Abstract
Tendon is vital to musculoskeletal function, transferring loads from muscle to bone for joint motion and stability. It is an anisotropic, highly organized, fibrous structure containing primarily type I collagen in addition to tenocytes and other extracellular matrix components contributing to maintenance and function. Tendon is generally loaded via normal stress in a longitudinal direction. However, certain situations, including fiber breakage, enzymatic remodeling, or tendon pathology may introduce various degrees of other loading modalities, such as shear-lag at the fiber level, potentially affecting cellular response and subsequent function. Fascicles from rat tail tendon were dissected and placed in one of three paired groups: intact, single laceration, or double laceration. Each pair had a mechanically tested and control specimen. Single laceration fascicles contained one transverse laceration to mimic a partial tear. Double laceration fascicles had overlapping, longitudinally separated lacerations on opposite sides to cause intra-fascicular shear transfer to be the primary mechanism of loading. Elastic properties of the fascicle, e.g. peak load, steady state load, and stiffness, decreased from intact to single laceration to double laceration groups. Surprisingly, 45% of the intact strength was maintained when shear was the primary internal load transfer mechanism. Cellular viability decreased after mechanical testing in both laceration groups; cell death appeared primarily in a longitudinal plane where high shear load transfer occurred. This cell death extended far from the injury site and may further compromise an already damaged tendon via enzymatic factors and subsequent remodeling associated with cell necrosis.
Keywords: Tendon, Mechanics, Shear, Cellular Viability, Viscoelasticity
Introduction
Tendon connects muscle to bone and functions to transfer muscle load for the movement and stabilization of joints. It consists primarily of the structural protein type I collagen but also contains tendon cells (tenocytes), proteoglycans, and elastin. Tendon structure is highly organized and hierarchical in nature, starting with the collagen molecule which combines to form collagen fibrils, fibers, fascicles, and finally the tendon (Screen, Bader, Lee, and Shelton, 2004). Collagen fibrils have been reported as the tendon’s primary load-bearing structures (Szczesny and Elliott, 2014).
Tendon is principally loaded in the longitudinal direction, parallel to its fibers. Therefore, axial loading is extensively investigated in vitro, with testing designed to apply a load as uniform as possible across the tendon’s cross-section (Abrahams, 1967; Rigby, Hirai, Spikes, and Eyring, 1959). Whole tendon and smaller levels of its hierarchy are viscoelastic (Duenwald, Vanderby, and Lakes, 2009; Kondratko, Duenwald-Kuehl, Lakes, and Vanderby, 2012; Screen, 2008; Woo, Johnson, and Smith, 1993), nonlinear (Lake, Miller, Elliott, and Soslowsky, 2009; Lynch, Johannessen, Wu, Jawa, and Elliott, 2003), and anisotropic in nature (Lynch et al., 2003) when tested in this orientation.
Whole tendon can stretch up to 4%–6% strain in vivo (Gardiner, Weiss, and Rosenberg, 2001; Kongsgaard, Nielsen, Hegnsvad, Aagaard, and Magnusson, 2011; Lochner, Milne, Mills, and Groom, 1980). However, studies comparing strain at different levels of tendon hierarchy have shown that lower hierarchical levels stretch to lower strains than higher levels. For instance, fascicles have been shown to stretch to 55–90% of the whole tendon strain during loading, depending on the tendon type (Thorpe, Udeze, Birch, Clegg, and Screen, 2012). Screen et al. have similarly shown that tendon fibers only display 15% of the strain seen in the fascicle during testing (Screen et al., 2004), and Puxkankl et al. have shown that fibrils only display 10–40% of the strain seen in whole tendon (Puxkandl et al., 2002). This discrepancy in strain between hierarchical levels has been explained by sliding of fascicles, fibers, and fibrils relative to one another (Ahmadzadeh, Connizzo, Freedman, Soslowsky, and Shenoy, 2013; Puxkandl et al., 2002; Screen, Bader, et al., 2004; Szczesny and Elliott, 2014; Thorpe et al., 2012). This sliding could generate shear loading between tendon hierarchical components during normal, physiological stretches.
With injury tendon strength and optimal function are compromised (Bishop, Cooney, and Wood, 1986; Child, Bryant, Clark, and Crossley, 2010; Dobyns, Cooney, and Wood, 1982; Duenwald-Kuehl, Kondratko, Lakes, and Vanderby, 2012; Duenwald-Kuehl, Lakes, and Vanderby Jr., 2012; Hariharan, Diao, Soejima, and Lotz, 1997; Kondratko et al., 2012; Mazzocca et al., 2008; McCarthy et al., 1995; Tan, Wang, Tan, Xu, and Tang, 2003). Specifically, tendon laceration or tear decreases ultimate load (Bishop et al., 1986; Dobyns et al., 1982; Hariharan et al., 1997; Mazzocca et al., 2008; McCarthy et al., 1995; Tan et al., 2003), stiffness (Bey, Ramsey, and Soslowsky, 2002; Bishop et al., 1986; Kondratko et al., 2012; Mazzocca et al., 2008; McCarthy et al., 1995), and viscoelastic parameters (Kondratko et al., 2012) in vitro. Mechanical compromise due to laceration is disproportional to laceration area (Kondratko et al., 2012), indicating that hierarchical levels of tendon do not behave as independent load bearing structures as depicted in simplified structural models. Instead, levels of shear-lag and load transfer above those seen during normal loading must occur between fibrils and fascicles, as suggested in other studies (Ahmadzadeh et al., 2013; Kondratko-Mittnacht, Duenwald-Kuehl, Lakes, and Vanderby Jr., 2015; Pensalfini, Duenwald-Kuehl, Kondratko-Mittnacht, Lakes, and Vanderby Jr., 2014; Szczesny and Elliott, 2014). Although some studies conclude that shear force transmission capability is negligible between isolated tendon fascicles (Haraldsson et al., 2008; Purslow, 2009), Kondratko et al. report a larger shear load transfer potential between fascicles when observing whole tendon behavior (Kondratko-Mittnacht et al., 2015). Authors used transversely overlapping cuts (each 60% of the tendon depth) such that no fascicles were contiguous grip-to-grip. In this configuration, tendons maintained 20% of their intact strength and stiffness (Kondratko-Mittnacht et al., 2015).
Beyond affecting mechanical function, abnormal levels of tendon loading mechanisms, such as shear, as utilized by Kondratko-Mittnacht et al. in (Kondratko-Mittnacht et al., 2015) or in vivo by partial tears, tendinopathy, or local remodeling, would affect nearby cells. In response to altered mechanical load, tenocytes change tendon structure, composition, and mechanical properties by adjusting extracellular matrix (ECM) protein expression (Banes et al., 1999; Chiquet, 1999; M. Kjær et al., 2009; Kjær, 2004). Additionally, excess stretch or stress of tenocytes may cause apoptosis, necrosis, or plasma membrane disruption (Millar, Wei, Molloy, Bonar, and Murrell, 2009; Scott et al., 2005), potentially releasing inflammatory factors into the ECM (Hosaka, Teraoka, Yamamoto, Ueda, and Takehana, 2005; Lian et al., 2007; Yuan, Murrell, Wei, and Wang, 2002; Yuan, Wang, and Murrell, 2003). Therefore, an increased level of the shear internal loading mechanism would significantly modify cell signaling or viability and likely degrade mechanical behavior and function or cause tendinopathy over time.
