Abstract
Human immunodeficiency virus type 1 (HIV-1)-infected mononuclear phagocytes (brain macrophages and microglial cells) release proinflammatory cytokines and chemokines. Elevated levels of chemokine CC motif ligand 2 (CCL2, known previously as monocyte chemoattractant protein-1) have been detected in serum and cerebrospinal fluid (CSF) of HIV-1-infected individuals and the raised CCL2 in the CSF correlates with HIV-1-associated neurocognitive disorders. To understand how elevated CCL2 induces HIV-1-associated neuropathy, we studied effects of CCL2 on excitatory postsynaptic current (EPSCs) in the CA1 region of rat hippocampal brain slices using whole-cell patch recording techniques. The AMPA receptor (AMPAR)-mediated EPSC (EPSCAMPAR) and N-Methyl-D-aspartate (NMDA) receptor (NMDAR)-mediated EPSCs (EPSCNMDAR) were isolated pharmacologically. Bath application of CCL2 produced a significant enhancement of the amplitudes of EPSCs, EPSCAMPAR and EPSCNMDAR. Further studies revealed that CCL2 potentiated NMDAR subtype NR2A-mediated EPSC (EPSCNR2AR) and NR2B-mediated EPSC (EPSCNR2BR). To determine the site of action, we recorded spontaneous mini EPSCs (mEPSC) before and during bath application of CCL2. Our results showed that CCL2 decreased inter event interval (IEI) and increased the frequency of mEPSCs without change on the amplitude, suggesting a presynaptic site of CCL2 action. CCL2 was also found to injure primary rat hippocampal neuronal cultures and neuronal dendrites in the CA1 region of hippocampal slices. The CCL2-associated neuronal and dendritic injuries were blocked by a specific NMDAR antagonist or by a CCR2 receptor antagonist, indicating that CCL2-associated neural injury was mediated via NMDARs and/or CCR2 receptors. Taken together, these results suggest a potential role CCL2 may play in HIV-1-associated neuropathology.
Keywords: chemokine, CCL2, NMDA receptors, EPSCs, hippocampus, HIV-1-associated neuropathy
Introduction
Brain infection with human immunodeficiency virus type-1 (HIV-1) often provokes neurocognitive impairment, termed collectively as HIV-1-associated neurocognitive disorders (HAND) (Antinori et al., 2007). The severity of HAND can vary, from asymptomatic to mild neurocognitive impairment and in its most severe form, a debilitating dementia commonly called HIV-associated dementia or HAD (Antinori et al., 2007; Grant, 2008). Although the introduction of combination antiretroviral therapy (cART) has significantly decreased the incidence of HAD, the prevalence of less severe forms of HAND has been on the rise even in the cART era (Heaton et al., 2011; Alfahad and Nath, 2013; Nath, 2015; Watkins and Treisman, 2015). Nevertheless, the precise mechanisms for HAND pathogenesis are still not fully understood. Many studies have shown that HIV-1-infected cells produce soluble immune/inflammatory factors with neurotoxic potential, leading to neural injury and HAND pathogenesis. Among the potential neurotoxic factors is chemokine CC motif ligand 2 (CCL2), formerly known as monocyte chemoattractant protein-1.
CCL2 is a well-characterized β or CC family member of chemokines that plays a crucial role in the recruitment of monocytes into tissues and organs, including the brain (Dhillon et al., 2008; Deshmane et al., 2009). It is produced in response to immune/inflammatory or other nociceptive stimuli by a variety of cell types including, but not limited to, macrophages, microglia, astrocytes and endothelial cells (Deshmane et al., 2009; Yadav et al., 2010; Kim et al., 2014). In addition to its well-established roles in regulating monocyte activation and providing the directional cues for the transmigration of leukocytes, CCL2 also plays an important role in the central nervous system (CNS). In the CNS, CCL2 is expressed in microglial cells, astrocytes and neurons and the levels of expression are often up-regulated in a variety of neuropathological conditions including HAND/HAD. Indeed, elevated levels of CCL2 (5–20ng/ml) have been detected in the cerebrospinal fluid (CSF) of HIV-1-infected individuals (Cinque et al., 1998; Conant et al., 1998; Kelder et al., 1998; Marzocchetti et al., 2005; Shiramizu et al., 2006; Yuan et al., 2013; Thames et al., 2015) and viral infection is one of the main stimuli for CCL2 production (Kim et al., 2014). The elevated levels of CCL2 in the CNS are associated with HIV-1-associated neurological disorders (Marzocchetti et al., 2005; Dhillon et al., 2008; Muratori et al., 2010; Yuan et al., 2013). Nonetheless, how elevated CCL2 induces neuronal injury in HIV-1-infected brain and how the CCL2-mediated neuronal injury contributes to the HAND pathogenesis and progression are still equivocal.
Several lines of evidence indicate that CCL2 is a mediator of excitotoxic brain injury (Sheehan et al., 2007; Yao and Tsirka, 2014). It has been shown that co-administration of CCL2 and a low dose of N-methyl-D-aspartic acid (NMDA) exacerbate NMDA-induced neural injury and functional inhibition of CCL2 protects against NMDA-induced neurotoxicity, suggesting CCL2 potentiation of NMDA-induced neural injury (Galasso et al., 2000). We hypothesize that the elevated CCL2 potentiates NMDA receptor (NMDAR)-mediated responses, leading to neuronal injury. To test this hypothesis, we studied effects of CCL2 on excitatory postsynaptic currents (EPSCs) in the CA1 region of rat hippocampal slices. Our results showed that bath application of CCL2 enhanced the NMDAR-mediated EPSCs (EPSCNMDAR) via a presynaptic mechanism and incubation of hippocampal neurons and slices with CCL2 resulted in neuronal and dendritic injuries that could be blocked with a specific NMDAR antagonist.
