Abstract
Background
Exposure to ethanol abuse and severe oxidative stress are risk factors for hepatocarcinoma. The aim of this study was to evaluate the effects of S-adenosylmethionine (SAMe) and its combinations with taurine and/or betaine on the level of glutathione (GSH), a powerful antioxidant in the liver, in acute hepatotoxicity induced by ethanol.
Methods
To examine the effects of SAMe and its combinations with taurine and/or betaine on ethanol-induced hepatotoxicity, AML12 cells and C57BL/6 mice were pretreated with SAMe, taurine, and/or betaine, followed by ethanol challenge. Cell viability was detected with an MTT assay. GSH concentration and mRNA levels of GSH synthetic enzymes were measured using GSH reductase and quantitative real-time reverse transcriptase-PCR. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured with commercially available kits.
Results
Pretreatment of SAMe, with or without taurine and/or betaine, attenuated decreases in GSH levels and mRNA expression of the catalytic subunit of glutamate-cysteine ligase (GCL), the rate-limiting enzyme for GSH synthesis, in ethanol-treated cells and mice. mRNA levels of the modifier subunit of GCL and glutathione synthetase were increased in mice treated with SAMe combinations. SAMe, taurine, and/or betaine pretreatment restored serum ALT and AST levels to control levels in the ethanol-treated group.
Conclusions
Combinations of SAMe with taurine and/or betaine have a hepatoprotective effect against ethanol-induced liver injury by maintaining GSH homeostasis.
Keywords: S-adenosylmethionine, Taurine, Betaine, Ethanol, Glutathione
INTRODUCTION
Alcohol abuse is a common cause of liver disease and contributes to fibrosis and cirrhosis, which can lead to liver cancer.1 Liver disease should be controlled to prevent the occurrence of cancer. Liver damage is associated with an impairment of methionine metabolism.2,3 Several studies have reported that ethanol-induced injury leads to a reduction in methionine and its metabolic products, S-adenosylmethionine (SAMe), S-adenosylhomocysteine (SAH), and glutathione (GSH).4,5 SAMe is the key metabolite for synthesis of GSH. The simple administration of methionine cannot restore these alterations induced by ethanol. It has been reported that even a high level of methionine administration could not significantly alter the hepatic SAMe level.6 Furthermore, excessive methionine supplementation may even have a toxic effect.6 Accordingly, SAMe, which is the product of a reaction catalyzed by methionine adenosyltransferase (MAT), becomes more important. Chronic or acute ethanol administration decreases the hepatic SAMe concentration in rats and mice.7–9 Interestingly, the depleted hepatic concentration of SAMe has also been demonstrated in alcoholic hepatitis patients.5 The utilization of SAMe is increased to synthesize GSH to compensate for alcohol-induced oxidative stress. Animal and human studies have suggested that SAMe treatment may ameliorate acute alcohol-induced liver injury by restoring SAMe and GSH concentrations.10
High concentrations of taurine are found in the liver. The liver is a major site for the synthesis of taurine from methionine and cysteine.11 It has been reported that patients with liver injury have a low serum concentration of taurine, cysteine, and GSH.12,13 Most of the cysteine is used for GSH synthesis and some is used for taurine and protein synthesis depending on the need of the cells.14 It could be suggested that lowering the rate of catabolism of cysteine to taurine could preserve the cysteine supply for GSH synthesis.15 Also, It has been suggested taurine supplementation is beneficial because of its antioxidative effect.16
Betaine is an oxidative metabolite of choline.17,18 Betaine participates in homocysteine remethylation. Betaine donates its methyl group to homocysteine and homocysteine is then converted to methionine by betaine-homocysteine methyltransferase (BHMT).17,18 Therefore, betaine can maintain the hepatic methionine level and regulate the intracellular SAH level.19 Homocysteine is a product of the metabolism of SAH. When hepatic SAH accumulates, the SAM:SAH ratio decreases.19,20 The SAM:SAH ratio is a major regulator of the activity of methyltransferases; therefore, a decreased SAM:SAH ratio could reduce the methylation of homocysteine.10 One of the defects caused by ethanol consumption is an inhibition of methionine synthase (MS) activity in the liver. This blocks the remethylation of homocysteine.21,22 In order to compensate for the impaired activity of MS by alcohol consumption, BHMT, the alternative enzyme, becomes more important and the use of betaine is increased. The BHMT/betaine system is thus crucial for homocysteine homeostasis.23 Betaine supplementation enhances tranS-sulfuration by increasing methionine and SAMe in liver.15 Moreover, betaine influences the metabolism of sulfur-containing amino acids (SCAA) in the liver by conserving SAMe levels and preventing the accumulation of homocysteine, which increases the risk for hepatic diseases and consequently prevents oxidative stress.18,24
Acetaldehyde and reactive oxygen species are generated during the metabolism of ethanol.25,26 It has been proposed that oxidative stress and the production of free radicals are linked to the development of alcoholic liver disease.27 Since the impaired metabolism of SCAA is often found in patients with alcoholic liver disease, the existence of antioxidants is very critical to maintain a healthy liver in the face of ethanol challenge.28 In particular, high concentrations of GSH attenuate oxidative stress.10 Therefore, one way of preventing liver damage is to conserve the hepatic GSH level. However, hepatic GSH concentrations are reduced by the inactivation of hepatic MAT and the depletion of SAMe. Low concentrations of hepatic GSH are responsible for increased susceptibility to hepatic damage. SAMe can restore the concentration of GSH.29,30 In this regard, we used SAMe as a major component in our experiments. Both taurine and betaine are essential endogenous compounds in SCAA metabolism. Administration of betaine increases the concentration of hepatic SAMe and decreases the concentration of SAH and homocysteine, thereby attenuating liver injury induced by alcohol in animal studies.17–19,24 Additionally, taurine supplementation has been reported to attenuate impaired SCAA metabolism by ethanol.31 Given the importance of the above-described players in the methionine metabolism pathways, the purpose of this study was to examine the effects of SAMe, taurine, and/or betaine on GSH homeostasis in ethanol-treated cells and mice.
