Abstract
Membrane receptors play important roles in regulating cellular activities. Targeting membrane receptors in cancer cells and understanding their interactions with specific ligands are key for cancer prognosis and therapeutics. However, there is a need to develop new technologies to provide molecular insight into ligand-receptor binding chemistry in cell membrane. Integrin receptors are important membrane receptors that regulate cellular migration, invasion and proliferation in tumors. Integrins have a well-known affinity towards small peptide ligands containing arginine-glycine-aspartate (RGD) sequence and are therefore an attractive model system to study ligand-receptor interactions. We have recently reported a method to detect integrin receptors and study their binding chemistry with cyclic-RGDfC ligand using tip-enhanced Raman scattering (TERS). We have demonstrated that two integrins with similar structures can be differentiated in intact cell membrane, due to the differences in their RGD ligand binding sites, showing the potential of this TERS methodology to study other membrane receptors and their interactions in live cells.
Keywords: Tip-enhanced Raman scattering, integrin, ligand-receptor binding, binding chemistry
While cellular membrane receptors are key molecules that initiate signaling pathways to regulate cellular activities and have been strongly associated with cancer progression, the tools available to identify relevant chemical reactions are limited. Recently we demonstrated that tip enhanced Raman scattering (TERS) may provide new insights into understanding the chemical interactions between specific extracellular molecules (so-called “ligands”).
Many overexpressed membrane receptors have been identified as hallmarks of various types of tumors, leading to diagnosis and treatment strategies that target these receptors [1, 2]. One widely studied example is integrin receptors. These receptors are important cell adhesion receptors that act as bridge molecules for cell-cell and cell-extracellular matrix (ECM) interactions. By binding with ECM ligands, integrins activate intracellular signal transduction pathways in tumors and mediate tumor cell migration and invasion [3]. Because of these biological roles in tumors, integrins have been recognized as molecular markers for targeted cancer imaging [4, 5]. For example, arginine-glycine-aspartate (RGD) peptide conjugated magnetic nanoparticles were found to provide significantly enhanced MRI image contrast at tumor areas, mediated through RGD-integrin interaction, in a xenograft model [6]. In addition, integrins have been targeted for drug developments in therapeutics of human cancers. Preclinical studies have shown that integrin antagonists, including monoclonal antibodies and RGD peptides, can inhibit tumor growth [7, 8]. For example, Cilengitide, a cyclic-RGD based drug molecule, which is a highly potent antagonist of integrins αvβ3, αvβ5 and α5β1, reached clinical phase III trials for treatments of glioblastomas [9], although some limitations of the drug have been reported recently [10].
The pharmacological potential of targeting integrin receptors has drawn great attention. To develop novel drugs with high efficacy and reduced side effects, understanding the interactions between integrins and ligand antagonists (e.g. RGD molecules) is critical [11]. Generally, understanding ligand-receptor binding and targeting pathological cell membrane receptors is key for regulating cellular signaling and activity [12, 13], and for imaging and treating diverse human pathologies such as cancers [14, 15]. In recent years, significant progress has been made on the use of radiolabeled RGD peptides, as radiotracers, to recognize and monitor integrins in cancer patients [16]. Furthermore, interactions between RGD peptides and integrin receptors have been investigated at cellular and molecular levels using advanced microscopic techniques such as fluorescence microscopy [17] and atomic force microscopy [18, 19]. Although these techniques are able to visualize ligand-receptor binding events with high spatial resolution, and bring insights into binding properties like binding affinity and kinetics, none of them provide molecular structural information at ligand-receptor binding sites, which is a key factor in mediating binding properties. Chemical elucidation of ligand-receptor binding sites is typically obtained by characterizing crystal structures of purified receptors using x-ray diffraction (XRD) and nuclear magnetic resonance (NMR), and building computational models for analysis [20, 21]. New technologies are needed to provide molecular insight into ligand-receptor binding chemistry in cell membranes, where the cellular environment can impact receptor-ligand interactions.