Materials and Methods
Animals
Fifteen to 35 d old male Sprague-Dawley rats used for experiments were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed at constant temperature (22°C) and relative humidity (50%) under a regular light-dark cycle (light on at 7:00 AM and off at 5:00 PM) with free access to food and water. All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (IACUC No. 00-062-07).
Chemicals and reagents
Drugs used in this study were CCL2 (R&D Systems, Minneapolis, MN), picrotoxin, tetrodotoxin(TTX), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 2-amino-5-phosphonovalerate (APV), (R)-3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid (R-CPP), ifenprodil, RS102895, and picrotoxin. Picrotoxin and RS102895 were dissolved in dimethyl sulfoxide (DMSO) and the final DMSO concentration in artificial cerebrospinal fluid (ACSF) was less than or equal to 0.1%. CCL2, TTX, CNQX, APV, R-CPP, and ifenprodil were pre-prepared separately in 1000x stock solutions and stored at −20°C refrigerator, thawed on experimental day just before use and diluted to the test concentrations. Drugs were applied onto brain slices via bath perfusion and added to the culture media. For bath perfusion, the time needed for a drug to reach the chamber was about 1 min. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Primary hippocampal neuronal culture
Hippocampal neuronal cultures were prepared from rat embryos using the methods described previously (Blair et al., 2009). Briefly, female Sprague-Dawley rats with 18–19 days of gestation were anesthetized with isoflurane, and embryonic pups were surgically dissected out and decapitated. Hippocampi were harvested under sterile conditions. The hippocampal tissue was enzymatically dissociated in 0.125% trypsin II (Sigma-Aldrich). Isolated neural cells were placed in poly-D-lysine-coated 35 mm plastic culture dishes containing 2 ml of neurobasal medium to a culture surface cell density of 5 x 105/ml. The cultures were maintained in neurobasal medium supplemented with B27 (2%, v/v, Invitrogen), glutamine (0.5mM) and 1% penicillin/streptomycin for at least 7–10 days before being used for experiments.
Hippocampal brain slices
Hippocampal brain slices (400μm in thickness) were prepared as previously described (Xiong et al., 1996). Young animals were used for preparation of hippocampal slices mainly because the brain tissues from young animals are more resistant to hypoxic/ischemic challenge. Briefly, animals were anesthetized with isoflurane and decapitated. The brain was quickly removed from cranial cavity and placed into an ice-cold (~4°C) oxygenated ACSF contained (in mM): NaCl 124.0, KCl 3.0, CaCl2 2.0, MgCl2 2.0, NaH2PO4 1.25, NaHCO3 26.0, glucose 10.0 and TTX 0.1μM. ACSF was saturated with 95% O2 and 5% CO2 and had a pH of 7.35–7.45. The hippocampi were dissected free, and transverse hippocampal slices were cut using a tissue chopper. The slices were then incubated in oxygenated ACSF at room temperature for at least 1 h before use.
Electrophysiology
During electrophysiological experiments, a single hippocampal slice was transferred into the recording chamber each time and superfused with ACSF at a constant flow rate of 2.5 ml/min. The temperature of the ACSF was maintained at 30°C ± 1°C with an automatic temperature controller (Warner Instrument Corp., Hamden, CT). Whole-cell patch clamp recordings were made from CA1 neurons using a “blind” method. The neuronal cells recorded were voltage-clamed at −70mV and EPSCs were evoked by electrical stimulation of the Schaffer collateral/commissural pathway (0.05 Hz, 40 μs in duration, 30–100 μA in intensity) with a bipolar tungsten electrode and amplified by an Axopatch-1D amplifier (Molecular Devices, Union City, CA). The mini EPSCs (mEPSCs) were recorded in gap-free mode from cells voltage-clamed at −60 mV. The recording electrodes were made from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) and had a resistance of 6–9 MΩ when filled with pipette solution contained (in mM): K-gluconate 130.0, K-methysulphate 17.5, NaCl 8.0, HEPES 10.0, Mg-ATP 2.0, GTP 0.2, EGTA 0.1, pH 7.25–7.35 (adjusted with KOH), osmolality 292–308 mOsm. Picrotoxin (50μM) was routinely added to ACSF to block the potential inhibition of inhibitory input by CCL2.
Cells with resting membrane potential more negative than −55 mV were used for the study. Each recording trial consisted of an average of three consecutive sweeps. Data were filtered at 1 kHz and digitized at 5 kHz using a Digidata 1322A interface (Molecular Devices). pCLAMP 8.2 software (Molecular Devices) was used for data acquisition and analyses. The series resistance was constantly monitored by delivering a hyperpolarizing voltage pulse during recording and the cells with > 20% changes in access resistance were excluded from the analysis.
Microtubule associated protein 2(MAP-2) staining
Four Sprague-Dawley rats were used in this study. Hippocampal brain slices prepared from each animal was randomly divided into four groups (4 slices in each group) and incubated with either normal ACSF (control), or ACSF contained CCL2 (CCL2, 20ng/ml, the same concentration in the following groups unless indicated), or CCL2 and 50μM AP-V (CCL2+APV), or CCL2 and 10 μM RS102895, a selective CCR2 receptor antagonist (CCL2+ RS102895) at room temperature for 6–8 h. The ACSF contained the above mentioned reagents was continuously oxygenated (95% O2 and 5% CO2) during incubation. Then hippocampal slices were fixed in 4% paraformaldehyde for 24 h followed by dehydrations in 15% and 30% sucrose overnight, respectively. The slices were then embedded in Optimal Cutting Temperature (OCT) compound and stored in −80°C freezer. Serial frozen sections (30μm in thickness) were then cut and the dendritic arbors were labeled with rabbit anti rat antibodies against MAP-2 (Chemicon, Temecula, CA). The second antibody was goat anti rabbit IgG (Invitrogen, Molecular probes). Sections were viewed with an E800 Nikon Eclipse microscope (Nikon, Japan), and images were captured with a MagnaFire digital camera and software (Optronics, Goleta, CA).