There have been relatively few studies investigating shear behavior within tendon and its effect on cells, despite its importance. Shear is present within tendon as a primary loading mechanism to redistribute load near insertion sites during joint movement, during fiber breakage and remodeling due to fatigue, and exaggerated under abnormal circumstances, such as around tendon partial tear, laceration, or tendinopathy. Shear load transfer is also an integral component of tendon lengthening procedures used to treat diabetic plantar forefoot ulceration (Mueller, Sinacore, Hastings, Strube, and Johnson, 2003), where multiple transections are created on alternating sides of the tendon (Hoke, 1931; Mueller et al., 2003; Salamon, Pinney, Bergeyk, and Hazelwood, 2006). Therefore, the purpose of this study is to investigate the mechanical and cellular response, in the form of tenocyte viability, to excess shear and axial loading (which includes physiologic levels of shear in the form of sliding between hierarchical components) within tendon fascicles. We hypothesize that cellular viability and mechanical properties will be adversely affected more by the excess shear loading mechanism than axial loading of the fascicles.
Methods
Specimen Preparation
Forty-eight (48) rat tail tendon fascicles from 10 2–3 month old male Wistar rats with a mass of about 300g were used in this study. Fascicles were carefully dissected from tail tendons of rats that were humanely euthanized immediately before fascicle removal, following a protocol approved by the University of Wisconsin Institutional Animal Use and Care Committee. No more than 5 paired fascicles of similar apparent diameters were removed from each rat to minimize cell death. The study had 3 paired groups (n=8 pairs per group): 1) double laceration (obtained from 4 different rats), 2) single laceration (obtained from 5 rats), and 3) intact fascicles (obtained from 6 rats), each with fascicles assigned to a mechanically tested and not mechanically tested sub-group (Table 1). The group assignments of fascicle pairs obtained from a single rat had some overlap between groups; however no rats contained pairs from all groups (explaining the discrepancy between the total rats utilized for each group). Laceration patterns for the double and single laceration pairs are shown in Figure 1. The double lacerations were created to ensure shear load transfer, while the single laceration was created to more clearly observe load transfer around a clinically relevant partial laceration/tear.
Table 1.
Description of experimental pairs used in the study.
| Experimental sub-groups | |
|---|---|
| Pair 1 | Double laceration +mechanical testing |
| Double laceration | |
| Pair 2 | Single laceration+mechanical testing |
| Single laceration | |
| Pair 3 | Intact + mechanical testing |
| Intact |
Figure 1.
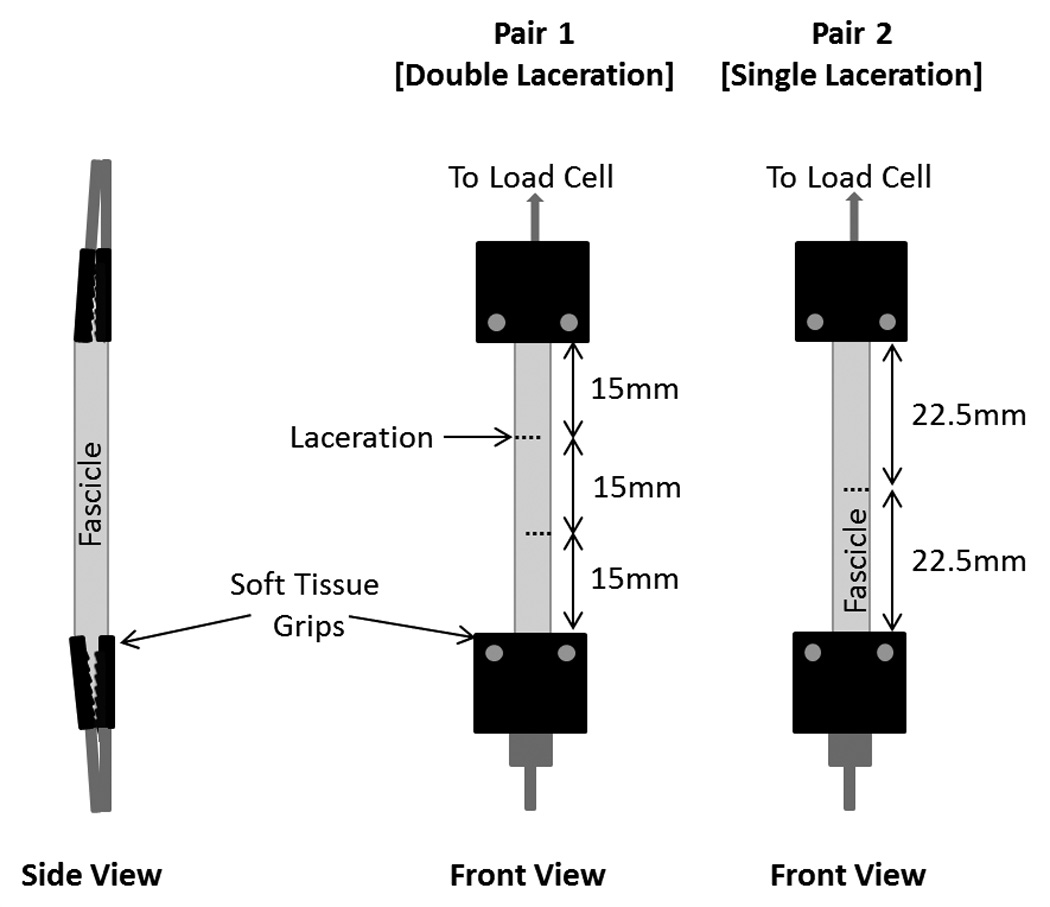
Specimen preparation and mechanical testing setup. Pair 1 (double laceration) was prepared with 2 overlapping lacerations on opposite sides of the fascicle, one specimen was mechanically loaded, one was not. Pair 2 (single laceration) was prepared with a single, mid-substance, transverse laceration, one specimen was loaded, one was not. Both ends of the fascicles were gripped in soft-tissue grips with rough, interlocking plates on both sides.
Lacerations were created in the transverse direction with a razor blade prior to loading samples in the testing device. A razor blade was used to ensure cutting, not tearing of fibers to maintain fascicle integrity and cellular viability in the surrounding area. Lacerations were created to a depth of about 50–70% of the fascicle width in the locations shown in Figure 1 for each fascicle pair. The overlapping lacerations ensured discontinuity of the majority of fibers between grips, thereby requiring shear to be the primary mechanism required to resist axial loads. Measurements of the fascicle diameter or exact laceration depth were not made because the goal was only to create a shear loading situation and to prevent cell death. Hydration was maintained throughout the setup and testing by frequently spraying with physiologic buffered saline (PBS) as fascicles were unsubmerged during testing. Protocol optimization verified that maintaining hydration with PBS at room temperature demonstrated minimal cell death during the time required for dissection and testing.
Mechanical Testing
Fascicles in mechanical testing sub-groups were mounted into a servohydraulic mechanical testing system (MTS) (Bionix 858; MTS, Minneapolis, Minnesota). Fascicles were gripped on both ends with two soft tissue grips made of rough, interlocking plates. Care was taken to prevent undesired loading and damage prior to beginning the testing protocol by ensuring no more than the weight of the fascicle itself was placed on the specimen.