Raman spectroscopy measures the energy of the vibrational modes associated with the structure of molecules, which provides an intrinsic contrast mechanism for identifying molecules in biological systems (e.g. cells and tissues). Raman spectra encode chemical-specific information regarding the identity (so-called “chemical fingerprints”) of the molecules. Raman bands arising from different functional groups identify biomolecules, but the unique spectra arising from the combination of biomolecules in a cell provide characteristic patterns that enable classification of different cell types. For example, Raman spectroscopy can classify benign and cancerous human lung cells in response to external stimuli [22, 23], and monitor differentiation from stem-like cells in different culture conditions [24, 25]. However, since Raman scattering is an inefficient process by nature, spontaneous Raman spectroscopy is challenged to detect specific membrane receptors in cells.
Plasmonic nanostructures produce significant enhancements in the Raman scattering from molecules in close proximity—an effect known as surface-enhanced Raman scattering (SERS), which provides improved sensitivity for biomedical applications [26, 27]. By conjugating specific ligands onto plasmonic nanoparticles (e.g. Au, Ag), SERS can detect and locate specific receptors in cell membrane. One typical approach that has been widely applied is to construct SERS tags, where the intense and distinct spectrum of a Raman reporter molecule enhanced by the nanoparticle provides the detected signal for localization and mapping of specific membrane receptors, such as cancer markers, in vitro and in vivo [28–31]. For example, Fabris et al. constructed an RGDfC peptides-conjugated SERS nanotag to achieve specific targeting of integrin αvβ3 in human glioblastoma cells, by detecting the signal of a Raman-active dithiolated linking reporter molecule [31]. With these SERS tags, the signal from a reporter molecule specifically attached to the nanoparticle is observed, overwhelming the intrinsic vibrational signal of biomolecules themselves, which is usually much weaker than the signals from reporter molecules. In other words, Raman reporter-based SERS tags are unable to detect vibrational modes of membrane receptors and cannot provide structural information of the ligand-receptor binding pockets. In recent years, SERS studies without the application of reporter molecules have been reported for detection and mapping of ligand-receptor interactions on SERS-active substrates [32, 33], and even on cell membrane surface [34]. Although this intrinsic SERS approach can provide structural information about the protein receptor, its imaging resolution is restricted by the diffraction limit, and therefore is unable to achieve single molecule imaging.
Tip-enhanced Raman scattering (TERS), utilizing a plasmonic nanostructure at the apex of a scanning probe microscopy (SPM) tip, integrates the chemical sensitivity of SERS and nanoscale spatial resolution of SPM to enable intrinsic chemical imaging of surfaces, such as biomembranes [35, 36]. Impressively, TERS was demonstrated to image components of individual molecules based on their vibrational modes [37], which makes it a promising technique to chemically characterize biomolecules, such as lipids and proteins, in cell membrane [36]. Our lab has conducted a series of studies to investigate chemistry of ligand-receptor interactions in recent years [38–43]. We have reported that Raman signals from immobilized protein receptors can be detected through a plasmonic coupling between a gold nanoparticle-coated TERS tip and a ligand-functionalized gold nanoparticle (GNPs) [38, 40]. Through protein mutation, we have been able to demonstrate that amino acids near the ligand binding sites are responsible for the observed TERS signal [42]. With controlled plasmonic coupling, we were able to obtain chemically specific information relevant to antibody-antigen interaction in cells, while achieve mapping of the binding events with spatial resolution below 50 nm [39]. An initial study on fixed SW620 cells (human colon cancer cell line) showed this detection mechanism can detect the amino acids involved in the specific interactions between αvβ3 integrin receptors and RGD peptides, in intact cell membrane [41]. These initial results suggest that TERS can provide chemical insights into cell membrane receptors, making it a promising new technology for investigating the chemical structure of membrane receptors and the chemical interactions that govern molecular recognition.