MATERIALS AND METHODS
1. Materials
SAMe was obtained in the form of S-adenosyl-L-methionine-sulfate-p-toluensulfonate from Samoh Pharm (Seoul, Korea). Taurine and betaine were purchased from Sigma Chemical (St. Louis, MO, USA).
2. Cell culture
A normal mouse hepatocyte cell line, AML12, was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). AML12 cells were cultured in DMEM:F12 (1:1) (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (v/v) (Hyclone), 100 U/mL penicillin and 100 μg/mL streptomycin (Hyclone), 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium (ITS) (BD Biosciences, Franklin Lakes, NJ, USA), 2.5 mL sodium pyruvate (Mediatech, Inc., Manassas, VA, USA), 0.6 g sodium bicarbonate (Daejung Chemicals Co., Siheung, Korea), and dexamethasone (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a 5% CO2 atmospheric condition.
3. Cell treatment
AML12 cells (0.2 × 106 cells/well, passage numbers: 10–18) were seeded on 6-well plates (Corning Coastar Corp., Cambridge, MA, USA) and incubated for 6 hours. SAMe was freshly dissolved in 1.8 M Tris solution and taurine and betaine were dissolved in culture medium. Cells were pretreated with SAMe (0.5 mM), taurine (10 mM), and/or betaine (1 mM) and incubated overnight. After pretreatment, they were treated with ethanol (100 mM) for 24 hours. Experimental concentrations of SAMe, taurine, and betaine were determined in previous studies.32–35 These concentrations showed beneficial effects in correcting damages induced by ethanol32 and did not affect cell viability. However, treatment of cells with a higher concentration of SAMe (1 mM) significantly lowered cell viability to under 80% (data not shown) in our experiments. The 100 mM dose of ethanol was used by other investigators to induce oxidative damage in cells to simulate the effect of heavy drinking on an individual.34,35
4. Cell viability
Cell viability was measured using a MTT assay. AML12 cells (0.5 × 105 cells/well) were seeded in 96-well plates and incubated at 37°C in a 5% CO2 for 6 hours. Cells were pretreated with SAMe (0.5 mM), taurine (10 mM), and/or betaine (1 mM) and incubated overnight. After pretreatment, the cells were treated with ethanol (100 mM) for 24 hours. MTT reagent (10% of media; Sigma-Aldrich) was added to each well and further incubated at 37°C for 2 hours. The supernatant was gently removed and 100 μL of DMSO was added into each well to dissolve formazan. The absorbance at 560 nm was measured using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
5. Animal experiments
Five-week-old male C57BL/6 mice were purchased from Samtako (Osan, Korea). The mice were acclimated for 10 days maintained with a relative humidity of 50% ± 5% and a 12-hour light/dark cycle at 22°C ± 2°C. Chow (22GJ30060; Cargill Agri Purina, Seongnam, Korea) and deionized water were freely accessible by mice. The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Ewha Womans University (approval number 15–059).
The mice were randomly divided into eight groups (n = 5–6/group). SAMe, taurine, and betaine were freshly dissolved in PBS. The mice were administered SAMe, taurine, betaine, SAMe + taurine, SAMe + betaine, and SAMe + taurine + betaine every day for a week by intragastric gavage. SAMe (100 mg/kg body weight [BW]),36 taurine (200 mg/kg BW),37 and/or betaine (500 mg/kg BW)38 were used. Control and ethanol groups were administered PBS (0.1 mL/kg BW). BW was recorded daily throughout the experimental period. At 6 hours after the last pretreatment, the mice, except control mice, were administrated 6 g/kg BW ethanol by intragastric gavage. This dose of ethanol has been reported to cause liver damage in mice.39 Mice were first languid but conscious and recovered usual behavior within about 18 hrs of ethanol administration. After exposure to ethanol for 18 hours, the mice were anesthetized with 2% isoflurane (isoflurane; Piramal Inc., Bethlehem, PA, USA) and sacrificed humanely. Blood was collected from the vena cava, and serum was obtained by centrifugation at 1,500 ×g for 20 minutes at room temperature. Liver was collected for further experiments. The liver washed with PBS, blotted with tissue paper, and weighed.