Prepared fascicles were placed into the MTS at an unloaded length of 45mm (Fig. 1). Specimens were preloaded to 0.01N, and initial grip-to-grip length was measured using a digital caliper. Load was measured with a 50lb load cell (Lebow Products Model 3397-50; Toronto, Ontario). Displacement was controlled and measured with the MTS. All data were output to a PC with Labtech Notebook software (Laboratory Technology Corporation; Fort Collins, Colorado).
Mechanical loading consisted of one sinusoidal, cyclic test (10 cycles at 0.5Hz) from 0 to 4% strain. The strain level was selected to not cause mechanical damage but fall near the upper limit of physiologic stretch in tendon (Gardiner et al., 2001; Kongsgaard et al., 2011; Lochner et al., 1980). Each control specimen remained unloaded and hydrated during the time required for mechanical testing of its paired, treated specimen.
LIVE/DEAD® Incubation
Upon completion of mechanical testing, the LIVE/DEAD® Viability/Cytotoxicity (Molecular Probes®; Eugene, Oregon) assay was prepared. In this assay, ethidium homodimer-1 penetrates cells with compromised plasma membrane integrity to fluoresce the cell nuclei red (non-viable cells appear red in the images), while calcein-AM fluoresces cells with intracellular esterase activity green (viable cells appear green). Prior to experimental testing, the assay was optimized for fascicle incubation to contain 1ml PBS, 5µl ethidium homodimer-1, and 1µl calcein-AM. The fascicles were placed in the media and incubated at 37°C for 45min. Following incubation, fascicles were submerged in PBS to maintain hydration until imaging.
Imaging
Imaging was completed on a Nikon A1RS high-speed confocal microscope equipped with green and red excitation lasers. Images were recorded using NIS Elements viewer software v 4.13 (Nikon Corporation, Tokyo, Japan). Fascicles were laid flat on a slide with a cover slip on top to prevent dehydration. Imaging was through the 4× Plan Fluor/0.13 air immersion objective lens; all lacerations were imaged, but locations near grips that may have been damaged due to handling during setup were avoided. Scope settings remained the same for all fascicles to allow normalized comparisons.
Mechanical Parameter Calculations
Peak load, i.e. the maximum load recorded during testing, occurred slightly prior to the peak strain of the initial cycle for all tests due to the viscoelastic nature of the fascicles. Additionally, a “steady state” load was calculated as the average of the peak load of the final three cycles, representing the fascicle’s pseudo-elastic behavior. A viscoelastic parameter, load decay, was presented as the ratio of the decrease in load from the peak of the initial cycle to the peak of the final cycle to the peak load determined for that specimen. Stiffness was calculated as the slope of the linear region of the load-displacement curve recorded during the rise of the final cycle. The linear region was determined visually and was slightly variable between tests but was generally found to be between 1.5 and 3.7% strain. Due to the lack of preconditioning in the protocol to minimize cellular death, peak load and load decay ratio were likely influenced by the fascicle’s loading history, including in vivo movement and handling.
Image Analysis
Images of the fascicles were analyzed using ImageJ (NIH; Bethesda, Maryland). Using a constant color threshold, the amount of red (‘non-viable’) and green (‘viable’) fluorescence were quantified as the percent area of the whole image (calculated based on pixel numbers). To normalize the fascicles for comparison, a ratio of the green percent area to the combined red and green percent areas was calculated and defined as the cellular viability ratio.
Fascicle diameters were not measured from confocal images as the cover slip used to prevent dehydration caused the fascicles to flatten, increasing the apparent diameter.
Statistics
For the mechanical analysis, a one-way ANOVA with a Fisher’s least significant difference (LSD) post hoc test was completed to compare groups for each mechanical parameter. In the LIVE/DEAD® analysis, a one-way ANOVA with a Tukey post hoc test was completed to compare the cellular viability ratios between sub-groups. Significance was defined as p≤0.05 for all statistics. All ANOVA calculations were completed based on the number of fascicles per sub-group being compared.
Results
Mechanical Analysis
As anticipated, mechanical integrity for most parameters, i.e. peak load, steady state load, and stiffness, decreased with increasing laceration number (intact to single laceration to double laceration). For these parameters the ANOVA demonstrated statistical significance (p<0.0001) between groups, and the post hoc analysis showed a statistical difference between all three groups (p<0.005) (Fig. 2a–c). The viscoelastic parameter, load decay ratio, demonstrated an increase in viscoelastic behavior with laceration (p<0.0001) (Fig. 2d). The double laceration group had a significantly larger ratio than both other groups (p<0.0001), but the single laceration group was not different than the intact group (p=0.34).
Figure 2.
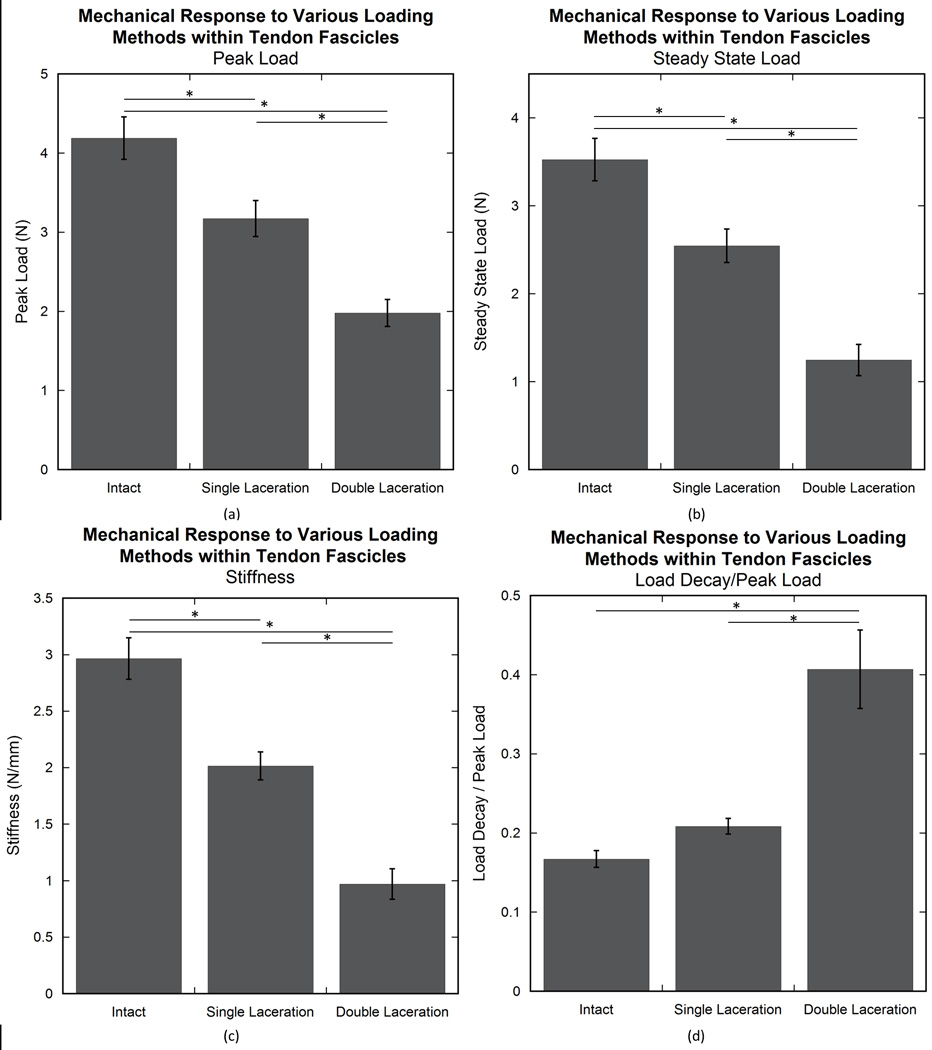
Mechanical response of experimental groups (intact, single laceration, and double laceration). The addition of a laceration decreased the mechanical properties in peak load (a), steady state load (b), and stiffness (c). Load decay ratio demonstrated larger value in the double laceration group compared to the other two groups (d). An asterisk (*) indicates significance (p≤0.05).