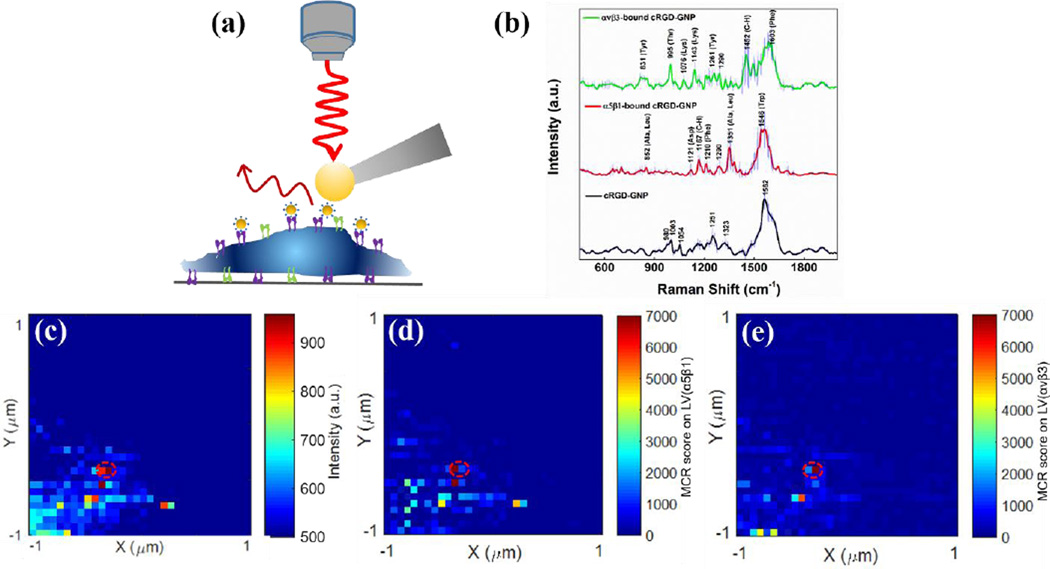
The TERS signal consists of the intrinsic Raman scattering from the amino acids closest to the nanoparticle. When binding to a ligand on the nanoparticle surface these amino acids are associated with the ligand-receptor binding site. We hypothesize that TERS is able to differentiate between different membrane receptors (e.g. αvβ3, αvβ5 and α5β1 integrins) that bind to same RGD sequences [11, 44], due to the slight differences in their respective ligand binding sites. In a recent report, we used two integrin receptors, α5β1 and αvβ3, with reported affinity for cyclic-RGDfC (cRGD) peptide, to examine whether RGD-integrin binding can be differentiated in intact human colon cancer cells (SW480) using TERS [43]. With this method, cRGD peptide-conjugated gold nanoparticles (cRGD-GNPs) were used to target integrins α5β1 and αvβ3 on SW480 cells. While the gold-coated SPM tip scans on the cell surface, the plasmonic coupling between TERS tip and gold nanoparticle produce enhanced Raman signals, which can be used to identify the receptors bound to the nanoparticles. As shown in this and previous work [38–41, 43, 45], the receptor remains in the cell membrane and does not necessarily reside in the tip-particle gap (Figure 1a).
Figure 1.
(a) Schematic illustration of TERS detection of integrin receptors in cell membrane. (b) Average SERS spectra of cRGD-GNPs (n=23), α5β1-bound cRGD-GNPs (n=12), and αvβ3-bound cRGD-GNPs (n=11). Shaded area represents standard deviation. (c) TERS map of SW480 cells incubated with cRGD-GNPs, constructed with single-peak intensity at 1003 cm−1. Pixel step: 62.5 nm. (d, e) MCR score maps reflecting the localization of (d) α5β1 and (e) αvβ3 on the same cell membrane area as (c). Adapted with permission from Reference [43] (Xiao L, Wang H, Schultz ZD. Selective Detection of RGD-Integrin Binding in Cancer Cells Using Tip Enhanced Raman Scattering Microscopy. Anal Chem 2016; 88: 6547–6553). Copyright (2016) American Chemical Society.