Measurement of neuronal cell viability
Experiments were performed in triplicate and total survival hippocampal neural cells in culture were determined by Hoechst 33342 (Sigma) staining and counted from five different visual fields. CCL2, CCL2+CNQX(10μM), CCL2+AP-V(100μM), CCL2+CNQX(10μM)+AP-V(100μM) were added to hippocampal neuronal cultures for 24 h, respectively, and then the cultured cells were incubated at 37°C with H 33342 (5μg/ml) for additional 30min, washed with PBS 3 times, and fixed for 15 min with 4% paraformaldehyde. After fixation, cultures were gently washed 3 times and photographed under a fluorescence microscope. Cells with bright blue fragmented nuclei, representing condensation of chromatin, were identified as injured cells and analyzed quantitatively by cell counting.
Data Analyses
Data were analyzed and displayed using ClampFit 8 (Molecular Devices, Mini Analysis Program (Synaptosoft Inc.,Decatur, GA) and Origin 8.5 (Northampton, MA). All numerical data were expressed as mean ± SEM unless otherwise indicated. Statistical significance was determined using ANOVA, two-tailed Student’s t-tests, or the Kolmogorov-Smirnov-test. The level of significance was determined at p < 0.05.
Results
CCL2 Enhancement of NMDA receptor- and AMPA receptor-mediated EPSCs in the CA1 region of hippocampal slices
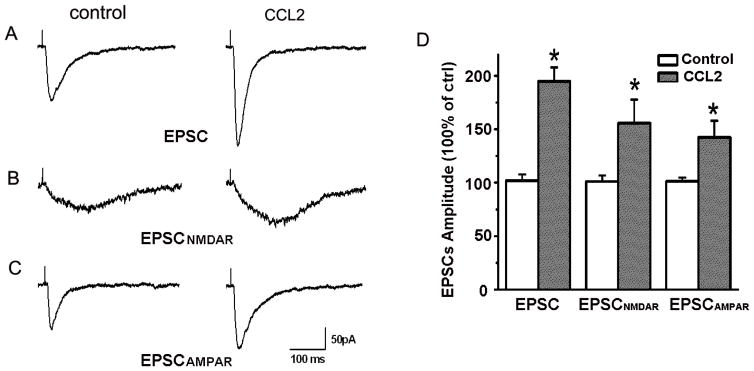
In a previous study, we demonstrated that CCL2 (20ng/ml) enhanced EPSCs recorded in the CA1 region of rat hippocampal slices in a concentration-dependent manner (it had no apparent effect at concentrations of 0.2ng/ml and 2ng/ml) (Zhou et al., 2011). In consistent with previous results, we found that bath application of CCL2 (20ng/ml) produced an increase of EPSCs to 194.82±13.05% of basal level (n=17, p<0.05; Fig.1 A, D). As the hippocampal excitatory synaptic transmission is mainly mediated by AMPA receptors and NMDA receptors, we further examined the effects of CCL2 on pharmacologically isolated AMPA receptor-mediated EPSCs (EPSCAMPAR) and NMDA receptor-mediated EPSCs (EPSCNMDAR) in the CA1 region of rat hippocampal slices.
Figure 1. CCL2 enhancement of EPSCs in the CA1 region of hippocampal slices.
Panels A–C are representative EPSCs recorded during control (left) and bath perfusion of CCL2 (right) in the absence (A) or presence of AMPA receptor antagonist CNQX (B) or NMDA receptor antagonist AP-V (C) in the perfusate. Note the bath perfusion of slices with CCL2 produced an enhancement of EPSCs, EPSCNMDAR and EPSCAMPAR. Panel D shows the average amplitudes of EPSCs, EPSCNMDAR and EPSCAMPAR expressed as percentages of corresponding controls. Note that CCL2 significantly enhanced EPSCs (n=17), EPSCNMDAR(n=13) and EPSCAMPAR (n=9). *p < 0.05, vs control, #p < 0.05 vs CCL2.
To record EPSCAMPAR and EPSCNMDAR, the cells were held at −70 mV and −50mV, respectively. Our results showed bath application of CCL2 significantly increased the EPSCAMPAR to 142.35±15.57% of basal level (M±SD, n=9, p<0.05, Fig.1 C, D) in presence of AP-V (50μM) in normal ACSF. In contrast, application of CCL2 significantly increased EPSCNMDAR to 155.60±22.12% of basal level (M±SD, n=13, p<0.05; Fig.1 B, D) in the presence of 10 μM CNQX and 1μM glycine in Mg2+ free ACSF. These results indicate that CCL2 enhanced both EPSCAMPAR and EPSCNMDAR in the hippocampus. Although CCL2 exhibited a stronger effect on EPSCNMDAR than EPSCAMPAR, the difference is not statistical significant (t(20)=0.11).