LIVE/DEAD® Analysis
The average cellular viability ratio was lower in mechanically tested sub-groups than their control, untested counterparts for the single (p=0.005) and double (p<0.0001) laceration groups. The intact pair did not demonstrate a difference between sub-groups (p=0.998) (Fig. 3). The double (p<0.0001 for all comparisons) and single (p<0.03 for all comparisons) laceration mechanical tested sub-groups were significantly different than all other sub-groups (excluding each other; p=0.28). No differences were seen between not mechanically tested sub-groups (p>0.99 for all comparisons).
Figure 3.
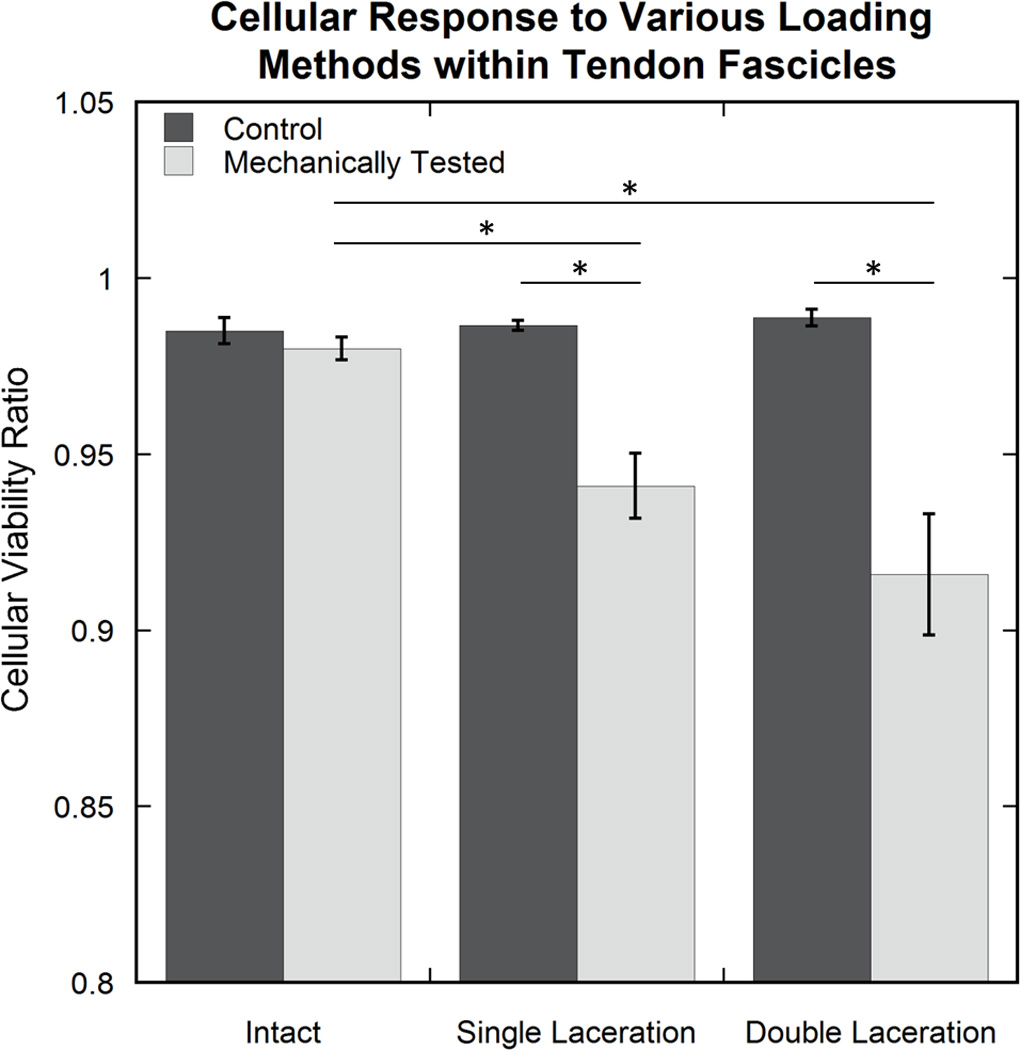
Cellular response in each experimental sub-group. Average cellular viability ratio values resulted in differences between the single and double laceration mechanically tested sub-groups and all other sub-groups, excluding each other (not all differences shown on the graph). An asterisk (*) indicates significance (p≤0.05).
Confocal images of the intact specimens showed no consistent areas of cell death due to mechanical testing. Small areas of concentrated cell death were occasionally seen in both control and mechanically tested specimens (Fig. 4). Conversely, both laceration groups demonstrated a pattern of significant, localized cell death within the fascicle. As seen in Fig. 5 and 6, mechanical testing of fascicles in either laceration group typically resulted in an axial plane of cell death. The plane of cell death in the single laceration fascicles was normal to the tip of the laceration (Fig. 5) and either stretched along the length of the fascicle (n=5) or faded to look similar to the control, not mechanically stretched, fascicle as it progressed away from the central laceration (n=3). Similarly, the plane of cell death in the double laceration fascicles connected the tip of one laceration to the tip of the opposite laceration (Fig. 6). Notably, in one double laceration fascicle, two planes of cell death occurred, potentially due to a lack of overlap in the lacerations.
Figure 4.
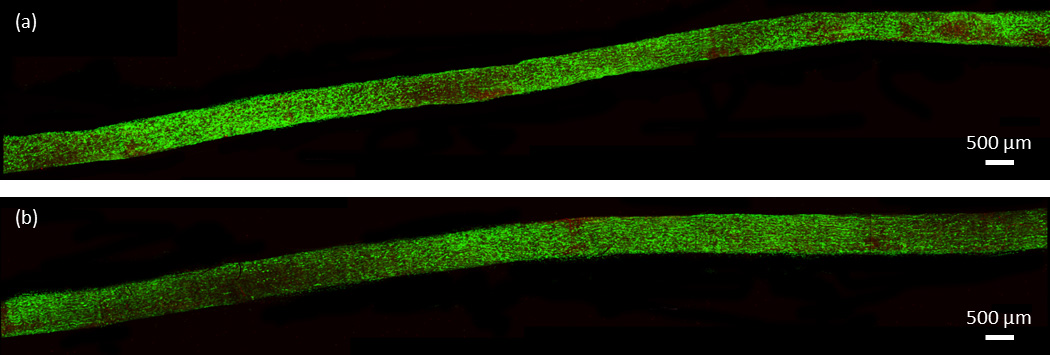
Confocal images of a representative intact pair. (a) Shows the control, not tested specimen and (b) shows the intact, mechanically tested specimen.
Figure 5.
