolidation were 5 months (range: 0–15) and 11.5 months (range: 0–31) in the allo-HCT and HDT-ASCT groups respectively. Additional patient characteristics are offered in Table I.
Table I.
Patient Characteristics
| HDT-ASCT n=22 | Allo-HCT n=22 | |
|---|---|---|
| Median age, years (range) | 51 (34–71) | 56 (34–69) |
| Median number of previous lines of therapy (range) |
2 (2–4) | 3 (2–5) |
| Median time from diagnosis to transplant consolidation, months (range) |
35 (19–134) | 45 (19–362) |
| Chemosensitive | 21/22 (95%) | 19/22 (86%) |
| CR | 10 (all FDG-PET(−)) | 9 (6/7 FDG-PET(−)) |
| PR | 11 (5/9 FDG-PET(−)) | 10 (5/10 FDG-PET(−)) |
| Clinical Stage at Rel/Ref | ||
| 2–3 | 12 | 6 |
| 4 | 10 | 16 |
| Bone Marrow Involved at Rel/Ref | ||
| yes | 8 | 16 |
| no | 12 | 6 |
| sFLIPI | ||
| ≤ 2 | 10 | 9 |
| > 2 | 9 | 7 |
| Median remission duration prior to pre- transplant re-induction therapy, months (range) |
11.5 (0*–31) | 6 (0*–15) |
| Remission duration ≤ 12 months from pre- transplant re-induction therapy |
12/22 (55%) | 21/22 (95%) |
chemorefractory disease;
