Abstract
OBJECTIVES
Pharmacotherapy is a mainstay in functional gastrointestinal (GI) disorder (FGID) management, but little is known about patient attitudes toward medication regimens. Understanding patient concerns and adherence to pharmacotherapy is particularly important for off-label medication use (e.g., anti-depressants) in FGID.
METHODS
Consecutive tertiary GI outpatients completed the Beliefs About Medications questionnaire (BMQ). Subjects were categorized as FGID and structural GI disease (SGID) using clinician diagnoses and Rome criteria; GI-specific medications and doses were recorded, and adherence to medication regimens was determined by patient self-report. BMQ domains (overuse, harm, necessity, and concern) were compared between FGID and SGID, with an interest in how these beliefs affected medication adherence. Psychiatric measures (depression, anxiety, and somatization) were assessed to gauge their influence on medication beliefs.
RESULTS
A total of 536 subjects (mean age 54.7±0.7 years, range 22–100 years; n=406, 75.7% female) were enrolled over a 5.5-year interval: 341 (63.6%) with FGID (IBS, 64.8%; functional dyspepsia, 51.0%, ≥2 FGIDs, 38.7%) and 142 (26.5%) with SGID (IBD, 28.9%; GERD, 23.2%). PPIs (n=231, 47.8%), tricyclic antidepressants (TCAs) (n=167, 34.6%), and anxiolytics (n=122, 25.3%) were common medications prescribed. FGID and SGID were similar across all BMQ domains (P>0.05 for overuse, harm, necessity, and concern). SGID subjects had higher necessity-concern framework (NCF) scores compared with FGID subjects ( P=0.043). FGID medication adherence correlated negatively with concerns about medication harm (r=−0.24, P<0.001) and overuse (r=−0.15, P=0.001), whereas higher NCF differences predicted medication compliance (P=0.006). Medication concern and overuse scores correlated with psychiatric comorbidity among FGID subjects (P<0.03 for each). FGID patients prescribed TCAs (n=142, 41.6%) expressed a greater medication necessity (17.4±0.4 vs. 16.2±0.4, P=0.024) and found their GI regimen to be more helpful (P=0.054). FGID subjects not on TCAs expressed a greater apprehension about medication overuse (10.7±0.3 vs. 9.7±0.2, P=0.002) on the BMQ.
CONCLUSIONS
FGID subjects report medication necessity and concern scores comparable to patients with SGID but have negative perceptions about medications, particularly in the presence of psychiatric comorbidity; these factors may affect treatment adherence and willingness to initiate neuromodulator regimens.
The irritable bowel syndrome (IBS) and other functional gastrointestinal (GI) disorders (FGIDs) are common and pose significant burdens to patients (1,2). Although pharmacotherapy forms one of the pillars of FGID treatment, high-quality evidence to support the use of many FGID regimens is lacking (3–5). Drugs approved for use in other conditions (e.g., tricyclic antidepressants (TCAs)) often are implemented, leading to off-label use of medications with substantial side effect burdens. Psychotropic medications also carry stigmas, which may further interfere with patient acceptance of treatment regimens (6,7). These factors may lead to medication non-adherence; understanding and preemptively addressing FGID patients’ concerns with regard to pharmacotherapeutic regimens could potentially improve adherence.
The Beliefs About Medicines questionnaire (BMQ) is a validated tool used to assess patients’ attitudes regarding their prescribed medication regimens (8–14). The BMQ “necessity-concern framework (NCF)” examines the balance between the perceived beneficial and worrisome aspects of medication use and has been demonstrated as a reliable predictor of adherence to prescribed regimens in multiple different disease states (15,16).
The aim of the current study was to assess how beliefs about medications intersect with medication adherence in FGID. Further, we sought to determine whether off-label use of antidepressants and other psychotropic agents affects compliance and medication beliefs. Through a better understanding of FGID patients’ concerns about medications, selection of optimal therapeutic strategies that improved medication adherence might be realized.