6. Serum alanine aminotransferase and aspartate aminotransferase levels
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured using commercial kits (Asan Pharm, Hwaseong, Korea) based on Retiman-Frankel method.40
7. Glutathione concentration
GSH concentration was determined by using GSH reductase (Sigma-Aldrich). Liver was homogenized (0.1 g/mL) in PBS and cell was scrapped with 150 μL PBS. Then they were centrifuged at 10,000 ×g for 30 minutes at 4°C. The supernatant of 0.1 mL was added to 0.1 mL 0.6 M perchloric acid (Junsei Chemical, Tokyo, Japan). The rest supernatant was used to measure total protein concentration. After removal of the protein, 0.1 mL GSH standards and samples (Sigma-Aldrich) were added to 2.5 mL reaction buffer (0.15 mM NADPH [Sigma-Aldrich], 0.1 mM 5,5′-dithio-bis-(2-nitrobenzoic acid) [Sigma-Aldrich], 50 mM NaPO4 [Junsei Chemical], 1.5 mM EDTA [E5124; Sigma-Aldrich], and 0.1 mL GSH reductase [10 units/mL]). GSH content was determined by measuring change in the absorbance for 1 minute at 412 nm using a Biochrom Libra S50 spectrophotometer (Biochrom Ltd., Cambridge, UK). The concentrations of GSH were calculated and expressed as nmol/mg protein.
8. RNA isolation and quantitative real-time reverse transcriptase-PCR
Total RNA were isolated from liver tissues or cells using Trizol (Life Technologies [a retired brand of Thermo Fisher Scientific], Grand Island, NY, USA) according to the manufacturer’s instructions. The RNA concentration and 260/280 nm ratio were evaluated using a spectrophotometer (Nanodrop Lite; Thermo Fisher Scientific, Wilmington, MA, USA). The 1 μg RNA was reverse transcribed into complementary DNA (cDNA) using first strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) and cDNA was used as a template for quantitative real-time PCR (qPCR). qPCR was performed using Maxima SYBR Green qPCR Master Mixes (K0222; Thermo Fisher Scientific, Waltham, MA, USA). qPCR was conducted in duplicate with Rotor Gene Q machine (Qiagen, Hilde, Germany). Amplification was started with a template denaturation step at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The primer sequences used for this experiment are as follows: glutamate-cysteine ligase (GCL) catalytic subunit (NM_010295, GCLC): 5′-ACA CCT GGA TGA TGC CAA CGA G-3′ (forward), 5′-CCT CCA TTG GTC GGA ACT CTA C-3′ (reverse), GCL modifier subunit (NM_008129, GCLM): 5′-TCC TGC TGT GTG ATG CCA CCA G-3′ (forward), 5′-GCT TCC TGG AAA CTT GCC TCA G-3′ (reverse), GSH synthase (NM_0008180, GS): 5′-CCA GGA AGT TGC TGT GGT GTA C-3′ (forward), 5′-GCT GTA TGG CAA TGT CTG GAC AC-3′ (reverse), hypoxanthine guanine phosphoribosyl transferase (NM_013556, Hprt1): 5′-CAG GCC AGA CTT TGT TGG AT-3′ (forward), 5′-TTG CGC TCA TCT TAG GCT TT-3′ (reverse). The relative amounts of these mRNAs were calculated using comparative CT method and normalized to the amounts of hprt1. All data are expressed as a relative quantity to each control value.
9. Statistical analysis
Results are expressed as the SEM. The data were analyzed using the Statistical Analysis Systems package ver. 9.3 (SAS Institute, Cary, NY, USA). The differences among groups were analyzed by one-way ANOVA with post-hoc Duncan’s test. P-values less than 0.05 were considered statistically significant.
RESULTS
1. Effects of S-adenosylmethionine, taurine, and/or betaine on cell viability in ethanol-treated AML12 cells
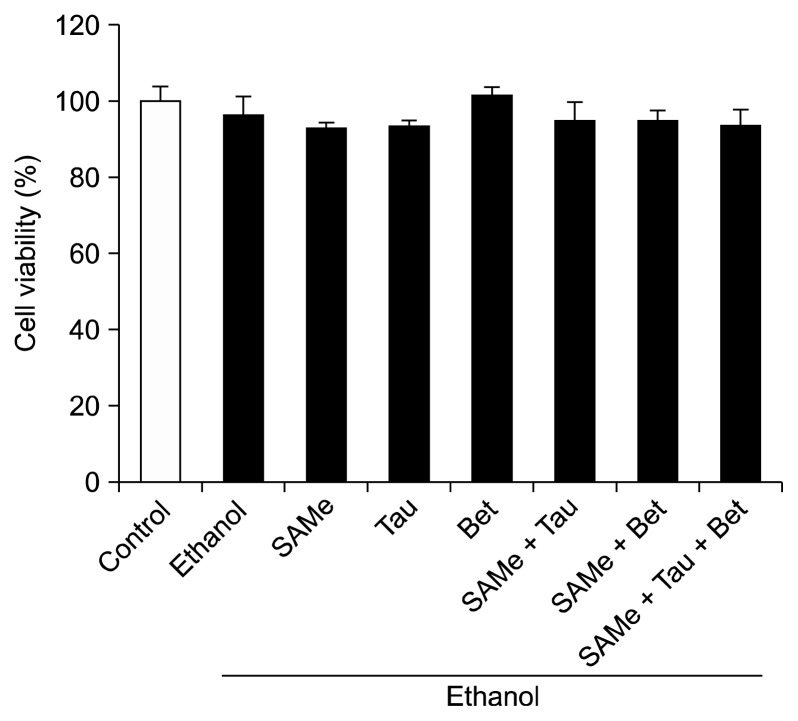
The effects of SAMe, taurine, betaine, ethanol, and their combinations on cell viability were assessed by an MTT assay (Fig. 1). Cell viability was more than 80% in all groups and there was no significant difference among groups. The results indicate that 0.5 mM of SAMe, 10 mM of taurine, and/or 1 mM of betaine are not cytotoxic to AML12 cells stimulated with 100 mM ethanol.