allo-HCT, allogeneic hematopoietic cell transplantation; CR, complete remission; FDG-PET, 18F-fludeoxyglucose positron emission tomography; HDT-ASCT, high-dose therapy and autologous stem cell transplantation; PR, partial remission; ref, refractory; rel, relapsed; sFLIPI, secondary follicular lymphoma international prognostic index
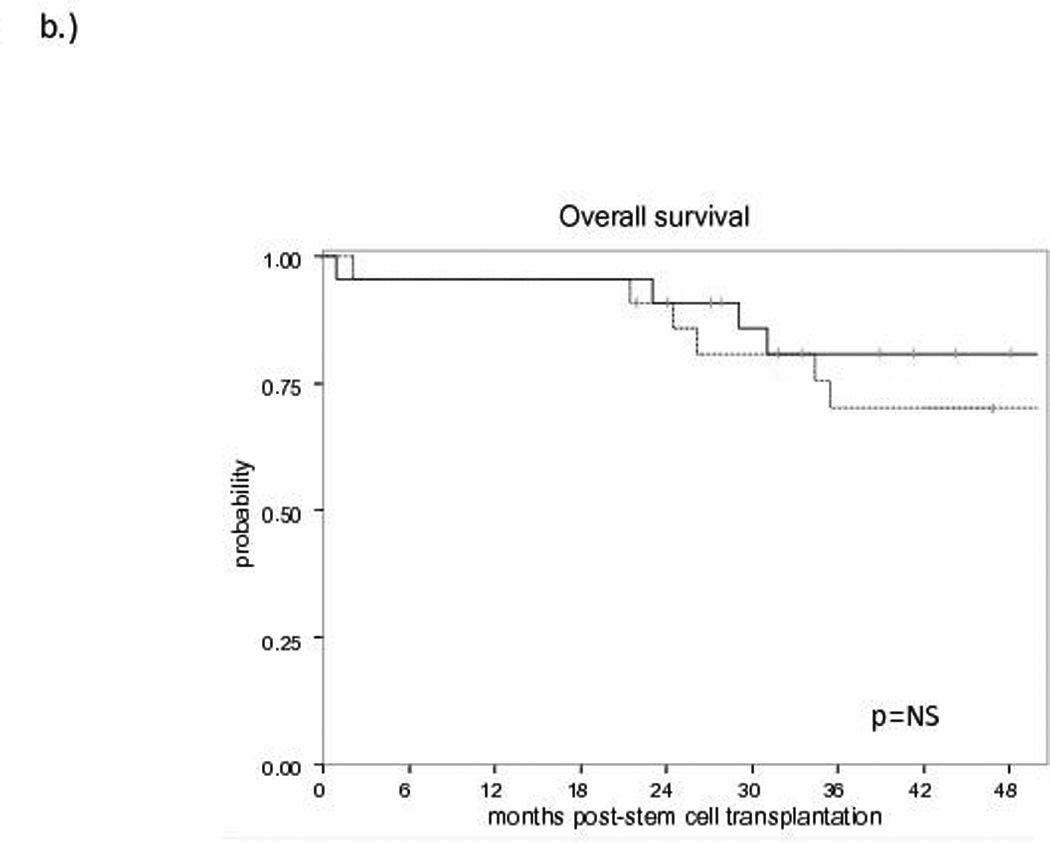
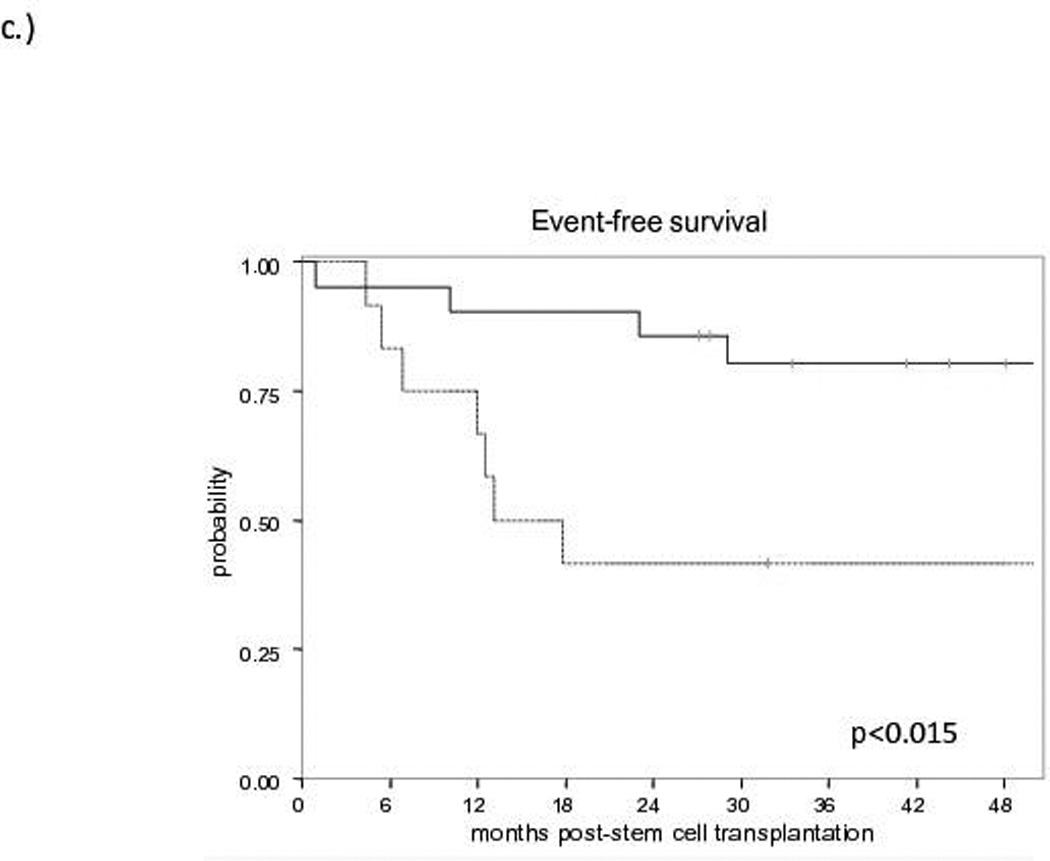
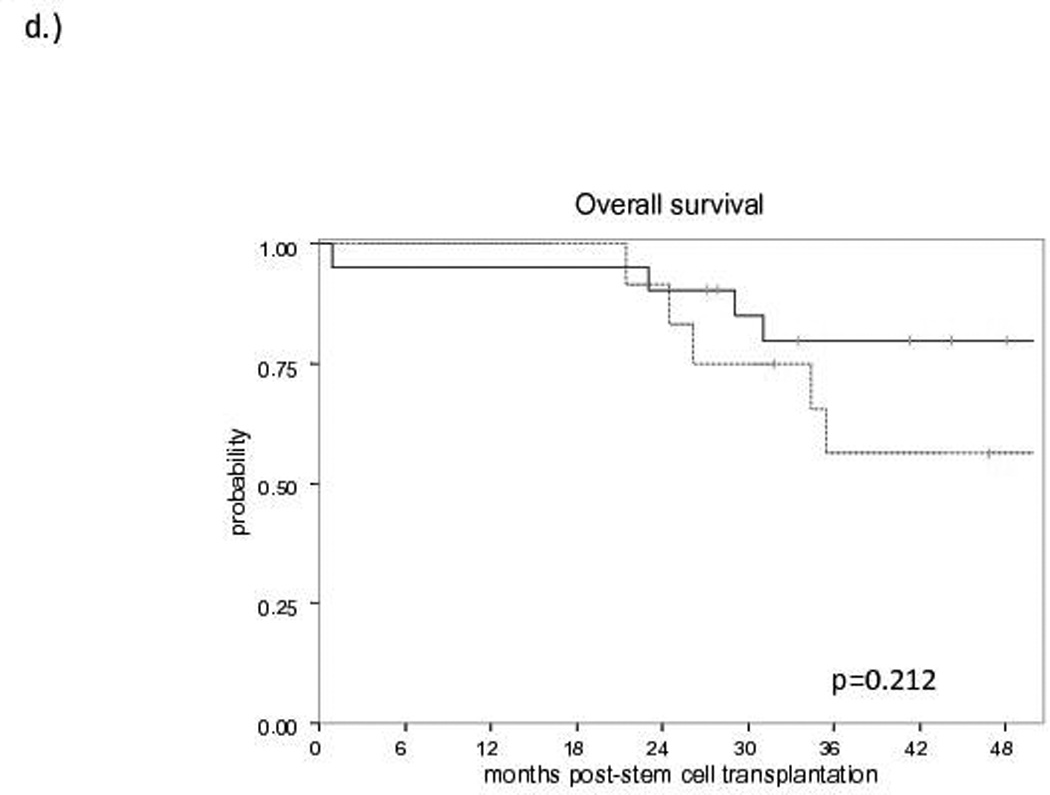
The median follow-up for survivors is 60 months (range: 21.8–96). No difference in EFS (Figure 1a) or OS (Figure 1b) was observed between allo-HCT and HDT-ASCT (OS: 81% and 70%, EFS: 81% and 64% at 3 years, respectively). At the time of analysis, no progressions or deaths have occurred in either group beyond 3 years. Of the 10 patients proceeding to HDT-ASCT with prior remission duration >12 months, one patient experienced non-relapse mortality (NRM) at 2 months post-HDT-ASCT while the remaining 9 patients continue to experience remission at a median follow-up of 5 years. Of the 22 allo-HCT patients, the 4 events included NRM secondary to graft-versus-host disease (GVHD, n=3) and one patient with progression of disease (POD) at 10 months post-allo-HCT. Furthermore, when HDT-ASCT and allo-HCT patients with a previous remission duration ≤ 12 months prior to salvage therapy were compared, the estimated 3-year EFS was 80% for allo-HCT and 42% for HDT-ASCT (p<0.015, Figure 1c). With relatively short follow-up, there was no difference in OS between allo-HCT and HDT-ASCT with prior remission duration ≤12 months, although a trend toward improved survival in the allo-HCT group was observed with relatively small cohorts (p=0.212, Figure 1d). No differences in EFS or OS were observed when analysed for pre-transplant variables including: clinical stage (2–3 vs. 4), bone marrow involvement prior to salvage therapy, complete versus partial remission or stable disease, FDG-PET response, secondary follicular lymphoma international prognostic index (sFLIPI) or number of previous lines of therapy (data not shown).
Figure 1. Survival curves for Allogeneic Transplantation (solid line) versus Autologous Transplantation (dashed line).