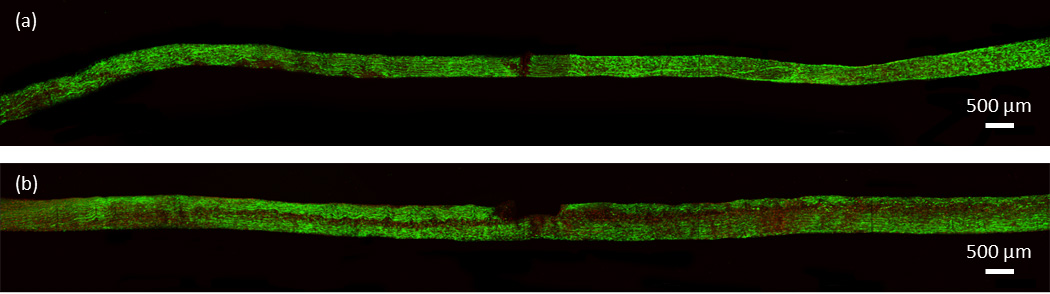
Confocal images of a representative single laceration pair. (a) Shows the control, not tested specimen and (b) shows the lacerated, mechanically tested specimen. Horizontal dark band corresponds to region of non-viable cells.
Figure 6.
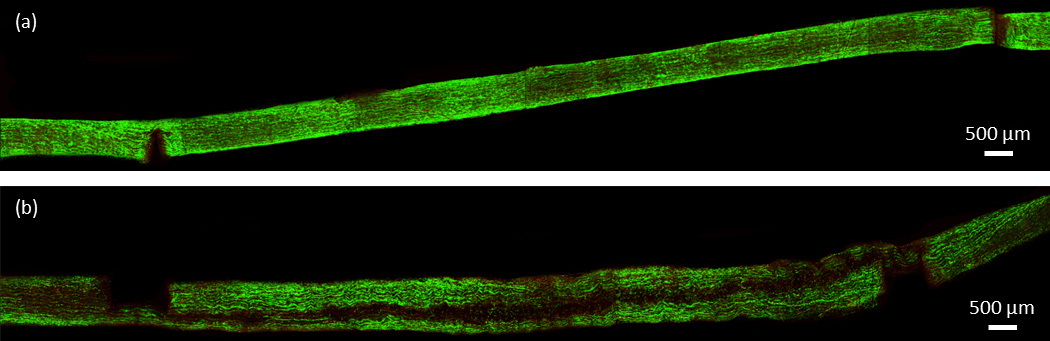
Confocal images of a representative double laceration pair. (a) Shows the control, not tested specimen and (b) shows the lacerated, mechanically tested specimen. Horizontal dark band corresponds to region of non-viable cells.
Discussion
Various loading scenarios were created in this study to investigate differences in mechanical and cellular response. In the single laceration group a 25% decrease in peak load compared to the intact control was much smaller than the decrease seen by Kondratko et al. in whole tendons (Kondratko et al., 2012), where a laceration of approximately 60% reduced the peak load to 55%. The double laceration group tested here also demonstrated a smaller drop in peak load than seen by Kondratko-Mittnacht et al. using a similar testing protocol in whole tendon (55% compared to 80%) (Kondratko-Mittnacht et al., 2015). The variation in these laceration scenarios is likely a result of differing shear transfer mechanisms at the two hierarchical levels. Previous studies have suggested that fiber sliding is the dominant mechanism of elongation within tendon fascicles (Khodabakhshi et al., 2013; Li, Fessel, Georgiadis, and Snedeker, 2013; Screen, Lee, Bader, and Shelton, 2004; Thorpe et al., 2013; Thorpe et al., 2012) as well as the main mechanism for transferring load within tendon (Khodabakhshi et al., 2013). Other studies have reported negligible lateral force transmission between fascicles (Haraldsson et al., 2008; Purslow, 2009). The combination of these two observations may suggest that there is more shear load transfer potential between fibers than fascicles. This suggestion is reinforced by the differences seen in the shear transfer of the current study and the whole tendon study completed by Kondratko-Mittnacht et al. (Kondratko-Mittnacht et al., 2015). The whole tendon study performed by Kondratko-Mittnacht et al., exploiting inter-fascicular shear, caused a greater decrease in load than the current experiment which tests the intra-fascicular shear (Kondratko-Mittnacht et al., 2015).
Viscoelastic response, quantified by the load decay ratio, increased upon introduction of the double laceration. This was surprising because both single and double laceration at the whole tendon level had a lesser viscoelastic response compared to the intact value (Kondratko et al., 2012; Kondratko-Mittnacht et al., 2015). A comparison of the double laceration data collected in this study and whole tendon tested by Kondratko-Mittnacht in (Kondratko-Mittnacht et al., 2015) is shown in Figure 7, demonstrating more viscoelastic decay in lacerated fascicles than tendon. The differences between the hierarchical levels suggest that the inter-fibrillar interface has a greater impact on the viscoelastic response of whole tendon than the inter-fascicular interface. This is supported by studies demonstrating that relaxation primarily occurs due to sliding between fibers within tendon fascicles (Gupta, Seto, Krauss, Boesecke, and Screen, 2010; Screen, 2008; Screen, Toorani, and Shelton, 2013).
Figure 7.
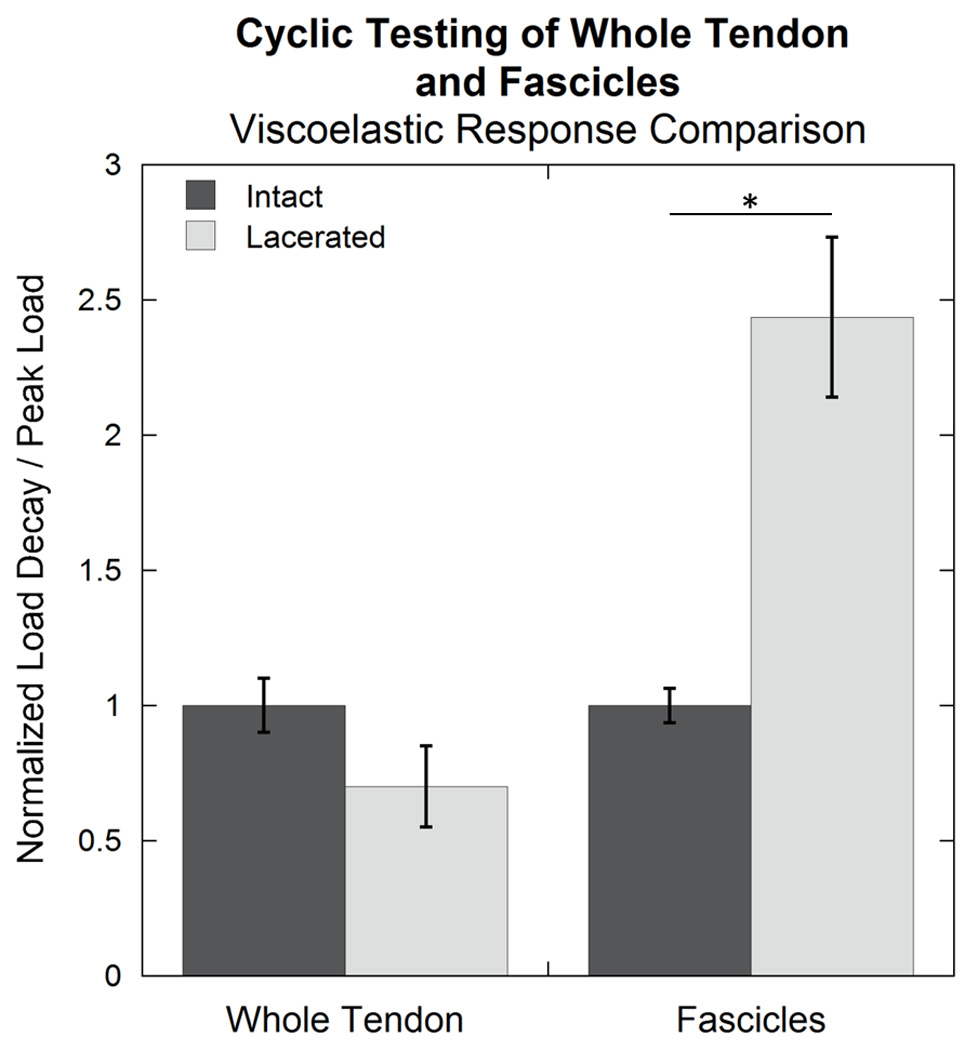
Load decay ratio, normalized to the average intact value, for intact rat tail tendons and fascicles and those tested after introduction of overlapping lacerations. The lacerated fascicles demonstrated an increase in load decay ratio compared to the intact fascicle, while the lacerated tendon had a smaller load decay ratio than the intact tendon (though not significant; p=0.22). Data for whole tendon were measured by Kondratko-Mittnacht as a part of (Kondratko-Mittnacht et al., 2015). An asterisk (*) indicates significance (p≤0.05).