Figure 1.
Effects of S-adenosylmethionine, taurine, and/or betaine on the viability of ethanol-treated AML12 cells. AML12 cells were treated with SAMe (0.5 mM), Tau (10 mM) and/or Bet (1 mM). After 18 hours, the cells were treated with ethanol (100 mM) for 24 hours. The cytotoxicity of SAMe, Tau, Bet, and/or ethanol were evaluated by an MTT assay. Values represent mean ± SEM of six independent experiments. SAMe, S-adenosylmethionine; Tau, taurine; Bet, betaine.
2. Effects of S-adenosylmethionine, taurine, and/or betaine on intracellular glutathione levels and mRNA levels of glutathione-synthesizing enzymes in ethanol-treated AML12 cells
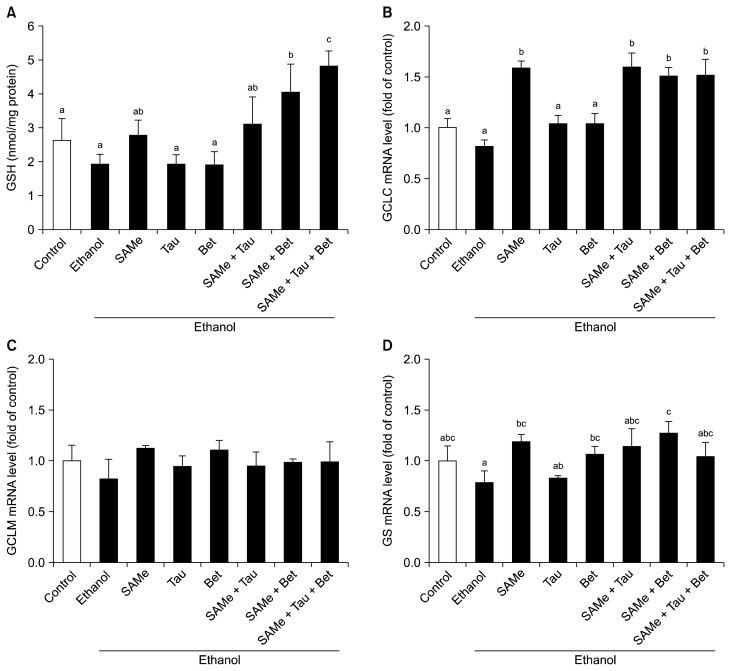
To evaluate the effect of SAMe and its combinations with taurine and/or betaine on GSH levels, intracellular GSH levels were assessed. Pretreatment with one of SAMe, taurine, and betaine had no significant effect on GSH levels (Fig. 2A). Conversely, pretreatment with a combination of SAMe and betaine in the absence or presence of taurine increased the levels of GSH in ethanol-treated AML12 cells. The mRNA levels of GCLC (Fig. 2B) and GS (Fig. 2D) were also significantly increased in AML12 cells pretreated with SAMe or its combinations as compared to the control, whereas these pretreatments had no significant effect on GCLM (Fig. 2C).
Figure 2.
Effects of S-adenosylmethionine, taurine, and/or betaine on intracellular GSH levels and mRNA levels of GSH synthesizing enzymes in ethanol-treated AML12 cells. Cells were pretreated with SAMe (0.5 mM), Tau (10 mM), and/or Bet (1 mM) for 18 hours, followed by ethanol (100 mM) treatment for 24 hours. (A) Intracellular GSH levels and mRNA levels of (B) GCLC, (C) GCLM, and (D) GS were measured using RT-qPCR. Values represent mean ± SEM of three independent experiments. SAMe, S-adenosylmethionine; Tau, taurine; Bet, betaine; GSH, glutathione; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GS, GSH synthase; RT-qPCR, quantitative real-time PCR. abcValues with different alphabets are significantly different at P < 0.05 level by Duncan’s multiple range test.
3. Effects of S-adenosylmethionine, taurine, and/or betaine on body weight, liver weight, and ratio of liver weight to body weight in ethanol-treated mice
Normally, liver weight (LW) relative to BW tends to increase when the liver is damaged or physiologically compromised. The LW to BW ratio was higher due to increased LW in ethanol-treated group compared to control group (Table 1). However, SCAA treatment did not restore the ratio to the control group level.
Table 1.