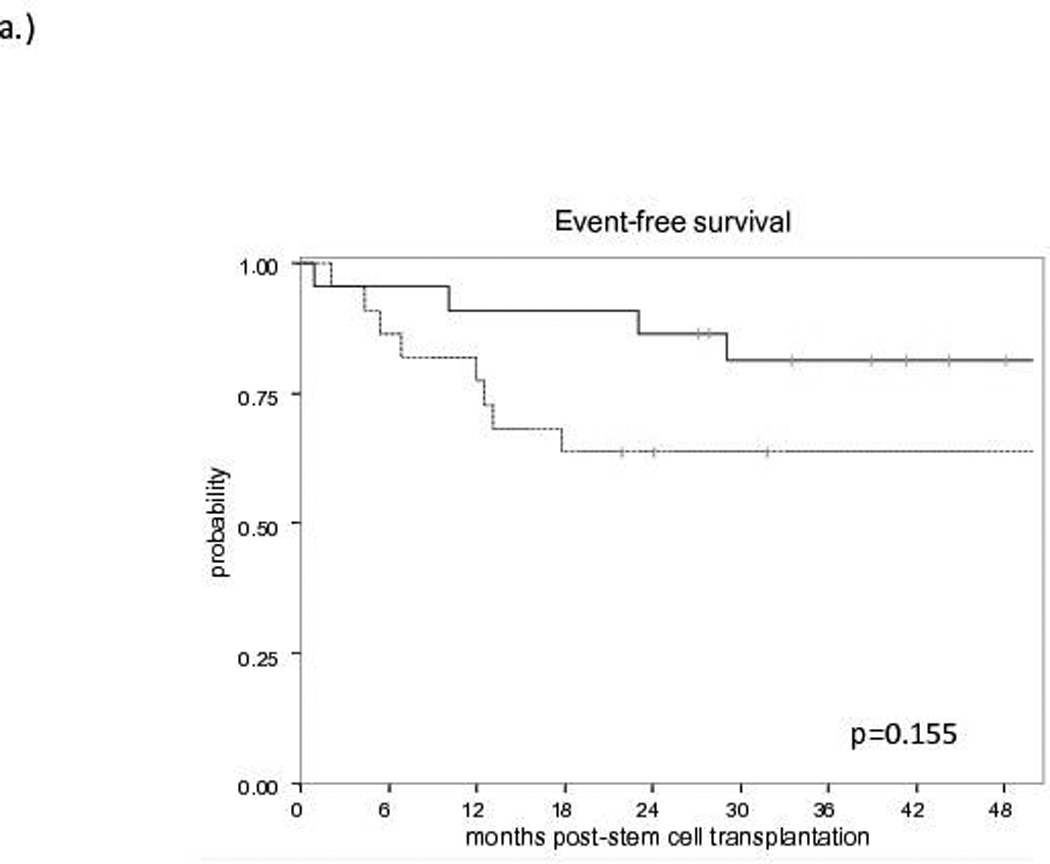
a) Event-Free Survival
b) Overall Survival
c) Event-Free Survival according to prior remission duration ≤ 12 months
d) Overall Survival according to prior remission duration ≤ 12 months
NS, not significant
We are the first to report remission duration of ≤12 months prior to re-induction chemotherapy is suggestive of inferior disease control with HDT-ASCT, with >50% patients experiencing progression of disease shortly following consolidation, the majority of these progressions being <1 year following HDT-ASCT. This could have implications toward the potential inability to proceed to subsequent salvage allo-HCT secondary to either accumulating co-morbidity following HDT-ASCT or difficulty achieving remission with further induction therapy. To this effect, Kornacker et al (2009) previously demonstrated significantly inferior OS in patients with progressive disease <1 year following HDT-ASCT, again a prognostic characteristic of the majority of patients in our series with early relapsed FL (≤ 12 months) proceeding to HDT-ASCT. Exceptional disease control in the allo-HCT cohort was demonstrated with only 1/22 patients experiencing progressive disease post-allo-HCT.