Mechanical testing of the intact fascicle did not result in a decrease in the cellular viability ratio. This was inconsistent with the observation that low levels of strain cause cellular damage in whole ligament (Provenzano, Heisey, Hayashi, Lakes, and Vanderby, 2002). However, the lack of cell death here may be explained by the hierarchical level and the location of the imaged cells. While our cells were near the fascicle surface (limited by the imaging technique), the fascicles were not located directly on the surface of the tendon because the epitenon and endotenon were removed to allow better imaging of fascicular cells. Provenzano et al. imaged cells on the surface of a whole ligament (Provenzano et al., 2002). Cells within tendon fascicles may be more robust than those on the exterior of a ligament (Alberts et al., 2002).
Although mechanical testing of intact specimens did not decrease cellular viability, both single and double laceration caused considerable cell necrosis after mechanical testing compared to control sub-groups. Cell death in both laceration groups was localized to a longitudinal plane spanning the length of the fascicle. Comparing the double laceration group, which allows primarily shear load transfer, to the single laceration group, suggests that a partial laceration creates a plane of high shear load. This result supports previous studies that conclude shear load transfer must be present around partial lacerations to explain the disproportional drop in mechanical properties as a function of laceration depth (Kondratko et al., 2012; Pensalfini et al., 2014).
Although cells can generally withstand small amounts of normal strains, axial or shear, during tendon loading, cells are not well adapted for an excessive amount of these loading mechanisms. Therefore, cell death due to the exaggerated shear loading environment created here may be caused by high strain on the cell plasma membrane resulting in its disruption, necrosis, or subsequent apoptosis (Miller, Connizzo, Feeney, and Soslowsky, 2012; Provenzano et al., 2002; Scott et al., 2005). Upon cell death, factors stored in the cytosol may be released causing inflammation (Hetts, 1998; McNeil, 1993; Wyllie, Kerr, and Currie, 1980), potentially affecting remodeling and repair of the tissue. However, early mobilization of partially lacerated tendons has been reported to be beneficial to repair (Gelberman, Woo, Lothringer, Akeson, and Amiel, 1982; Tanaka, Manske, Pruitt, and Larson, 1995).
This study provides insight into the mechanical and cellular response of tendon fascicles to excess levels of shear loading; however, there are limitations to discuss. All protocols were performed on fascicles from rat tail tendon due to the ease of fascicle removal and the magnitude of literature for comparison; similar information should be studied on other tendons. While the double laceration group was included to investigate shear load transfer capability and cellular response between fibers, without measuring the fiber alignment within the fascicle, the fascicle diameter, or depth of lacerations, the authors cannot be confident that axial loading of some remaining intact fibers did not provide a portion of the strength of those specimens. It has been reported that fibrils within equine energy storing and positional tendon fascicles exhibit a helical orientation; however, the positional tendon fascicle, which is more similar to the rat tail tendon, exhibits less of a helical pitch angle (Thorpe et al., 2013). This helical orientation suggests that a portion of the fibrils or fibers may bypass the overlapping lacerations. Although, it is clear through the cellular viability portion of this study, that an abnormal loading mechanism (excess shear) is causing significant cell death in that experimental group. During mechanical testing and setup, evaporation of PBS from the surface of the fascicle occurred quickly due to the fascicle size, affecting cellular viability in some instances and resulting in small drift during preload. As a result, preload was not held long to allow for complete stabilization. However, due to the low preload compared to values seen during testing and the short testing time, the small drift seen while preloading was unlikely to affect the overall mechanical results. Another limitation, that the control, untested specimens were not loaded into the mechanical testing system, was incorporated into the protocol to minimize handling and potential dehydration but may have caused methodological differences between the groups. However, because no difference in cellular viability was seen between intact mechanically loaded and their not loaded control specimens, it is believed that little effect on cell death was actually present. Finally, despite care taken to prevent unnecessary handling of the fascicles, some localized areas of cell death were seen in the LIVE/DEAD analysis. Since these areas were random and occurred in all groups, they were assumed to not affect results in this manuscript. If a fascicle had large areas of cell death deemed due to dehydration or handling, it was removed from the study.
Despite these experimental limitations, this study provides insight into the shear loading mechanism in tendon. Shear occurs as a primary loading mechanism during normal loading situations (e.g. sliding between hierarchical levels, load transfer around fiber breakage or enzymatic remodeling, or during insertion site rotation during joint movement). It is also an integral aspect of loading during tendon injury, such as partial tear, laceration, or tendinopathy, where it is present at an exaggerated level. Shear is the main loading mechanism after tendon lengthening procedures used to treat diabetic plantar forefoot ulceration (Mueller et al., 2003). Experimentally, the double laceration group provided mechanical and cellular insight into these lengthening procedures and a baseline for the behaviors seen in the single laceration case which more closely mimics partial tear injuries. Although surgical repair of injured tendon is primarily based upon strength of the damaged tendon (Bishop et al., 1986; Dobyns et al., 1982; Hariharan et al., 1997; Manning, Spiguel, and Mass, 2010; McCarthy et al., 1995; Tan et al., 2003), this study suggests that cellular response should not be overlooked, and needs further investigation.
Conclusions
This study describes mechanical and cellular response to a laceration model focusing on shear loading within rat tail tendon fascicles. With the exception of the viscoelastic parameter, which increased after laceration, mechanical properties decreased with increasing laceration number (single to double laceration). Surprisingly, even with overlapping lacerations in the fascicles, eliminating the majority of full length intact fibers, the fascicle retained about 45% of its intact load capacity when tested at the same grip-to-grip strain level, suggesting that the ability of whole tendon to bear shear load (Kondratko-Mittnacht et al., 2015) is likely limited by mechanisms at other hierarchical levels. Despite the surprising loading capacity maintained during exaggerated shear loading, cellular necrosis increased in both laceration groups, demonstrating significant necrosis in a longitudinal plane, corresponding to the plane of greatest shear. Interestingly, cell damage due to a localized injury, such as the laceration created here, can span a significant distance in both directions from the site of injury.