Effects of CCL2 on subtype NMDA receptor-mediated EPSCs in CA1 region of hippocampal slices
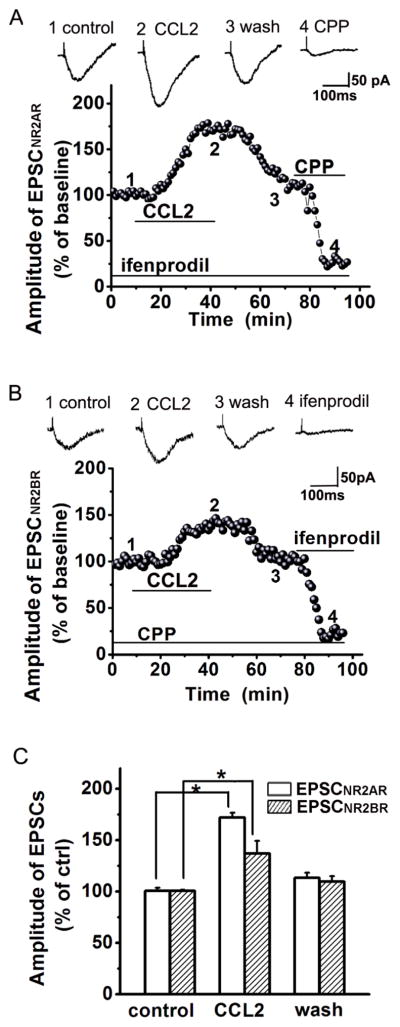
Studies have shown that NR2A receptors (NR2AR) and NR2B receptors (NR2BR) are the major subtypes of NMDARs in the hippocampus (Monyer et al., 1994; Kim et al., 2005; Chen et al., 2007). To evaluate the effects of CCL2 on NR2AR-mediated EPSCs (EPSCNR2AR) and NR2BR-mediated EPSCs (EPSCNR2BR), we recorded EPSCNR2AR in the presence of NR2BR antagonist ifenprodil in the perfusate (10μM, which has a high affinity for NR2BRs with an IC50=0.34μM and IC50 =146μM for NR2ARs) (Williams, 1993) and EPSCNR2BR in the presence of NR2AR antagonist R-CPP in the perfusate (1μM, which has ~7-fold greater selectivity for NR2ARs compared with NR2BRs) (Feng et al., 2004; Feng et al., 2005). In the presence of ifenprodil or R-CPP in the perfusate, bath application of CCL2 increased EPSCNR2AR to 160.97 ± 18.32% of control level (n=10, p<0.05 vs control) or EPSCNR2BR to 137.19 ± 12.09% of control level (n=12, p<0.05 vs control), respectively (Fig. 2). CCL2 seems to have a stronger effect on enhancing EPSCNR2AR than EPSCNR2BR, but the difference was not statistically significant (Fig. 2, t(22)=0.28).
Figure 2. Enhancement of EPSCNR2AR and EPSCNR2BR by CCL2.
Panels A and B illustrate the time course and amplitude (% of baseline) of the EPSCNR2AR and EPSCNR2BR recorded, in the presence of ifenprodil (Panel A, to block NR2BR, n=10) and R-CPP (Panel B, to block NR2AR, n=12), from two different neuronal cells in the CA1 region of two different slices taken from the same animal in response to constant current stimulation of Schaffer-collateral fibers (80μA, 40μS, 0.05Hz). Each data point plots the average of three consecutive EPSCs. Note that bath application of CCL2, as indicated a horizontal bar, increased the EPSCNR2AR (A) and EPSCNR2BR (B). Above each time course graph are representative individual EPSCNR2AR (A) and EPSCNR2BR (B) taken from different time points as marked by numbers 1, 2, 3, and 4, respectively. The CCL2-induced increase of EPSCNR2AR or EPSCNR2BR was almost completely blocked by a specific NA2AR antagonist R-CPP (A) or a specific NR2BR blocker ifenprodil (B). Panel C is a bar graph exhibiting the average amplitudes of EPSCNR2AR or EPSCNR2BR before (control), during (CCL2) and post (Wash) bath application of CCL2. CCL2 enhanced both EPSCNR2AR or EPSCNR2BR. All experiments were carried out in the presence of CNQX (10μM) in the perfusate and the cells were voltage clamped at −50mV. *p<0.05.
CCL2 increased the spontaneous occurrence of mEPSCs in the CA1 region of the hippocampal slices
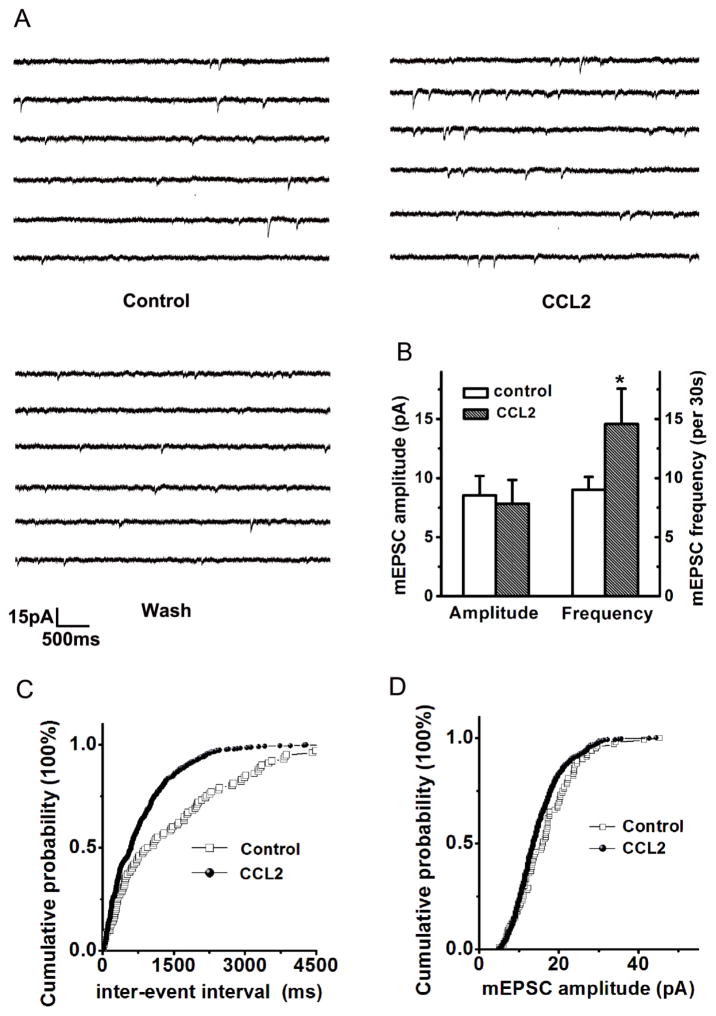
To evaluate the site of action for CCL2 in the enhancement of EPSCs, we recorded spontaneous miniature excitatory postsynaptic currents (mEPSCs) in the hippocampal CA1 pyramidal neurons in the absence and presence of CCL2 in the perfusate. When applied through bath perfusion, CCL2 (20ng/ml) significantly increased spontaneous mEPSCs occurrence from 9.01±1.12 counts/30s before (control) to 14.58 ± 2.97 counts/30s during CCL2 application (CCL2) (Fig. 3, p<0.05, n=9). In contrast, CCL2 had no apparent effect on the amplitude of spontaneous mEPSCs. The average amplitudes before and during bath application of CCL2 were 8.54±1.64pA and 7.84±1.99 pA, respectively (p>0.05, n=9, K-S test, Fig.3 B, D). These results suggest that CCL2 enhancement of EPSCs via a presynaptic mechanism. Analysis of the inter-event interval (IEI) revealed that CCL2 decreased the IEI duration from 1462.42 ± 136.05 ms in control (before bath persufion of CCL2) to 784.71 ± 31.18ms during bath perfusion of CCL2 (CCL2, p<0.05, n=9). The decrease of IEI following CCL2 treatment suggest an increase of either functional synapses and/or probability of synaptic quantal release.