The management of early rel/ref FL remains a controversial subject. In the light of OS benefit for HDT-ASCT in consolidation compared to chemotherapy alone (Schouten et al., 2003) this has become an accepted practice in early rel/ref FL with the intent of prolonging remission duration. Most recently, RIC/NMA allo-HCT has afforded improved disease control with the potential for cure at the expense of increased risk of treatment-related morbidity and mortality, most notably secondary to complications of GVHD. There is limited data toward identifying prognostic variables informing the decision between to two transplantation modalities in consolidation. Other groups have compared outcomes of first transplantation for rel/ref FL with either allo-HCT or HDT-ASCT in contemporary retrospective studies (Reddy et al., 2012; Evens et al., 2013; Robinson et al., 2013) and one prospective clinical trial.(Tomblyn et al., 2011) A single-centre study demonstrated superiority of HDT-ASCT compared to allo-HCT with small numbers of patients and relatively short follow-up (4 years from transplantation).(Reddy et al., 2012) A National Comprehensive Cancer Network retrospective analysis determined age >60 years and 3 or more prior lines of therapy as adverse prognostic factors in the HDT-ASCT group and the allo-HCT cohort experiencing a greater risk of death in multivariate analysis of 184 patients.(Evens et al., 2013) Of the five HDT-ASCT patients with > 3 prior lines of therapy in our series, one patient died of NRM and another experienced POD at 2 months and 18 months post-HDT-ASCT, respectively. The largest report of nearly 900 patients from the European Bone Marrow Transplant demonstrated expectantly increased incidence of POD with HDT-ASCT and increased NRM with allo-HCT resulting in no significant difference in OS between the groups.(Robinson et al., 2013) Most recently, a Center for International Blood and Marrow Transplant Research study demonstrated significant decrease in the incidence of POD for patients with first remission duration of ≥ 1 year without differentiation between HDT-ASCT or allo-HCT in relation to this variable.(Klyuchnikov et al., 2015) Unfortunately, the one prospective study, the Blood and Marrow Transplant Clinical Trial Network (BMT CTN) 0202 resulted in under accrual with only 8 patients being biologically randomized, with a HLA-matched sibling donor, to the allo-HCT arm and reported with short follow-up of 36 months for survivors.(Tomblyn et al., 2011) None of the aforementioned studies analysed outcomes according to prior remission duration between the two transplantation modalities.
There are limitations to our single-centre series. Small patient numbers in the HDT-ASCT and allo-HCT cohorts’ limits determination of significant pre-transplantation variables. Additionally, longer follow-up is necessary to determine the OS impact of prior remission duration, though it should be noted that no events have occurred after 3 years. We acknowledge that patient selection at a tertiary cancer centre may have factored into the favourable outcomes of the allo-HCT patients.
Given the relatively unfavourable pre-transplant characteristics of the allo-HCT cohort in our series (older, more heavily pre-treated and shorter prior remission durations), FL appears to be exquisitely sensitive to an allogeneic effect, as previously reported by other groups, with very low incidence of progression of disease. Complications of allo-HCT, most notably morbidity and NRM relatable to GVHD, continue to limit the benefit of allo-HCT. Hopefully, novel GVHD prophylaxis regimens under prospective evaluation in a multi-centre randomized phase II study (NCT02208037, BMT CTN 1203) will result in improved outcomes related to this devastating complication.
Acknowledgments
Grant Support: P30 CA008748
The authors thank the staff of the Lymphoma and Adult BMT Services at MSKCC for their enduring dedication to patient care.
Footnotes
Authorship
M.L. and C.S. performed the research, designed the research study, analysed the data and wrote the paper. J.M. performed the research. P.H. and S.D. analysed the data. H.C.M, M.A.P, A.Z., and C.M. wrote the paper.
The authors declare no conflicts of interest.