Acknowledgments
Support by the National Science Foundation (Award CMMI-1432937) and National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health (Award EB008548) are gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also thank Ron McCabe for his technical assistance.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- Abrahams M. Mechanical behaviour of tendon In vitro. Medical and Biological Engineering. 1967;5(5):433–443. doi: 10.1007/BF02479137. http://doi.org/10.1007/BF02479137. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh H, Connizzo BK, Freedman BR, Soslowsky LJ, Shenoy VB. Determining the contribution of glycosaminoglycans to tendon mechanical properties with a modified shear-lag model. Journal of Biomechanics. 2013;46(14):2497–2503. doi: 10.1016/j.jbiomech.2013.07.008. http://doi.org/10.1016/j.jbiomech.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th. Garland Science; 2002. [Google Scholar]
- Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, Miller L. Mechanical load stimulates expression of novel genesin vivoandin vitroin avian flexor tendon cells. Osteoarthritis and Cartilage. 1999;7(1):141–153. doi: 10.1053/joca.1998.0169. http://doi.org/10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- Bey MJ, Ramsey ML, Soslowsky LJ. Intratendinous strain fields of the supraspinatus tendon: Effect of a surgically created articular-surface rotator cuff tear. Journal of Shoulder and Elbow Surgery. 2002;11(6):562–569. doi: 10.1067/mse.2002.126767. [DOI] [PubMed] [Google Scholar]
- Bishop AT, Cooney WP, 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. The Journal of Trauma. 1986;26(4):301–312. doi: 10.1097/00005373-198604000-00001. [DOI] [PubMed] [Google Scholar]
- Child S, Bryant AL, Clark RA, Crossley KM. Mechanical Properties of the Achilles Tendon Aponeurosis Are Altered in Athletes With Achilles Tendinopathy. The American Journal of Sports Medicine. 2010;38(9):1885–1893. doi: 10.1177/0363546510366234. http://doi.org/10.1177/0363546510366234. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biology. 1999;18(5):417–426. doi: 10.1016/s0945-053x(99)00039-6. http://doi.org/10.1016/S0945-053X(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minnesota Medicine. 1982;65(1):27–32. [PubMed] [Google Scholar]
- Duenwald-Kuehl S, Kondratko J, Lakes R, Vanderby R. Damage Mechanics of Porcine Flexor Tendon: Mechanical Evaluation and Modeling. Annals of Biomedical Engineering. 2012;40(8):1692–1707. doi: 10.1007/s10439-012-0538-z. http://doi.org/10.1007/s10439-012-0538-z. [DOI] [PubMed] [Google Scholar]
- Duenwald-Kuehl S, Lakes R, Vanderby R., Jr Strain-induced damage reduces echo intensity changes in tendon during loading. Journal of Biomechanics. 2012;45(9):1607–1611. doi: 10.1016/j.jbiomech.2012.04.004. http://doi.org/10.1016/j.jbiomech.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenwald SE, Vanderby R, Lakes RS. Viscoelastic Relaxation and Recovery of Tendon. Annals of Biomedical Engineering. 2009;37(6):1131–1140. doi: 10.1007/s10439-009-9687-0. [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Weiss JA, Rosenberg TD. Strain in the human medial collateral ligament during valgus loading of the knee. Clinical Orthopaedics and Related Research. 2001;391:266–274. doi: 10.1097/00003086-200110000-00031. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Woo SL-Y, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. The Journal of Hand Surgery. 1982;7(2):170–175. doi: 10.1016/s0363-5023(82)80083-x. http://doi.org/10.1016/S0363-5023(82)80083-X. [DOI] [PubMed] [Google Scholar]
- Gupta HS, Seto J, Krauss S, Boesecke P, Screen HRC. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. Journal of Structural Biology. 2010;169(2):183–191. doi: 10.1016/j.jsb.2009.10.002. http://doi.org/10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Qvortrup K, Bojsen-Moller J, Krogsgaard M, Koskinen S, Magnusson SP. Lateral force transmission between human tendon fascicles. Matrix Biology. 2008;27(2):86–95. doi: 10.1016/j.matbio.2007.09.001. http://doi.org/10.1016/j.matbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. The Journal of Hand Surgery. 1997;22(6):1011–1015. doi: 10.1016/S0363-5023(97)80040-8. http://doi.org/10.1016/S0363-5023(97)80040-8. [DOI] [PubMed] [Google Scholar]
- Hetts SW. To die or not to die: An overview of apoptosis and its role in disease. The Journal of the American Medical Association. 1998;279(4):300–307. doi: 10.1001/jama.279.4.300. http://doi.org/10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- Hoke M. An Operation for the Correction of Extremely Relaxed Flat Feet. The Journal of Bone & Joint Surgery. 1931;13(4):773–783. [Google Scholar]
- Hosaka Y, Teraoka H, Yamamoto E, Ueda H, Takehana K. Mechanism of Cell Death in Inflamed Superficial Digital Flexor Tendon in the Horse. Journal of Comparative Pathology. 2005;132(1):51–58. doi: 10.1016/j.jcpa.2004.06.006. http://doi.org/10.1016/j.jcpa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Khodabakhshi G, Walker D, Scutt A, Way L, Cowie RM, Hose DR. Measuring three-dimensional strain distribution in tendon. Journal of Microscopy. 2013;249(3):195–205. doi: 10.1111/jmi.12009. http://doi.org/10.1111/jmi.12009. [DOI] [PubMed] [Google Scholar]
- Kjær M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiological Reviews. 2004;84(2):649–698. doi: 10.1152/physrev.00031.2003. http://doi.org/10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kjær M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scandinavian Journal of Medicine & Science in Sports. 2009;19(4):500–510. doi: 10.1111/j.1600-0838.2009.00986.x. http://doi.org/10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Kondratko J, Duenwald-Kuehl S, Lakes R, Vanderby R. Mechanical Compromise of Partially Lacerated Flexor Tendons. Journal of Biomechanical Engineering. 2012;135(1) doi: 10.1115/1.4023092. 011001–1–011001–8. http://doi.org/10.1115/1.4023092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratko-Mittnacht J, Duenwald-Kuehl S, Lakes R, Vanderby R., Jr Shear load transfer in high and low stress tendons. Journal of the Mechanical Behavior of Biomedical Materials. 2015;45:109–120. doi: 10.1016/j.jmbbm.2015.01.021. http://doi.org/10.1016/j.jmbbm.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsgaard M, Nielsen CH, Hegnsvad S, Aagaard P, Magnusson SP. Mechanical properties of the human Achilles tendon, in vivo. Clinical Biomechanics. 2011;26(7):772–777. doi: 10.1016/j.clinbiomech.2011.02.011. http://doi.org/10.1016/j.clinbiomech.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. Journal of Orthopaedic Research. 2009;27(12):1596–1602. doi: 10.1002/jor.20938. http://doi.org/10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Ø, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive Apoptosis in Patellar Tendinopathy in Athletes. The American Journal of Sports Medicine. 2007;35(4):605–611. doi: 10.1177/0363546506295702. http://doi.org/10.1177/0363546506295702. [DOI] [PubMed] [Google Scholar]
- Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biology. 2013;32(3–4):169–177. doi: 10.1016/j.matbio.2013.01.003. http://doi.org/10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Lochner FK, Milne DW, Mills EJ, Groom JJ. In vivo and in vitro measurement of tendon strain in the horse. American Journal of Veterinary Research. 1980;41(12):1929–1937. [PubMed] [Google Scholar]