Methods
Subjects and clinical characteristics
Adult patients (>18 years) presenting to two referral gastroenterology clinics (CPG, GSS) from January 2007 to June 2012 were prospectively enrolled into this observational cohort study. Patients were approached by a study coordinator to participate, after which consent was obtained. Participants were provided the study questionnaires to complete and return via a self-address, stamped envelope. Questionnaires included a multi-dimensional survey, which included demographic information, mood and somatization instruments (detailed below), as well as the Rome III Diagnostic Questionnaire for the Adult FGID (17). Chart review was undertaken (BC) to identify the primary GI diagnosis and other secondary diagnoses as documented by the treating gastroenterologist. Patients were categorized as having FGID if (i) the treating clinician recorded a FGID as their primary GI diagnosis, or (ii) FGID was suspected clinically (i.e., “likely”, “probable” FGID) and the patient met the Rome III criteria for a FGID. Structural GI disease (SGID) was established if an active structural diagnosis was recorded as the primary GI condition in the absence of a clinical and/or a Rome III FGID diagnosis. Patients with clinical ambiguity as to the primary GI diagnosis (FGID vs. SGID) were excluded from further analysis. The study was approved by the Washington University Human Research Protection Office.
Medication usage
Medication use contemporaneous to completion of the survey was assessed by medical record review. Patients were asked to rate their degree of adherence to their medication regimen on a 10-cm visual analog scale in response to the question “How well have you been following the treatment recommendations given to you by your doctor for your gastrointestinal (GI) disorder?” anchored by “Not at all”=0 and “Following exactly what my doctor recommended”=10. Patient responses on the 10-cm VAS were converted to a percentage adherence using the formula VAS score/10 *100. A threshold of 80% (8-cm) was used to define good medication adherence (18).
BMQ
The BMQ (Table 1) is composed of 18 statements with two subsets; 10 items address patients’ beliefs about their particular regimen (“specific” subset), and 8 items focus on the attitudes toward medications and prescribing practices generally (“general” subset). The “specific” statements further subdivide into those that examine perceived dependence on medications for health (“necessity” domain) and apprehensions about their regimen (“concern” domain). The “general” subdomains assess beliefs about the “overuse” of medications and “harms” potentially caused by pharmacotherapies. Study participants were asked to rate agreement with these statements using a 6-point Likert scale where 1=strongly disagree and 6=strongly agree (5-point scale with “uncertain” option expanded to “agree somewhat” or “disagree somewhat”). For the purpose of comparison with the cited literature, the 5-point scale was used in calculation of the BMQ domains. The NCF was calculated by subtracting the scores on the “concern” domain from the “necessity” domain scores.
Table 1.
Overview of the Beliefs About Medicines questionnaire (BMQ)
| BMQ category | BMQ domain | Number of statements | Example statement |
|---|---|---|---|
| Specific | Necessity | 5 | “My health, at present, depends on my medications.” |
| Concern | 5 | “I sometimes worry about being too dependent on my medicines.” | |
| General | Overuse | 4 | “Doctors use too many medicines.” |
| Harm | 4 | “Medicines do more harm than good.” |
Psychiatric and somatization measures
Psychiatric comorbidity in the form of depression and anxiety was assessed using BDI (The Beck Depression Inventory) (19,20) and BAI (Beck Anxiety Inventory). BDI scores of ≥19 and BAI scores of ≥16 were used as cutoffs for the presence of depression and anxiety, respectively, based on the described thresholds (21,22). Somatization was assessed using the PHQ-15 instrument, as well as the PHQ-12, which is the PHQ-15 without the three GI-specific questions (20,23). PHQ-15 scores of 5, 10, 15 represented cut-off points for low, medium, and high somatic symptom severity, whereas PHQ-12 scores of ≥10 designate “high somatization” (24).
Statistical analysis
Grouped values are reported as mean±s.e.m., and between-group comparisons were performed using Student's t-tests; χ2 analyses were performed for binomial data as appropriate. In each case, P<0.05 was required for statistical significance. Median values for nonparametric data were compared using Mann-Whitney U tests. Pearson's correlations were performed to assess relationships of psychiatric and BMQ measures with reported medication compliance. A multiple linear regression model was developed to establish the independent predictive value of the patients’ demographic factors, psychiatric state, and medication beliefs in predicting medication compliance. Given the previous literature supporting the use of the NCF, this was the primary medication belief construct examined in this model. All statistical analyses were carried out using SPSS Statistics v.20 (IBM, Armonk, NY).