Figure 3. CCL2 Enhancement of EPSCs via a presynaptic mechanism.
Panel A shows the example traces of spontaneous mEPSCs recorded from a neuronal cell in the CA1 region of a hippocampal slice. Bath application of CCL2 significantly increased frequency of spontaneous mEPSCs without apparent effect on the amplitude (Panel B, n=9). Panel C exhibits cumulative distribution of mEPSCs inter-event interval (IEI) showing that CCL2 significantly decreased the IEI, indicating a significant increase in mEPSC frequency during bath application of CCL2 (p<0.05 vs control, n=9). A representative amplitude cumulative histogram is shown in panel D, showing no significant change (Kolmogorov-Smirnov-test) on mEPSC amplitude during bath application of CCL2.
CCL2 induced hippocampal neuronal injury
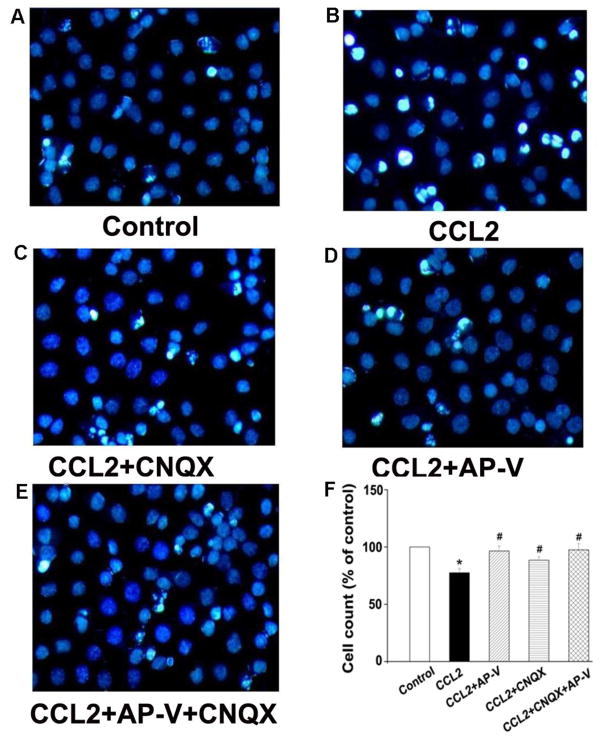
As over-activation of NMDA receptors causes neuronal injury and our results showed CCL2 increased EPSCNMDAR, we further examined the effects of CCL2 on cell viability in cultured rat hippocampal neurons. To mimic its chronic action under diseased conditions, CCL2 (20ng/ml) was added to neuronal cultures for 24 h before analysis of cell viability. As shown in Fig 4, the amount of cellular survival in the control group was defined as survival rate 100%. Addition of CCL2 to the culture media substantially enhanced the number of bright blue fragmented nuclei showing condensation of chromatin for injured neurons, and produced a significant reduction on neuronal viability (67.5±2.7%) in comparison with the control group which was 100% (p<0.05, n=3). The CCL2-induced reduction of neuronal viability was attenuated by pretreatment of the neuronal cultures with AP-V, CNQX or AP-V+CNQX (AP-V group: 96.5±3.1%; CNQX group: 78.6±2.2%; AP-V+CNQX group: 99.5±2.9%; vs. CCL2 group: 67.5±2.7%, p<0.05, n=3), demonstrating that CCL2 induces neuronal injury mainly via both NMDA and AMPA receptors.
Figure 4. Attenuation of CCL2-induced neuronal injury by Hoechst staining.