Effects of SAMe, taurine, betaine, and the combinations of three sulfur containing amino acids on body weight and liver weight in ethanol-treated mice
| Group | Control | Ethanol | Ethanol | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| SAMe | Tau | Bet | SAMe + Tau | SAMe + Bet | SAMe + Tau + Bet | |||
| BW (g) | 22.28 ± 0.68ab | 22.12 ± 1.02b | 21.84 ± 0.72b | 21.66 ± 1.01b | 21.55 ± 0.44b | 22.69 ± 0.75ab | 23.55 ± 0.47a | 21.29 ± 0.46b |
| LW (g) | 0.85 ± 0.05a | 1.03 ± 0.09b | 0.94 ± 0.07ab | 0.97 ± 0.05b | 0.99 ± 0.03b | 1.02 ± 0.05b | 1.03 ± 0.02b | 0.96 ± 0.03ab |
| LW/BW ratio | 0.38 ± 0.02a | 0.46 ± 0.03b | 0.43 ± 0.03b | 0.45 ± 0.01b | 0.46 ± 0.01b | 0.45 ± 0.02b | 0.44 ± 0.01b | 0.45 ± 0.01b |
Values are presented as mean ± SEM (n = 5). SAMe, S-adenosylmethionine; Tau, taurine; Bet, betaine; BW, body weight; LW, liver weight.
Values with different superscripted alphabets are significantly different at P < 0.05 level compared with control group.
4. Effects of S-adenosylmethionine, taurine, and/or betaine on serum alanine aminotransferase and aspartate aminotransferase levels in ethanol-treated mice
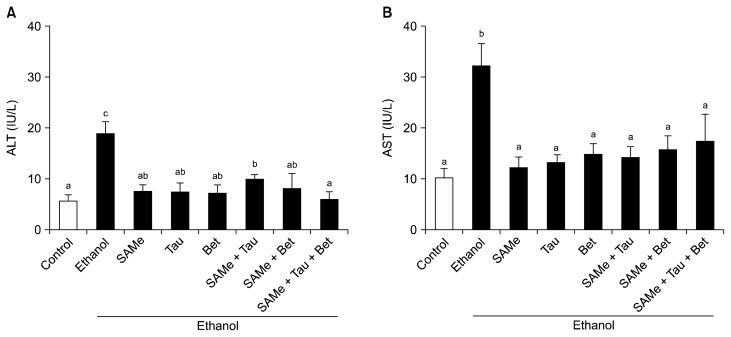
Serum ALT and AST levels were measured to determine the degree of liver injury. Ethanol-treated group showed significantly increased serum ALT (Fig. 3A) and AST (Fig. 3B) levels compared with the control group. SAMe, taurine, and/or betaine pretreatment restored serum ALT and AST levels to control levels compared with the ethanol alone-treated group.
Figure 3.
Plasma alanine aminotransferase and aspartate aminotransferase levels in ethanol-treated mice. SAMe (100 mg/kg BW), Tau (200 mg/kg BW), and/or Bet (500 mg/kg BW) were orally administered to mice every day for a week. At 6 hours after the last treatment, the mice were administrated 6 g/kg BW ethanol by intragastric gavage. Control groups were administered 0.1 mL PBS. (A) Plasma ALT and (B) AST levels were measured using commertially available kits. Values represent mean ± SEM (n = 5). ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAMe, S-adenosylmethionine; Tau, taurine; Bet, betaine; BW, body weight. abcValues with different alphabets are significantly different at P < 0.05 level by Duncan’s multiple range test.
5. Effects of S-adenosylmethionine, taurine, and/or betaine on hepatic glutathione levels and mRNA expression levels of glutathione synthetic enzymes in mice
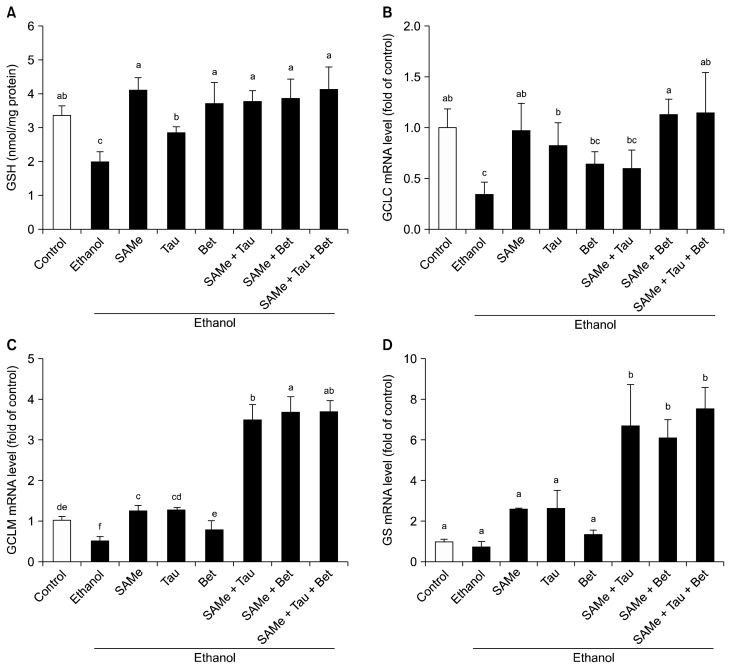
Hepatic GSH concentrations were decreased in ethanol-treated group compared to control group. Pretreatment of SAMe and SAMe combinations significantly attenuated the decrease in hepatic GSH in ethanol-treated group (Fig. 4A). The mRNA levels of the GCLC (Fig. 4B) and GCLM (Fig. 4C) were reduced 18 hours after ethanol administration. These results are in agreement with the fall in GSH and mRNA levels of GSH synthetic enzymes in our experiment. SAMe pretreatment maintained the mRNA level of GCLC in ethanol-treated group. Interestingly, GCLM (Fig. 4C) and GS mRNA levels (Fig. 4D) were dramatically increased by SAMe combinations with taurine and/or betaine in ethanol-treated group.