Results
Subject demographic and clinical characteristics
A total of 1,434 GI outpatients consented for study participation over the 5.5-year period. Of those agreeing to participate, 536 (37.4%) returned their completed instruments (mean age 54.7±0.7 years, range 22–100 years, 406 (75.7%) female), 341 (63.6%) patients were diagnosed with FGID, 142 (26.5%) had SGIDs, whereas 53 (9.9%) had an uncertain diagnosis and were excluded from further analysis. Of the FGID subjects, the Rome criteria for two or more FGIDs were satisfied in 132 (38.7%) individuals. IBS and functional dyspepsia were most frequently diagnosed (64.8% and 51.0%, respectively). The most common SGID diagnoses were gastroesophageal reflux disease (n=33, 23.2%) and inflammatory bowel disease (n=41, 28.9%). FGID patients reported more severe, bothersome, and frequent GI symptoms. FGID participants also had higher psychiatric comorbidity, with higher depression, and a greater degree of somatization (PHQ-12/15) compared with SGID (Table 2).
Table 2.
Baseline demographics and clinical characteristics
| FGID subjects (n=341) | SGID subjects (n=142) | P-value | |
|---|---|---|---|
| Mean age | 52.6±0.8 | 57.0±1.2 | 0.005 |
| Female gender, n (%) | 279 (81.8%) | 90 (63.4%) | <0.001 |
| Bowel symptom severity (VAS) | 7.1±0.1 | 4.8±0.3 | <0.001 |
| Bowel symptom bother (VAS) | 7.0±0.1 | 5.1±0.3 | <0.001 |
| Median bowel symptom frequency (days/last 2 week) | 10 (0–14) | 6 (0–14) | <0.001 |
| BAI | 12.4±0.5 | 8.7±0.7 | <0.001 |
| BDI | 11.4±0.4 | 8.5±0.7 | 0.002 |
| Somatization symptoms (PHQ-15) | 14.1±3.1 | 11.2±0.7 | <0.001 |
| Non-GI somatization symptoms (PHQ-12) | 9.4±0.4 | 8.0±0.6 | 0.038 |
BAI, Beck anxiety inventory; BDI, Beck depression inventory; FGID, functional gastrointestinal disorder; GI, gastrointestinal; SGID, structural gastrointestinal disorder.
Score ranges for instruments in table: BAI and BDI: 0–63, PHQ-15: 0–30, PHQ-12: 0–24.
Medication regimens and adherence
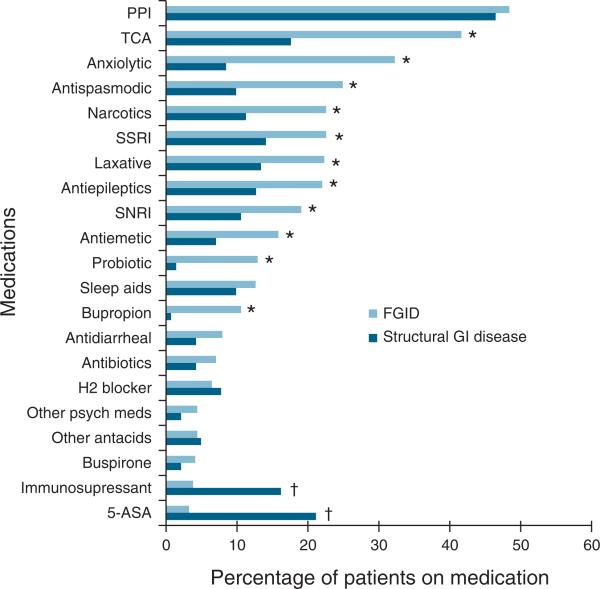
The major classes of medicines used by study subjects are detailed in Figure 1, with additional details reported in Tables 1a and 1b in the Supplementary Appendix online. The medication regimens most commonly used among the study population included proton pump inhibitors (n=231, 47.8%), TCAs (n=167, 34.6%), and anxiolytics (n=122, 25.3%). Patients with FGID were significantly more likely to be using psychoactive medications. FGID subjects used significantly more symptom-directed therapy (i.e., antispasmodics, anti-emetics, and laxatives). Opiate pain medications were used by over one-fifth of the FGID cohort (n=77, 22.6%), significantly higher than in SGID patients (n=16, 11.3%, P=0.004).