Panels A–E are primary hippocampal neurons untreated (control) or incubated with CCL2, CCL2+CNQX, CCL2+AP-V, or CCL2+AP-V+CNQX as indicated. The injured cells were quantified after staining with Hoechst 33342. Survival rates were calculated by counting Hoechst-stained cells (bright blue) and total cells from five different visual fields in each dish contained cultured neurons are shown in panel F. CCL2 significantly decreased neuronal survival rates and the CCL2-induced reduction of survival rates were blocked by a NMDA receptor antagonist AP-V and by an AMPA receptor blocker CNQX as well. Addition of CNQX did not further improve the neuronal survival rates under existence of CCL2 and AP-V in the culture media, suggesting the CCL2-induced neuronal injury was largely mediated via NMDA receptors. * p<0.05 vs control, #p<0.05 vs CCL2. Experiments were done in three triplicates. Objective magnification: 40×
Attenuation of CCL2-induced loss of MAP2 from CA1 dendrites by an NMDAR antagonist or a CCR2 receptor antagonist
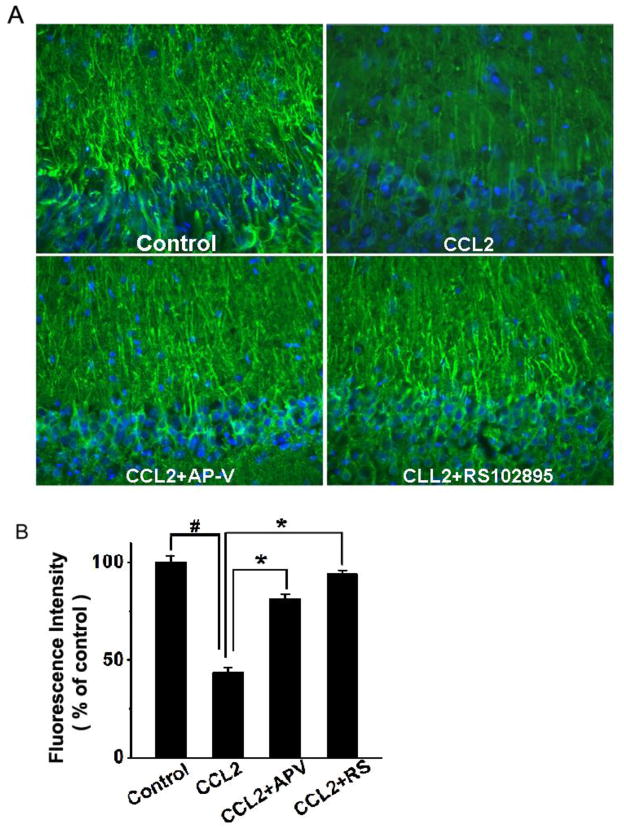
To further examine CCL2 injury of hippocampal neurons via NMDA receptors, we assayed its detrimental effect on rat hippocampal brain slices and evaluated the protective effects of AP-V (50μM) and RS102895 (10μM, a specific CCR2 receptor antagonist). Incubation of hippocampal slices with CCL2 (20ng/ml) for 6 – 8 h produced a significant loss of MAP-2 in the CA1 region of the hippocampal slices as revealed by an evident reduction of immunoreactivity for MAP2 (Fig. 5). Notable difference in MAP-2 staining was visible in apical dendrites. As MAP2 is localized in the neuronal dendritic compartment and it is considered a marker of structural integrity(Di Stefano et al., 2001; Hoskison and Shuttleworth, 2006; Hoskison et al., 2007), the loss of dendritic MAP2 suggests a detrimental effect of CCL2 on neuronal dendrites in the CA1 region of the hippocampal slices. In contrast, addition of AP-V or RS102895 to the incubation solution, which was the ACSF, significantly increased MAP-2 expression in the CA1 region, demonstrating that blockade of NMDARs or CCR2 receptors attenuated CCL2-induced loss of MAP2 (Fig. 5). Densitometry analyses revealed the expression levels of MAP-2 in the CA1 dendrite region were 81.1±2.4% or 93.8±1.9% of control (untreated) when the slices were treated with CCL2+ AP-V or CCL2+RS102895, respectively. In comparison with the MAP-2 expression level detected on CCL2-treated slices (43.2±3.0% of control) (Fig. 5 B), the differences were statistically significant, indicating blockade of NMDARs or CCR2 receptors attenuated CCL2-induced neuronal dendritic injury in the CA1 region of the hippocampus.
Figure 5. Attenuation of CCL2-induced dendritic injury in the CA1 region of hippocampal slices as revealed by MAP-2 staining.
Panel A shows the representative sections of the CA1 region immunostained with antibodies to MAP-2 in different experimental conditions as indicated. The same region of the CA1 was selected in all samples. MAP-2 expression was reduced in the CCL2-treated group (CCL2) as compared with the untreated group (control). The CCL2-induced reduction of MAP-2 expression was attenuated by either a NMDA receptor antagonist AP-V (CCL2+AP-V), or a CCR2 receptor antagonist RS102895 (CCL2+RS102895), demonstrating that CCL2 induces dendritic injury via NMDA receptor and CCR2 receptor which are expressed in the hippocampus. Quantification of fluorescence intensities using ImageJ software is shown in panel B. Note that CCL2 significantly reduced the fluorescence intensity and such a reduction was significantly attenuated by AP-V (CCL2+AP-V) or RS102895 (CCL2+RS). Values are expressed as mean±SE from five independent experiments. # p< 0.05 vs control, * p < 0.05 vs CCL2 group. Objective magnification: 20×
Discussion
CCL2 is one of the most studied chemokines of the CC chemokine family and plays an important role in the CNS, in addition to its well established role in the immune system (Deshmane et al., 2009; Reaux-Le Goazigo et al., 2013). CCL2 and its cognate receptor CCR2 are expressed in the CNS and elevated levels of CCL2 have been detected in the cerebrospinal fluid (CSF, 5–20ng/ml) (Cinque et al., 1998; Conant et al., 1998; Kelder et al., 1998; Marzocchetti et al., 2005; Shiramizu et al., 2006; Yuan et al., 2013; Thames et al., 2015) and brain of HIV-1-infected patients with HIV-1 encephalitis, implicating CCL2 may be associated with HAND pathogenesis. Indeed, clinical studies have revealed that elevated levels of CCL2 do correlate with neuropathology seen in patients with HAND (Cinque et al., 1998; Conant et al., 1998; Kelder et al., 1998; Ragin et al., 2006), even in the era of cART (Yuan et al., 2013; Thames et al., 2015). How does the raised CCL2 in the CSF and brain causes HAND remains to be determined. In the present study, we tested our hypothesis that elevated CCL2 potentiates NMDA receptor-mediated responses resulting in neuronal injury. We found that CCL2 enhanced EPSCAMPAR and EPSCNMDAR in the CA1 region of rat hippocampal slices. The CCL2-associated enhancement of EPSCs was mediated through a presynaptic mechanism as CCL2 decreased IEI duration and altered spontaneous mEPSC occurrence (frequency) without significant influence on the amplitude. Further studies revealed that CCL2 enhanced both EPSCNR2AR and EPACNR2BR, and such enhancements were blocked by a specific NR2AR blocker R-CPP and a specific NR2BR blocker ifenprodil, respectively, suggesting that CCL2 interacts with NMDA receptors. Attribution of CCL2-medied enhancement of EPSCNMDAR to neuronal injury was demonstrated by experimental results that the CCL2-associated neural injuries both in primary neuronal culture and hippocampal slices were attenuated by a specific NMDA receptor antagonist or a specific CCR2 receptor antagonist, suggesting a detrimental role CCL2 may play in the CNS, especially when its expression levels are upregulated under neurological conditions(Cinque et al., 1998; Ragin et al., 2006; Yuan et al., 2013).