SERS experiments were performed on the cRGD-GNPs incubated with purified integrin α5β1 or αvβ3 receptors, to generate a set of reference spectra for each receptor. Multiple spectra from just the cRGD-GNPs, α5β1-bound cRGD-GNPs, and αvβ3-bound cRGD-GNPs were acquired in 0.1X PBS solution where nanoparticle clusters formed plasmon-enhanced “hot spots”. Figure 1b shows the average SERS spectra of integrins α5β1 and αvβ3 bound with cRGD-GNPs. The observed vibrational bands can be assigned to amino acids found in the RGD binding sites of α5β1 and αvβ3 integrins [46, 47]. SERS spectra exhibit distinct differences in Raman bands (Figure 1b), arising from differences in ligand binding sites between α5β1 and αvβ3 receptors [48, 49]. The acquired SERS spectra were analyzed with multivariate curve resolution (MCR), a chemometric method that analyzes the variance in the spectra and generates pure components associated with the spectral composition. The MCR analysis produced 3 pure spectral components that can be attributed to cRGD-GNPs, α5β1-bound cRGD-GNPs, and αvβ3-bound cRGD-GNPs, respectively. This multivariate model accounted for approximately 90% of the total variance in the SERS data and was able to distinguish between spectra acquired from the distinct integrin receptors.
TERS experiments were performed on fixed SW480 colon cancer cells incubated with cRGD-GNPs. A TERS map was acquired by taking spectra every 62.5 nm over a 2 2 µm2 surface area of a SW480 cell membrane. The TERS map was constructed using the peak intensity at 1003 cm−1 (attributed to phenylalanine from cRGD-GNPs), in which high intensity pixels reflect the localization where the cRGD-GNPs bind, possibly to integrin receptors, on the cell membrane (Figure 1c). Given that both α5β1 and αvβ3 can be expressed in SW480 cells, the obtained TERS data were analyzed using the MCR model generated from the SERS data, in order to identify the receptors based on spectral similarity between TERS spectra and MCR pure components. MCR scores of α5β1 and αvβ3 were generated to estimate the spectral contribution from α5β1 and αvβ3 in each TERS spectrum [43]. Maps created from MCR scores reflect the localization of α5β1 (Figure 1d) and αvβ3 (Figure 1e) on the same cell membrane area as in Figure 1c. Upon the application of MCR regression model, the two integrin receptors were readily detected and differentiated from general TERS signals. We noticed that two adjacent pixels with high levels of α5β1 and αvβ3 (red dashed circles in Figures 1c–e) were observed in the TERS maps, suggesting the co-localization of α5β1 and αvβ3 within ~60 nm range, as they have been reported to co-localize on cell membranes to regulate different mechanochemical steps of cell-matrix adhesion [50]. In addition, the fact that exclusive spectral signatures are detected from co-localized integrin receptors α5β1 and αvβ3 indicates the TERS signal may arise from individual receptors.
The above findings indicate that TERS is capable of nanoscale imaging of individual integrin receptors within cell membranes, while providing chemical insight into the RGD-integrin binding structures. The chemical insight TERS provides is a new tool for studying drug targeting in vitro, which may aid in drug discovery. We successfully differentiated between two related integrin structures, suggesting the signature of other receptors could also be identified. Future direction will be applying the developed TERS methodology to provide an early stage screen to identify drug interactions with different receptors in living cells.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences, part of the United States National Institutes of Health, award R01 GM109988.
Abbreviations
- TERS
tip-enhanced Raman scattering
- RGD
arginine-glycine-aspartate
- ECM
cell-extracellular matrix
- MRI
magnetic resonance imaging
- XRD
X-ray diffraction
- NMR
nuclear magnetic resonance
- SERS
surface-enhanced Raman scattering
- SPM
scanning probe microscopy
- GNPs
gold nanoparticles
- cRGD (cRGDfC)
cyclic-arginine-glycine-aspartate-phenylalanine-cysteine
- MCR
multivariate curve resolution
Footnotes
Author Contributions
L.X. and Z.D.S. wrote the manuscript.
Conflicting interests
The authors have declared that no competing interests exist.