REFERENCES
- Avivi I, Montoto S, Canals C, Maertens J, Al-Ali H, Mufti GJ, Finke J, Schattenberg A, Fanin R, Cornelissen JJ, Vernant JP, Russell N, Beguin Y, Thomson K, Verdonck LF, Kobbe G, Tilly H, Socie G, Sureda A. Matched unrelated donor stem cell transplant in 131 patients with follicular lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2009;147(5):719–728. doi: 10.1111/j.1365-2141.2009.07905.x. [DOI] [PubMed] [Google Scholar]
- Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, Hainsworth JD, Maurer MJ, Cerhan JR, Link BK, Zelenetz AD, Friedberg JW. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Evens AM, Vanderplas A, LaCasce AS, Crosby AL, Nademanee AP, Kaminski MS, Abel GA, Millenson M, Czuczman MS, Rodriguez MA, Niland J, Zelenetz AD, Gordon LI, Friedberg JW. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: a comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119(20):3662–3671. doi: 10.1002/cncr.28243. [DOI] [PubMed] [Google Scholar]
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S, Fischer T, Kropff M, Reis HE, Freund M, Wormann B, Fuchs R, Planker M, Schimke J, Eimermacher H, Trumper L, Aldaoud A, Parwaresch R, Unterhalt M. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, Medeiros LJ, Fayad L, Samaniego F, Alousi A, Anderlini P, Couriel D, de Lima M, Giralt S, Neelapu SS, Ueno NT, Samuels BI, Hagemeister F, Kwak LW, Champlin RE. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyuchnikov E, Bacher U, Kroger NM, Hari PN, Ahn KW, Carreras J, Bachanova V, Bashey A, Cohen JB, D'Souza A, Freytes CO, Gale RP, Ganguly S, Hertzberg MS, Holmberg LA, Kharfan-Dabaja MA, Klein A, Ku GH, Laport GG, Lazarus HM, Miller AM, Mussetti A, Olsson RF, Slavin S, Usmani SZ, Vij R, Wood WA, Maloney DG, Sureda AM, Smith SM, Hamadani M. Reduced-Intensity Allografting as First Transplantation Approach in Relapsed/Refractory Grades One and Two Follicular Lymphoma Provides Improved Outcomes in Long-Term Survivors. Biol Blood Marrow Transplant. 2015;21(12):2091–2099. doi: 10.1016/j.bbmt.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornacker M, Stumm J, Pott C, Dietrich S, Sussmilch S, Hensel M, Nickelsen M, Witzens-Harig M, Kneba M, Schmitz N, Ho AD, Dreger P. Characteristics of relapse after autologous stem-cell transplantation for follicular lymphoma: a long-term follow-up. Ann Oncol. 2009;20(4):722–728. doi: 10.1093/annonc/mdn691. [DOI] [PubMed] [Google Scholar]
- Reddy N, Greer JP, Goodman S, Engelhardt B, Oluwole O, Jagasia MH, Savani BN. Long-term outcome after autologous or allogeneic stem cell transplantation in patients with recurrent follicular lymphoma. Bone Marrow Transplant. 2012;47(10):1318–1320. doi: 10.1038/bmt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SP, Canals C, Luang JJ, Tilly H, Crawley C, Cahn JY, Pohlreich D, Le Gouill S, Gilleece M, Milpied N, Attal M, Biron P, Maury S, Rambaldi A, Maertens J, Capria S, Colombat P, Montoto S, Sureda A. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48(11):1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- Sauter CS, Barker JN, Lechner L, Zheng J, Devlin SM, Papadopoulos EB, Perales MA, Jakubowski AA, Goldberg JD, Koehne G, Ceberio I, Giralt S, Zelenetz AD, Moskowitz CH, Castro-Malaspina H. A Phase II Study of a Nonmyeloablative Allogeneic Stem Cell Transplant with Peritransplant Rituximab in Patients with B Cell Lymphoid Malignancies: Favorably Durable Event-Free Survival in Chemosensitive Patients. Biol Blood Marrow Transplant. 2014a Mar;20(3):354–360. doi: 10.1016/j.bbmt.2013.11.029. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter CS, Lechner L, Scordo M, Zheng J, Devlin SM, Fleming SE, Castro-Malaspina H, Moskowitz CH. Pretransplantation fluorine-18-deoxyglucose--positron emission tomography scan lacks prognostic value in chemosensitive B cell non-hodgkin lymphoma patients undergoing nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014b;20(6):881–884. doi: 10.1016/j.bbmt.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, Doorduijn JK, Sydes MR, Kvalheim G. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21(21):3918–3927. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Tomblyn MR, Ewell M, Bredeson C, Kahl BS, Goodman SA, Horowitz MM, Vose JM, Negrin RS, Laport GG. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17(7):1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