NMDAR is a ligand-gated cation channel that comprises one of the major subclasses of glutamate receptors (Studzinski et al., 2015). It has a presumed role in excitatory synaptic transmission, learning, memory, and nociceptive pathways (Huo et al., 2015; Wang et al., 2015). Excessive stimulation of the NMDAR induces neuronal injury via an excitotoxic mechanism (Bonfoco et al., 1995; Prentice et al., 2015). Studies have shown that CCL2 potentiates NMDA-induced neural injury in the hippocampus and enhances AMPA and NMDA receptor currents in spinal neurons (Galasso et al., 2000; Gao et al., 2009). In a manner consistent with these aforementioned studies, we found that CCL2 enhanced AMPA and NMDA receptor currents in the CA1 region of rat hippocampal slices and induced neural injury on primary rat neuronal cultures which was blocked mainly by a specific NMDAR antagonist AP-V. Incubation of hippocampal slices with CCL2 produced neuronal dendritic injury in the CA1 region which was also blocked by AP-V. It has been demonstrated that NR2A and NR2B are the two major subtypes of NMDARs in the hippocampus; our further investigation revealed that CCL2 enhanced EPSCNR2AR and EPSCNR2BR. As activation of NR2BRs is believed to induce excitotoxicity and neuronal injury, the CCL2 enhancement of EPSCNR2BR may underlie CCL2-induced neuronal and dendritic injuries observed in this study. It is not clear at present whether CCL2 enhancement of EPSCNR2AR contributes to CCL2-induced neural injury. Considering the role that NR2ARs play in the regulation of normal synaptic transmission, the enhancement of EPSCNR2AR by CCL2 may reflect the modulatory effect of CCL2 on synaptic transmission in the hippocampal slices as observed in our previous study (Zhou et al., 2011).
It is worth pointing out that in this study we focused on CCL2-mediated enhancement of EPSCNMDAR although the EPSCAMPAR was also increased by CCL2 (Fig. 1). The reason for focusing on NMDARs is because activation of NMDARs and resultant Ca2+ influx is a well-established mechanism for neuronal excitotoxicity (Fan et al., 2014; Parsons and Raymond, 2014; Fujikawa, 2015). Though bath application of CCL2 produced a transient enhancement of EPSCNMDAR, such an enhancement may exist during a chronic disease condition like brain infection with HIV-1 leading to over-activation of NMDARs, increase of intracellular Ca2+ concentration, and consequently neuronal injury. Thus, the CCL2-associated potentiation of EPSCNMDAR may underlie HIV-1-associated neuropathology in HIV-1-infected brain since activation of NMDARs is believed to induce excitotoxicity. It is also possible that activation of AMPARs could contribute to CCL2/HIV-1-associated neuronal injury as AMPAR-mediated excitotoxicity has been observed on hippocampal neurons (Ohno et al., 1998; Tomita et al., 2007). We did observe that addition of AMPA receptor antagonist CNQX (10μM) to neuronal culture attenuated CCL2-associated neuronal injury as detected by Hoechst stain (Fig. 4C), though the potency of CNQX was less potent than NMDAR antagonist AP-V. These results suggest that the CCL2-associated neuronal injury was largely mediated by potentiation of NMDAR-mediated effect although AMPAR was also involved in CCL2-associated neuronal injury.
In summary, we have demonstrated that CCL2 enhanced EPSCAMPAR and EPSCNMDAR recorded in the CA1 region of rat hippocampal slices via a presynaptic mechanism. The enhancement of EPSCNMDAR might be associated with CCL2-associated neural injury because the CCL2-associated neural injury was attenuated either by a specific NMDAR antagonist or by a specific CCR2 receptor antagonist, suggesting NMDAR and CCR2 receptor were involved in CCL2-associated neural injury. As elevated levels of CCL2 have been detected in the CSF and brain of HIV-1-infected patients with HAND, the CCL2-mediated enhancement of EPSCNMDAR and resultant neural injury may have implications for the pathogenesis of HIV-1-associated neurological disorders such as HAND.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 NS063878 (HX), the National Natural Science Foundation of China 81360192 (YZ), Guangxi Natural Science Foundation 2012GXNSFCA053004 (YZ), Guangxi Education Department Foundation 201203YB041 (YZ).
Footnotes
Compliance with Ethical Standards All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of University of Nebraska Medical Center, Omaha, NE, USA.
Competing interests The authors declare that they have no competing interests.