Figure 4.
Effects of SAMe, taurine, and/or betaine on hepatic GSH levels and mRNA levels of GSH synthetic enzymes in ethanol-treated groups. SAMe (100 mg/kg BW), Tau (200 mg/kg BW), and/or Bet (500 mg/kg BW) were orally administered to mice every day for a week. At 6 hours after the last treatment, the mice were administrated 6 g/kg BW ethanol by intragastric gavage. Control groups were administered 0.1 mL PBS. (A) Hepatic GSH levels were measured using glutathione reductase. mRNA levels of (B) GCLC, (C) GCLM, and (D) GS were measured by RT-qPCR and normalized by hprt1. Values represents mean ± SEM (n = 5). SAMe, S-adenosylmethionine; Tau, taurine; Bet, betaine; GSH, glutathione; BW, body weight; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GS, GSH synthase; RT-qPCR, quantitative real time PCR. abcdefValues with different alphabets are significantly different at P < 0.05 level by Duncan’s multiple range test.
DISCUSSION
Alterations in methionine metabolism have been shown in patients with liver disease and this impairment has been associated with the development of liver disorders.2,3,10,41 Ethanol abuse can lead to liver diseases ranging from fatty liver to alcoholic hepatitis and even hepatocellular carcinoma.1 The disturbance in the metabolism of SCAA in the liver could be characterized mainly by the depletion of SAMe and GSH concentrations.42 In our study, we therefore focused on SAMe and GSH metabolism. Previous studies suggested that SAMe and betaine have the potential to attenuate liver injury by preserving normal methionine metabolism.10 Also, taurine administration has been shown to protect the liver from liver cirrhosis by decreasing oxidative stress.43 This study was performed to evaluate the effects of combinations of these compounds against ethanol-induced changes in the liver.
AML12 cells were used to investigate the effects of SAMe combinations with taurine and/or betaine on GSH levels in vitro. GSH is an important factor to protect the liver against oxidative stress and to reduce susceptibility to liver damage.44 GSH is synthesized by GS and GCL, which is a rate limiting enzyme in GSH synthesis. GCL contains two subunits, GCLC and GCLM.45 In animals, ethanol administration was shown to decrease GSH concentration.9,46 It has also been shown that ethanol decreases GSH concentration in isolated rat hepatocytes.47 However, we found that 100 mM ethanol treatment did not significantly decrease intracellular GSH levels and mRNA expression of GSH synthetic enzymes in AML12 cells. Although the results are not statistically significant, ethanol treatment tended to decrease GSH level by 27% and mRNA expression of GSH synthetic enzymes by about 20% compared to control. Intracellular GSH levels were increased in SAMe and betaine treatment groups with or without taurine in AML12 cells. In agreement with this result, Kharbanda et al.32 also reported that SAMe and betaine treatment corrected ethanol-induced impairment in methionine metabolism in isolated rat hepatocytes. Interestingly, intracellular GSH concentration and mRNA expression of GCLC were increased in SAMe-treated AML12 cells. Given the importance of GCLC which is the rate-limiting enzyme in GSH synthesis,45 SAMe might affect GSH levels by upregulating GCLC. SAMe administered in combination with taurine and betaine did not further increase GCLC mRNA expression compared to treatment with SAMe alone. It has been reported that increasing the transcription of GCLC could increase the amount of GSH.48 In this regard, SAMe may be an important key factor to preserve the GSH level by upregulating GCLC mRNA expression.
In mice, acute ethanol treatment significantly decreased GSH concentration compared to the control group. In agreement with our results, other investigators showed that ethanol administration reduced GSH levels.9,49 SAMe pretreatment prevented the fall in hepatic GSH levels induced by ethanol in our study. In addition, SAMe pretreatment completely restored the mRNA level of GCLC. Interestingly, pretreatment of SAMe combinations with taurine and/or betaine dramatically increased mRNA levels of GCLM and GS. This action of SAMe may be one of the mechanisms for hepatoprotection. Also, taurine or betaine treatment tended to prevent the fall in hepatic GSH levels induced by ethanol in mice. These observations are in accordance with other observations. Pushpakiran et al.49 showed that the content of GSH in the liver was significantly increased in rats treated with ethanol and taurine as compared to rats treated with ethanol alone. Betaine supplementation prevented the decrease in GSH levels in mice and rats.50 Therefore, we suggest that the effects of SAMe, taurine, and betaine on ethanol-induced acute liver injury might be associated with their regulatory role in the preservation of hepatic GSH levels.