Figure 1.
Medication usage among functional gastrointestinal (GI) disorder (FGID) and structural GI disease (SGID) subjects. *P<0.05 for FGID vs. SGID; †P<0.05 for SGID vs. FGID. 5-ASA, 5-aminosalicylic acid; PPI, proton pump inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Patients reported their medication regimens to be moderately helpful with their GI symptoms with greater relief of GI symptoms reported by SGID patients (5.7±0.3 vs. 4.2±0.2, P<0.001). The overall rate of reported adherence to prescribed regimens was high (mean 84.2% compliance). No differences were noted in adherence to medication regimens (P=0.67) between FGID and SGID. Patients taking TCAs reported similar adherence compared with those taking non-TCA regimens (P=0.54). However, FGID patients on TCAs reported their GI medication regimens to be more helpful at relieving their GI symptoms (P=0.054).
BMQ results
Mean BMQ scores across all domains among FGID patients were comparable to those with SGID. Higher concern scores resulted in a significantly higher necessity-concern differential (NCF) in the SGID group (5.1±0.5 vs. 3.8±0.4, P=0.043). BMQ-specific domain scores, notably concern and NCF, were higher in those with IBS compared with non-IBS FGIDs (13.5±0.3 vs. 11.8±0.4, P<0.001 and 5.0±0.5 vs. 3.1±0.5, P=0.01, respectively). BMQ scores between those meeting the criteria for more than one FGID were higher for concern (13.8±0.4 vs. 12.3±0.3, P=0.005) and harm (8.1±0.2 vs. 7.4±0.2, P=0.022). FGID subjects prescribed TCAs (n=142) expressed higher medication necessity, leading to greater NCF differences in these patients (necessity: 17.4±0.4 vs. 16.1±0.4, P=0.024; NCF: 4.6±0.5 vs. 3.1±0.5, P=0.046 for those on TCAs compared with non-TCA regimens, respectively). FGID subjects not on TCAs expressed greater apprehension about medication overuse (10.7±0.3 vs. 9.7±0.2, P=0.002) on the BMQ.
Psychiatric comorbidity and beliefs about medications in FGID patients
Depressed and/or anxious FGID patients reported higher necessity, concern, and harm scores compared with FGID patients without active mood symptoms (P<0.03 for each). FGID subjects with high somatization also were noted to have higher concern, overuse, and harm scores (P<0.007 for each).
Relationship between beliefs about medications and adherence
Overall, subject medication adherence correlated positively with perceived medication necessity (r= 0.11, P=0.017) and negatively with concerns about medication harm (r=−0.24, P<0.001) and overuse (r=−0.15, P=0.001). In FGID subjects, medication adherence was inversely related to harm and overuse (r=−0.22, P<0.001 and r=−0.15, P=0.011, respectively). In a multiple variable linear model examining demographic and psychiatric measures as covariates, only the NCF significantly predicted medication adherence (B=0.05, P=0.006; Supplementary Table 2).
Discussion
In this prospective observational cohort study, we found using the validated BMQ instrument that FGID and SGID patients overall express similar beliefs and concerns about medications. Medication adherence was high in both cohorts and correlated positively with perceived medication necessity, whereas correlating negatively with beliefs of medication harm or overuse. Among FGID patients, IBS sufferers endorsed higher medication concern and necessity-concern differentials compared with non-IBS FGID; further, those with multiple FGIDs, comorbid depression, and patients on antidepressant regimens had higher scores within these domains.