References
- Alfahad TB, Nath A. Update on HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep. 2013;13:387. doi: 10.1007/s11910-013-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Elphick MR, Martin BR, DeLorenzo RJ. Prolonged exposure to WIN55,212–2 causes downregulation of the CB1 receptor and the development of tolerance to its anticonvulsant effects in the hippocampal neuronal culture model of acquired epilepsy. Neuropharmacology. 2009;57:208–218. doi: 10.1016/j.neuropharm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007;27:542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Front Biosci. 2008;13:3913–3918. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano G, Casoli T, Fattoretti P, Gracciotti N, Solazzi M, Bertoni-Freddari C. Distribution of map2 in hippocampus and cerebellum of young and old rats by quantitative immunohistochemistry. J Histochem Cytochem. 2001;49:1065–1066. doi: 10.1177/002215540104900818. [DOI] [PubMed] [Google Scholar]
- Fan X, Jin WY, Wang YT. The NMDA receptor complex: a multifunctional machine at the glutamatergic synapse. Front Cell Neurosci. 2014;8:160. doi: 10.3389/fncel.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa DG. The role of excitotoxic programmed necrosis in acute brain injury. Comput Struct Biotechnol J. 2015;13:212–221. doi: 10.1016/j.csbj.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso JM, Liu Y, Szaflarski J, Warren JS, Silverstein FS. Monocyte chemoattractant protein-1 is a mediator of acute excitotoxic injury in neonatal rat brain. Neuroscience. 2000;101:737–744. doi: 10.1016/s0306-4522(00)00399-7. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008;20:33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskison MM, Shuttleworth CW. Microtubule disruption, not calpain-dependent loss of MAP2, contributes to enduring NMDA-induced dendritic dysfunction in acute hippocampal slices. Exp Neurol. 2006;202:302–312. doi: 10.1016/j.expneurol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hoskison MM, Yanagawa Y, Obata K, Shuttleworth CW. Calcium-dependent NMDA-induced dendritic injury and MAP2 loss in acute hippocampal slices. Neuroscience. 2007;145:66–79. doi: 10.1016/j.neuroscience.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo TG, Li WK, Zhang YH, Yuan J, Gao LY, Yuan Y, Yang HL, Jiang H, Sun GF. Excitotoxicity Induced by Realgar in the Rat Hippocampus: the Involvement of Learning Memory Injury, Dysfunction of Glutamate Metabolism and NMDA Receptors. Mol Neurobiol. 2015;51:980–994. doi: 10.1007/s12035-014-8753-2. [DOI] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus- associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, Voskuhl RR. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;274:53–61. doi: 10.1016/j.jneuroim.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzocchetti A, Cingolani A, Giambenedetto SD, Ammassari A, Giancola ML, Cauda R, Antinori A, Luca AD. Macrophage chemoattractant protein-1 levels in cerebrospinal fluid correlate with containment of JC virus and prognosis of acquired immunodeficiency syndrome--associated progressive multifocal leukoencephalopathy. J Neurovirol. 2005;11:219–224. doi: 10.1080/13550280590924539. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Muratori C, Mangino G, Affabris E, Federico M. Astrocytes contacting HIV-1-infected macrophages increase the release of CCL2 in response to the HIV-1-dependent enhancement of membrane-associated TNFalpha in macrophages. Glia. 2010;58:1893–1904. doi: 10.1002/glia.21059. [DOI] [PubMed] [Google Scholar]
- Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21:227–234. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Okada M, Tsutsumi R, Sakamoto S, Yamaguchi T. The AMPA-receptor antagonist YM90K reduces AMPA receptor-mediated excitotoxicity in rat hippocampal cultures. Jpn J Pharmacol. 1998;76:105–108. doi: 10.1254/jjp.76.105. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82:279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Prentice H, Modi JP, Wu JY. Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid Med Cell Longev. 2015;2015:964518. doi: 10.1155/2015/964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Monocyte chemoattractant protein-1 correlates with subcortical brain injury in HIV infection. Neurology. 2006;66:1255–1257. doi: 10.1212/01.wnl.0000208433.34723.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Zhou C, Gravanis I, Rogove AD, Wu YP, Bogenhagen DF, Tsirka SE. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J Neurosci. 2007;27:1738–1745. doi: 10.1523/JNEUROSCI.4987-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Lau E, Tamamoto A, Uniatowski J, Troelstrup D. Feasibility assessment of cerebrospinal fluid from HIV-1-infected children for HIV proviral DNA and monocyte chemoattractant protein 1 alleles. J Investig Med. 2006;54:468–472. doi: 10.2310/6650.2006.06007. [DOI] [PubMed] [Google Scholar]
- Studzinski AL, Barros DM, Marins LF. Growth hormone (GH) increases cognition and expression of ionotropic glutamate receptors (AMPA and NMDA) in transgenic zebrafish (Danio rerio) Behav Brain Res. 2015;294:36–42. doi: 10.1016/j.bbr.2015.07.054. [DOI] [PubMed] [Google Scholar]
- Thames AD, Briones MS, Magpantay LI, Martinez-Maza O, Singer EJ, Hinkin CH, Morgello S, Gelman BB, Moore DJ, Heizerling K, Levine AJ. The role of chemokine C-C motif ligand 2 genotype and cerebrospinal fluid chemokine C-C motif ligand 2 in neurocognition among HIV-infected patients. AIDS. 2015;29:1483–1491. doi: 10.1097/QAD.0000000000000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Byrd RK, Rouach N, Bellone C, Venegas A, O'Brien JL, Kim KS, Olsen O, Nicoll RA, Bredt DS. AMPA receptors and stargazin-like transmembrane AMPA receptor-regulatory proteins mediate hippocampal kainate neurotoxicity. Proc Natl Acad Sci U S A. 2007;104:18784–18788. doi: 10.1073/pnas.0708970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WT, Pan GQ, Zhang ZY, Suo ZW, Yang X, Hu XD. Ht31 peptide inhibited inflammatory pain by blocking NMDA receptor-mediated nociceptive transmission in spinal dorsal horn of mice. Neuropharmacology. 2015;89:290–297. doi: 10.1016/j.neuropharm.2014.09.031. [DOI] [PubMed] [Google Scholar]
- Watkins CC, Treisman GJ. Cognitive impairment in patients with AIDS - prevalence and severity. HIV AIDS (Auckl) 2015;7:35–47. doi: 10.2147/HIV.S39665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Xiong H, Baskys A, Wojtowicz JM. Brain-derived peptides inhibit synaptic transmission via presynaptic GABAB receptors in CA1 area of rat hippocampal slices. Brain Res. 1996;737:188–194. doi: 10.1016/0006-8993(96)00731-7. [DOI] [PubMed] [Google Scholar]
- Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–697. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, Smith D, Li N, Chen D. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol. 2013;19:144–149. doi: 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem. 2011;116:406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.