In our study, pretreatment with SAMe was shown to especially preserve GSH levels and gene expression of GSH synthetic enzymes. Treatment with SAMe was more effective to maintain normal GSH levels than treatment with taurine or betaine alone. Although further research is needed to elucidate the mechanisms of action of SAMe, taurine, and betaine, our study demonstrated the potential effects of SAMe in combination with taurine and betaine on GSH levels. SAMe combinations could have hepatoprotective effects. Therefore, SAMe combinations could protect the liver from various liver diseases caused by excess alcohol consumption, eventually helping to prevent the occurrence of liver cancer.
ACKNOWLEDGMENTS
Thanks to Samoh Pharm Co., Ltd. (Seoul, Korea) for providing SAMe powder.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 2.García-Tevijano ER, Berasain C, Rodríguez JA, Corrales FJ, Arias R, Martín-Duce A, et al. Hyperhomocysteinemia in liver cirrhosis: mechanisms and role in vascular and hepatic fibrosis. Hypertension. 2001;38:1217–21. doi: 10.1161/hy1101.099499. [DOI] [PubMed] [Google Scholar]
- 3.Duce AM, Ortíz P, Cabrero C, Mato JM. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology. 1988;8:65–8. doi: 10.1002/hep.1840080113. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Lee JW, Jung YS, Kwon DY, Park HK, Ryu CS, et al. Ethanol-induced liver injury and changes in sulfur amino acid metabolomics in glutathione peroxidase and catalase double knockout mice. J Hepatol. 2009;50:1184–91. doi: 10.1016/j.jhep.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Lee TD, Sadda MR, Mendler MH, Bottiglieri T, Kanel G, Mato JM, et al. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2004;28:173–81. doi: 10.1097/01.ALC.0000108654.77178.03. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–37. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 7.Barak AJ, Beckenhauer HC, Tuma DJ. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol. 1994;11:501–3. doi: 10.1016/0741-8329(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 8.Barak AJ, Beckenhauer HC, Tuma DJ. Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol. 1996;13:483–6. doi: 10.1016/0741-8329(96)00040-7. [DOI] [PubMed] [Google Scholar]
- 9.Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, et al. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem. 2003;14:591–7. doi: 10.1016/S0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 10.Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr. 2007;86:14–24. doi: 10.1093/ajcn/86.1.14. [DOI] [PubMed] [Google Scholar]
- 11.Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Curr Opin Clin Nutr Metab Care. 2006;9:728–33. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- 12.Cooper A, Betts JM, Pereira GR, Ziegler MM. Taurine deficiency in the severe hepatic dysfunction complicating total parenteral nutrition. J Pediatr Surg. 1984;19:462–6. doi: 10.1016/S0022-3468(84)80276-6. [DOI] [PubMed] [Google Scholar]
- 13.Mårtensson J, Foberg U, Fryden A, Schwartz MK, Sörbo B, Weiland O. Sulfur amino acid metabolism in hepatobiliary disorders. Scand J Gastroenterol. 1992;27:405–11. doi: 10.3109/00365529209000096. [DOI] [PubMed] [Google Scholar]
- 14.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SK, Kim YC. Effects of betaine supplementation on hepatic metabolism of sulfur-containing amino acids in mice. J Hepatol. 2005;42:907–13. doi: 10.1016/j.jhep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D. Immunonutrition: the role of taurine. Nutrition. 1998;14:599–604. doi: 10.1016/S0899-9007(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 17.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99. doi: 10.1016/S0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 18.Barak AJ, Beckenhauer HC, Kharbanda KK, Tuma DJ. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol. 2001;25:77–81. doi: 10.1016/S0741-8329(01)00168-9. [DOI] [PubMed] [Google Scholar]
- 19.Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ. Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr. 2003;133:2845–8. doi: 10.1093/jn/133.9.2845. [DOI] [PubMed] [Google Scholar]
- 20.Halsted CH, Villanueva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvili L, et al. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 1996;23:497–505. doi: 10.1002/hep.510230314. [DOI] [PubMed] [Google Scholar]
- 21.Barak AJ, Beckenhauer HC, Tuma DJ. Ethanol feeding inhibits the activity of hepatic N5-methyltetrahydrofolate-homocysteine methyltransferase in the rat. IRCS Med Sci. 1985;13:760–1. [Google Scholar]