The NCF (16) is central to the BMQ, as this construct quantitatively balances the patient's perceived dependence on their medications against their apprehensions about using this same regimen, and has proven to best predict adherence to medication regimens in other patient populations. Although we found no statistical difference in scores on the BMQ between subjects with FGID and SGID in this study, we did observe a statistically lower NCF among FGID subjects. SGID and FGID patients appeared to have similar levels of concerns relating to their medications but perhaps for different reasons: in FGID patients, many of the medications commonly used in the group have central side effects (e.g., sedation, disturbed sleep), whereas some of the SGID medications have potentially serious systemic side effects, such as infection and malignancy in the case of immunosuppressants.
Overall, the rate of adherence to prescribed medication regimens in this study was high, with approximately three in four subjects reporting good adherence. These rates are higher compared with those previously reported for IBD, excluding patients on anti-TNF therapies who had similarly high rates of adherence (25–27). Although data on medication adherence in FGID patients are sparse, our observed adherence exceeds the 29–45% reported for patients with non-GI functional disorders, such as fibromyalgia (28,29). We hypothesized that adherence would be low in the subgroup of FGID patients on TCAs given their stigmatization as “psychiatric medications” (6,7). However, we observed that subjects on TCAs reported a greater medication necessity along with higher scores on the NCF. Accordingly, TCA users trended toward reporting their medication regimen to be significantly more helpful with their GI symptoms. This observation might be interpreted as indirect evidence of the efficacy of TCAs in managing FGID symptoms (30). FGID subjects not on TCAs, however, were more likely to express greater concerns about medication overuse; these patients may perceive antidepressant use as an acknowledgment of an underlying mood disorder or personal weaknesses (“it's in my head”) (6,7,31). Among depressed patients, a priori positive and negative attitudes about antidepressants have been shown to best predict medication adherence (32,33). Greater insight into FGID patient attitudes regarding off-label medications such as TCAs offers the gastroenterologist an opportunity to address preconceived notions of the effectiveness (or lack thereof) of antidepressants and to de-stigmatize their use. At the very least, recognizing patients with greater medication concerns may highlight a group of patients better suited for non-pharmacologic therapies such as dietary intervention (e.g., low FODMAP diet), cognitive behavioral therapy, or other complementary approaches (34–36). Indeed, higher scores on the BMQ overuse and harm domains predict increased interest in alternative/complementary care among other patient populations (8).
Psychiatric comorbidity is another important factor in FGID medication beliefs. Higher scores on the negative domains (concern, overuse, and harm) were associated statistically with both mood and somatization. Higher scores in the concern domain have been shown to be associated with depression, anxiety, and neuroticism across varying disease states (11,12,25). Depression and anxiety also were associated with higher necessity scores. This relationship (high necessity and concern scores both) previously has been demonstrated among patients on antidepressants for depression (37) and may result from patients’ personal experience of benefit from these medications (high necessity) while still reflecting the stigma surrounding antidepressants and the anxious state inherent in their psychiatric illness (both leading to high concern). Coupled with our previous observations that psychiatric comorbidity and somatization symptoms are significant predictors of eventual antidepressant treatment discontinuation in FGID (38), the negative attitudes toward medications in those with psychiatric comorbidity observed in the current study further emphasize these factors as important barriers to the successful implementation of FGID medication regimens.
Limitations of the study include the use of self-report instruments for medication adherence and psychiatric comorbidity, potentially susceptible to over and underreporting, respectively (39). As subjects were recruited from a tertiary care university-based practice, our observations may be less applicable to community-based FGID populations. Although efforts were undertaken to avoid enrollment bias (e.g., recruitment from same patient pool, neutral study coordinator for enrollment), self-selection bias remains a potential limitation inherent in studies of this design. Nevertheless, we feel that these limitations are balanced by the large sample of subjects with gastroenterologist-confirmed clinical diagnoses, and the inclusion of a comprehensive assessment of GI and psychiatric comorbidities potentially key to understanding the complexities of medication adherence.