- Lynch HA, Johannessen W, Wu JP, Jawa A, Elliott DM. Effect of Fiber Orientation and Strain Rate on the Nonlinear Uniaxial Tensile Material Properties of Tendon. Journal of Biomechanical Engineering. 2003;125(5):726–731. doi: 10.1115/1.1614819. http://doi.org/10.1115/1.1614819. [DOI] [PubMed] [Google Scholar]
- Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. The Journal of Hand Surgery. 2010;35(1):11–18. doi: 10.1016/j.jhsa.2009.10.015. http://doi.org/10.1016/j.jhsa.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Mazzocca AD, Rincon LM, O’Connor RW, Obopilwe E, Andersen M, Geaney L, Arciero RA. Intra-articular Partial-Thickness Rotator Cuff Tears. The American Journal of Sports Medicine. 2008;36(1):110–116. doi: 10.1177/0363546507307502. http://doi.org/10.1177/0363546507307502. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. The Journal of Hand Surgery. 1995;20(5):795–800. doi: 10.1016/S0363-5023(05)80434-4. http://doi.org/10.1016/S0363-5023(05)80434-4. [DOI] [PubMed] [Google Scholar]
- McNeil PL. Cellular and molecular adaptations to injurious mechanical stress. Trends in Cell Biology. 1993;3(9):302–307. doi: 10.1016/0962-8924(93)90012-p. http://doi.org/10.1016/0962-8924(93)90012-P. [DOI] [PubMed] [Google Scholar]
- Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GaC. Cytokines and apoptosis in supraspinatus tendinopathy. Journal of Bone & Joint Surgery, British Volume. 2009;91-B(3):417–424. doi: 10.1302/0301-620X.91B3.21652. http://doi.org/10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- Miller KS, Connizzo BK, Feeney E, Soslowsky LJ. Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. Journal of Biomechanics. 2012;45(12):2061–2065. doi: 10.1016/j.jbiomech.2012.06.006. http://doi.org/10.1016/j.jbiomech.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles Tendon Lengthening on Neuropathic Plantar Ulcers: A Randomized Clinical Trial. The Journal of Bone & Joint Surgery. 2003;85(8):1436–1445. [PubMed] [Google Scholar]
- Pensalfini M, Duenwald-Kuehl SE, Kondratko-Mittnacht JR, Lakes R, Vanderby R., Jr Evaluation of Global Load Sharing and Shear-lag Models to Describe Mechanical Behavior in Partially Lacerated Tendons. Journal of Biomechanical Engineering. 2014 doi: 10.1115/1.4027714. http://doi.org/10.1115/1.4027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Heisey D, Hayashi K, Lakes R, Vanderby R. Subfailure damage in ligament: a structural and cellular evaluation. Journal of Applied Physiology. 2002;92(1):362–371. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- Purslow PP. The shear modulus of connections between tendon fascicles; Science and Technology for Humanity (TIC-STH), 2009 IEEE Toronto International Conference; 2009. pp. 134–136. http://doi.org/10.1109/TIC-STH.2009.5444520. [Google Scholar]
- Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philosophical Transactions of the Royal Society of London B. Biological Sciences. 2002;357(1418):191–197. doi: 10.1098/rstb.2001.1033. http://doi.org/10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby BJ, Hirai N, Spikes JD, Eyring H. The Mechanical Properties of Rat Tail Tendon. The Journal of General Physiology. 1959;43(2):265–283. doi: 10.1085/jgp.43.2.265. http://doi.org/10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon ML, Pinney SJ, Bergeyk AV, Hazelwood S. Surgical Anatomy and Accuracy of Percutaneous Achilles Tendon Lengthening. Foot & Ankle International. 2006;27(6):411–413. doi: 10.1177/107110070602700604. http://doi.org/10.1177/107110070602700604. [DOI] [PubMed] [Google Scholar]
- Scott A, Khan KM, Heer J, Cook JL, Lian O, Duronio V. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. British Journal of Sports Medicine. 2005;39(5):e25–e25. doi: 10.1136/bjsm.2004.015164. http://doi.org/10.1136/bjsm.2004.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screen HRC. Investigating load relaxation mechanics in tendon. Journal of the Mechanical Behavior of Biomedical Materials. 2008;1(1):51–58. doi: 10.1016/j.jmbbm.2007.03.002. http://doi.org/10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Bader DL, Lee DA, Shelton JC. Local Strain Measurement within Tendon. Strain. 2004;40(4):157–163. http://doi.org/10.1111/j.1475-1305.2004.00164.x. [Google Scholar]
- Screen HRC, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proceedings of the Institution of Mechanical Engineers, Part H. Journal of Engineering in Medicine. 2004;218(2):109–119. doi: 10.1243/095441104322984004. http://doi.org/10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Toorani S, Shelton JC. Microstructural stress relaxation mechanics in functionally different tendons. Medical Engineering & Physics. 2013;35(1):96–102. doi: 10.1016/j.medengphy.2012.04.004. http://doi.org/10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Szczesny SE, Elliott DM. Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomaterialia. 2014;10(6):2582–2590. doi: 10.1016/j.actbio.2014.01.032. http://doi.org/10.1016/j.actbio.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Manske PR, Pruitt DL, Larson BJ. Effect of cyclic tension on lacerated flexor tendons in vitro. The Journal of Hand Surgery. 1995;20(3):467–473. doi: 10.1016/S0363-5023(05)80109-1. http://doi.org/10.1016/S0363-5023(05)80109-1. [DOI] [PubMed] [Google Scholar]
- Tan J, Wang B, Tan B, Xu Y, Tang JB. Changes in Tendon Strength After Partial Cut and Effects of Running Peripheral Sutures. Journal of Hand Surgery (British and European Volume) 2003;28(5):478–482. doi: 10.1016/s0266-7681(03)00168-2. http://doi.org/10.1016/S0266-7681(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Klemt C, Riley GP, Birch HL, Clegg PD, Screen HRC. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomaterialia. 2013;9(8):7948–7956. doi: 10.1016/j.actbio.2013.05.004. http://doi.org/10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Thorpe C, Udeze CP, Birch HL, Clegg PD, Screen HRC. Specialization of tendon mechanical properties results from interfascicular differences. Journal of The Royal Society Interface. 2012:3108–3117. doi: 10.1098/rsif.2012.0362. http://doi.org/10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Johnson GA, Smith BA. Mathematical modeling of ligaments and tendons. Journal of Biomechanical Engineering. 1993;115(4B):468–473. doi: 10.1115/1.2895526. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell Death: The Significance of Apoptosis. In: D JF, Bourne KWJGH, editors. International Review of Cytology. Vol. 68. Academic Press; 1980. pp. 251–306. Retrieved from http://www.sciencedirect.com/science/article/pii/S0074769608623128. [DOI] [PubMed] [Google Scholar]
- Yuan J, Murrell GAC, Wei A-Q, Wang M-X. Apoptosis in rotator cuff tendonopathy. Journal of Orthopaedic Research. 2002;20(6):1372–1379. doi: 10.1016/S0736-0266(02)00075-X. http://doi.org/10.1016/S0736-0266(02)00075-X. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wang M-X, Murrell GAC. Cell death and tendinopathy. Clinics in Sports Medicine. 2003;22(4):693–701. doi: 10.1016/s0278-5919(03)00049-8. [DOI] [PubMed] [Google Scholar]