- 22.Trimble KC, Molloy AM, Scott JM, Weir DG. The effect of ethanol on one-carbon metabolism: increased methionine catabolism and lipotrope methyl-group wastage. Hepatology. 1993;18:984–9. doi: 10.1002/hep.1840180433. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg IS, Park E, Ballman KV, Berger P, Nunn M, Suh DS, et al. Investigations of a common genetic variant in betaine-homocysteine methyltransferase (BHMT) in coronary artery disease. Atherosclerosis. 2003;167:205–14. doi: 10.1016/S0021-9150(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 24.Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res. 1993;17:552–5. doi: 10.1111/j.1530-0277.1993.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 25.Müller A, Sies H. Role of alcohol dehydrogenase activity and the acetaldehyde in ethanol-induced ethane and pentane production by isolated perfused rat liver. Biochem J. 1982;206:153–6. doi: 10.1042/bj2060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch OR, Galeotti T, Bartoli GM, Boveris A. Alcohol-induced oxidative stress in rat liver. Xenobiotica. 1991;21:1077–84. doi: 10.3109/00498259109039547. [DOI] [PubMed] [Google Scholar]
- 27.Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994;29:513–22. [PubMed] [Google Scholar]
- 28.Kinsell LW, Harper HA, Barton HC, Michaels GD, Weiss HA. Rate of disappearance from plasma of intravenously administered methionine in patients with liver damage. Science. 1947;106:589–90. doi: 10.1126/science.106.2763.589. [DOI] [PubMed] [Google Scholar]
- 29.García-Ruiz C, Morales A, Colell A, Ballesta A, Rodés J, Kaplowitz N, et al. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–14. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- 30.Colell A, García-Ruiz C, Miranda M, Ardite E, Marí M, Morales A, et al. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–51. doi: 10.1016/S0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 31.Yang HT, Chien YW, Tsen JH, Chang CC, Chang JH, Huang SY. Taurine supplementation improves the utilization of sulfur-containing amino acids in rats continually administrated alcohol. J Nutr Biochem. 2009;20:132–9. doi: 10.1016/j.jnutbio.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Kharbanda KK, Rogers DD, 2nd, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, et al. A comparison of the effects of betaine and S-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J Nutr. 2005;135:519–24. doi: 10.1093/jn/135.3.519. [DOI] [PubMed] [Google Scholar]
- 33.Lam NV, Chen W, Suruga K, Nishimura N, Goda T, Yokogoshi H. Enhancing effect of taurine on CYP7A1 mRNA expression in Hep G2 cells. Amino Acids. 2006;30:43–8. doi: 10.1007/s00726-005-0244-3. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Hou W, Yao P, Zhang B, Sun S, Nüssler AK, et al. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol In Vitro. 2010;24:516–22. doi: 10.1016/j.tiv.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Katz GG, Shear NH, Malkiewicz IM, Valentino K, Neuman MG. Signaling for ethanol-induced apoptosis and repair in vitro. Clin Biochem. 2001;34:219–27. doi: 10.1016/S0009-9120(01)00218-1. [DOI] [PubMed] [Google Scholar]
- 36.Tomasi ML, Ramani K, Lopitz-Otsoa F, Rodríguez MS, Li TW, Ko K, et al. S-adenosylmethionine regulates dual-specificity mitogen-activated protein kinase phosphatase expression in mouse and human hepatocytes. Hepatology. 2010;51:2152–61. doi: 10.1002/hep.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey RG, Gang C, Joyce M, Bouchier-Hayes DJ. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J Vasc Res. 2007;44:31–9. doi: 10.1159/000097893. [DOI] [PubMed] [Google Scholar]
- 38.Kim SK, Kim YC, Kim YC. Effects of singly administered betaine on hepatotoxicity of chloroform in mice. Food Chem Toxicol. 1998;36:655–61. doi: 10.1016/S0278-6915(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 39.Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, et al. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 41.Corrales F, Alvarez L, Pajares MA, Ortiz P, Mato JM. Impairment of methionine metabolism in liver disease. Drug Investig. 1992;4:8–13. doi: 10.1007/BF03258359. [DOI] [Google Scholar]
- 42.Kwon DY, Jung YS, Kim SJ, Park HK, Park JH, Kim YC. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr. 2009;139:63–8. doi: 10.3945/jn.108.094771. [DOI] [PubMed] [Google Scholar]
- 43.Balkan J, Doğru-Abbasoğlu S, Kanbağli O, Cevikbaş U, Aykaç-Toker G, Uysal M. Taurine has a protective effect against thioacetamide-induced liver cirrhosis by decreasing oxidative stress. Hum Exp Toxicol. 2001;20:251–4. doi: 10.1191/096032701678227758. [DOI] [PubMed] [Google Scholar]
- 44.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–83. [PubMed] [Google Scholar]
- 45.Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–80. [PubMed] [Google Scholar]
- 46.Cravo ML, Camilo ME. Hyperhomocysteinemia in chronic alcoholism: relations to folic acid and vitamins B(6) and B(12) status. Nutrition. 2000;16:296–302. doi: 10.1016/S0899-9007(99)00297-X. [DOI] [PubMed] [Google Scholar]
- 47.Viña J, Estrela JM, Guerri C, Romero FJ. Effect of ethanol on glutathione concentration in isolated hepatocytes. Biochem J. 1980;188:549–52. doi: 10.1042/bj1880549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem. 1994;269:26512–7. [PubMed] [Google Scholar]
- 49.Pushpakiran G, Mahalakshmi K, Anuradha CV. Protective effects of taurine on glutathione and glutathione-dependent enzymes in ethanol-fed rats. Pharmazie. 2004;59:869–72. [PubMed] [Google Scholar]
- 50.Jung YS, Kim SJ, Kwon do Y, Ahn CW, Kim YS, Choi DW, et al. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem Toxicol. 2013;62:292–8. doi: 10.1016/j.fct.2013.08.049. [DOI] [PubMed] [Google Scholar]