In summary, this study provides important insight into FGID patient beliefs about their medications. Opinions about the necessity, harm, and potential overuse of medications have varying but predictable relationships to medication adherence in this population and are inextricably intertwined with psychiatric comorbidity, when present. Understanding patients’ perspectives regarding these belief domains should allow clinicians to address potential concerns and stigmas regarding medication indications and usage, ultimately leading to the formulation of treatment strategies that will result in the greatest potential for success in this challenging GI patient population.
Supplementary Material
Acknowledgments
Financial support: Grants NIDDK K23 DK84113 to GSS, NIDDK P30 DK052574 (Washington University DDRCC) to BDN, and Washington University School of Medicine Mentors in Medicine (MiM) Program to BC.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: None.
Guarantor of the article: Gregory S. Sayuk, MD, MPH.
Specific author contributions: Study concept, data acquisition, analysis and interpretation, and drafting of manuscript: Benjamin Cassell; analysis and interpretation of data, drafting and critical review of the manuscript: C. Prakash Gyawali; drafting and revision of the manuscript: Vladimir M. Kushnir; analysis and interpretation of data, drafting of manuscript: Britt M. Gott; study concept and design, data analysis and interpretation, drafting and revision of the manuscript and study supervision: Gregory S. Sayuk; data analysis and interpretation: Billy D. Nix.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
REFERENCES
- 1.Brandt LJ, Bjorkman D, Fennerty MB, et al. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S7–26. doi: 10.1016/s0002-9270(02)05657-5. [DOI] [PubMed] [Google Scholar]
- 2.Russo MW, Gaynes BN, Drossman DA. A national survey of practice patterns of gastroenterologists with comparison to the past two decades. J Clin Gastroenterol. 1999;29:339–43. doi: 10.1097/00004836-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Lembo A, Sultan S. American gastroenterological association technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1149–72. doi: 10.1053/j.gastro.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:9S–97S. doi: 10.1016/s0002-9343(99)00086-8. [DOI] [PubMed] [Google Scholar]
- 5.Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–65. doi: 10.1038/ajg.2014.148. [DOI] [PubMed] [Google Scholar]
- 6.Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72:827–34. doi: 10.4088/JCP.09m05528blu. [DOI] [PubMed] [Google Scholar]
- 7.Castaldelli-Maia JM, Scomparini LB, Andrade AG, et al. Perceptions of and attitudes toward antidepressants: stigma attached to their use–a review. J Nerv Ment Dis. 2011;199:866–71. doi: 10.1097/NMD.0b013e3182388950. [DOI] [PubMed] [Google Scholar]
- 8.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. [Google Scholar]
- 9.Beck EM, Cavelti M, Kvrgic S, et al. Are we addressing the ‘right stuff’ to enhance adherence in schizophrenia? Understanding the role of insight and attitudes towards medication. Schizophr Res. 2011;132:42–9. doi: 10.1016/j.schres.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Hedenrud T, Jonsson P, Linde M. Beliefs about medicines and adherence among Swedish migraineurs. Ann Pharmacother. 2008;42:39–45. doi: 10.1345/aph.1K354. [DOI] [PubMed] [Google Scholar]
- 11.Allen LaPointe NM, Ou FS, Calvert SB, et al. Changes in beliefs about medications during long-term care for ischemic heart disease. Am Heart J. 2010;159:561–9. doi: 10.1016/j.ahj.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Emilsson M, Berndtsson I, Lotvall J, et al. The influence of personality traits and beliefs about medicines on adherence to asthma treatment. Prim Care Respir J. 2011;20:141–7. doi: 10.4104/pcrj.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya D, Easthall C, Willoughby KA, et al. Capecitabine non-adherence: exploration of magnitude, nature and contributing factors. J Oncol Pharm Pract. 2012;18:333–42. doi: 10.1177/1078155211436022. [DOI] [PubMed] [Google Scholar]
- 14.de Thurah A, Norgaard M, Harder I, et al. Compliance with methotrexate treatment in patients with rheumatoid arthritis: influence of patients' beliefs about the medicine. A prospective cohort study. Rheumatol Int. 2010;30:1441–8. doi: 10.1007/s00296-009-1160-8. [DOI] [PubMed] [Google Scholar]
- 15.Horne R, Chapman SC, Parham R, et al. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8:e80633. doi: 10.1371/journal.pone.0080633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–67. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DACEDM, Spiller RC, Talley NJ, Thompson WG, Whitehead W, editors. Rome III: The Functional Gastrointestinal Disorders Degnon Associates Inc. McLean, VA: 2006. [Google Scholar]
- 18.Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–10. doi: 10.1185/03007990903126833. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Spiller RC, Humes DJ, Campbell E, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32:811–20. doi: 10.1111/j.1365-2036.2010.04402.x. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the beck depression inventory for primary care. Behav Res Ther. 1997;35:785–91. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Vu J, Kushnir V, Cassell B, et al. The impact of psychiatric and extraintestinal comorbidity on quality of life and bowel symptom burden in functional GI disorders. Neurogastroenterol Motil. 2014;26:1323–32. doi: 10.1111/nmo.12396. [DOI] [PubMed] [Google Scholar]
- 25.Selinger CP, Eaden J, Jones DB, et al. Modifiable factors associated with nonadherence to maintenance medication for inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2199–206. doi: 10.1097/MIB.0b013e31829ed8a6. [DOI] [PubMed] [Google Scholar]
- 26.Horne R, Parham R, Driscoll R, et al. Patients’ attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:837–44. doi: 10.1002/ibd.20846. [DOI] [PubMed] [Google Scholar]
- 27.Lopez A, Billioud V, Peyrin-Biroulet C, et al. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19:1528–33. doi: 10.1097/MIB.0b013e31828132cb. [DOI] [PubMed] [Google Scholar]
- 28.Cui Z, Zhao Y, Novick D, et al. Predictors of duloxetine adherence and persistence in patients with fibromyalgia. J Pain Res. 2012;5:193–201. doi: 10.2147/JPR.S31800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun P, Peng X, Sun S, et al. Direct medical costs and medication compliance among fibromyalgia patients: duloxetine initiators vs. pregabalin initiators. Pain Pract. 2014;14:22–31. doi: 10.1111/papr.12044. [DOI] [PubMed] [Google Scholar]
- 30.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 31.Malpass A, Shaw A, Sharp D, et al. “Medication career” or “moral career”? The two sides of managing antidepressants: a meta-ethnography of patients' experience of antidepressants. Soc Sci Med. 2009;68:154–68. doi: 10.1016/j.socscimed.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 32.Aikens JE, Kroenke K, Swindle RW, et al. Nine-month predictors and outcomes of SSRI antidepressant continuation in primary care. Gen Hosp Psychiatry. 2005;27:229–36. doi: 10.1016/j.genhosppsych.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Lin EH, Von Korff M, Ludman EJ, et al. Enhancing adherence to prevent depression relapse in primary care. Gen Hosp Psychiatry. 2003;25:303–10. doi: 10.1016/s0163-8343(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 34.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Xiong L, Zhang S, et al. Cognitive-behavioral therapy for irritable bowel syndrome: a meta-analysis. J Psychosom Res. 2014;77:1–12. doi: 10.1016/j.jpsychores.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Asare F, Storsrud S, Simren M. Meditation over medication for irritable bowel syndrome? On exercise and alternative treatments for irritable bowel syndrome. Curr Gastroenterol Rep. 2012;14:283–9. doi: 10.1007/s11894-012-0268-2. [DOI] [PubMed] [Google Scholar]
- 37.Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43:1203–7. doi: 10.1097/01.mlr.0000185733.30697.f6. [DOI] [PubMed] [Google Scholar]
- 38.Sayuk GS, Elwing JE, Lustman PJ, et al. Predictors of premature antidepressant discontinuation in functional gastrointestinal disorders. Psychosom Med. 2007;69:173–81. doi: 10.1097/PSY.0b013e318031391d. [DOI] [PubMed] [Google Scholar]
- 39.Pearson CR, Simoni JM, Hoff P, et al. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007;11:161–73. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.