References
- 1.Kampen KR. Membrane Proteins: The Key Players of a Cancer Cell. J Membr Biol. 2011;242:69–74. doi: 10.1007/s00232-011-9381-7. [DOI] [PubMed] [Google Scholar]
- 2.Grimm D, Bauer J, Pietsch J, Infanger M, Eucker J, Schoenberger CEaJ. Diagnostic and Therapeutic Use of Membrane Proteins in Cancer Cells. Curr Med Chem. 2011;18:176–190. doi: 10.2174/092986711794088344. [DOI] [PubMed] [Google Scholar]
- 3.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X. Integrin Targeted Imaging and Therapy. Theranostics. 2011;2011:28–29. doi: 10.7150/thno/v01p0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Chen K, Lee HY, Xu C, Hsu AR, Peng S, et al. Ultrasmall c(RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin alpha(v)beta3-rich tumor cells. J Am Chem Soc. 2008;130:7542–7543. doi: 10.1021/ja802003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–188. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisele G, Wick A, Eisele AC, Clement PM, Tonn J, Tabatabai G, et al. Cilengitide treatment of newly diagnosed glioblastoma patients does not alter patterns of progression. J Neurooncol. 2014;117:141–145. doi: 10.1007/s11060-014-1365-x. [DOI] [PubMed] [Google Scholar]
- 11.Mas-Moruno C, Fraioli R, Rechenmacher F, Neubauer S, Kapp TG, Kessler H. alphavbeta3- or alpha5beta1-Integrin-Selective Peptidomimetics for Surface Coating. Angew Chem Int Ed Engl. 2016;55:7048–7067. doi: 10.1002/anie.201509782. [DOI] [PubMed] [Google Scholar]
- 12.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 13.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 14.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 15.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15:173–183. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S. Radiolabeled cyclic RGD peptides as integrin alpha(v)beta(3)-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Ji S, Czerwinski A, Valenzuela F, Pennington M, Liu S. FITC-conjugated cyclic RGD peptides as fluorescent probes for staining integrin alphavbeta3/alphavbeta5 in tumor tissues. Bioconjug Chem. 2014;25:1925–1941. doi: 10.1021/bc500452y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehenkari PP, Horton MA. Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem Biophys Res Commun. 1999;259:645–650. doi: 10.1006/bbrc.1999.0827. [DOI] [PubMed] [Google Scholar]
- 19.Manna S, Senapati S, Lindsay S, Zhang P. A three-arm scaffold carrying affinity molecules for multiplex recognition imaging by atomic force microscopy: the synthesis, attachment to silicon tips, and detection of proteins. J Am Chem Soc. 2015;137:7415–7423. doi: 10.1021/jacs.5b03079. [DOI] [PubMed] [Google Scholar]
- 20.Shoichet BK. Virtual screening of chemical libraries. Nature. 2004;432:862–865. doi: 10.1038/nature03197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinelli L, Meyer A, Heckmann D, Lavecchia A, Novellino E, Kessler H. Ligand binding analysis for human alpha5beta1 integrin: strategies for designing new alpha5beta1 integrin antagonists. J Med Chem. 2005;48:4204–4207. doi: 10.1021/jm040224i. [DOI] [PubMed] [Google Scholar]
- 22.Tang M, Li Q, Xiao L, Li Y, Jensen JL, Liou TG, et al. Toxicity effects of short term diesel exhaust particles exposure to human small airway epithelial cells (SAECs) and human lung carcinoma epithelial cells (A549) Toxicol Lett. 2012;215:181–192. doi: 10.1016/j.toxlet.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao L, Tang M, Li Q, Zhou A. Non-invasive detection of biomechanical and biochemical responses of human lung cells to short time chemotherapy exposure using AFM and confocal Raman spectroscopy. Analytical Methods. 2013;5:874–879. [Google Scholar]
- 24.Li Q, Suasnavas E, Heywood S, Xiao L, Zhou A, Isom SC. Biochemical, biophysical, and genetic changes of porcine trophoblast-derived stem-like cells during differentiation as evaluated using Raman microspectroscopy, Atomic force microscopy, and quantitative polymerase chain reaction. Genesis. 2015;53:749–761. doi: 10.1002/dvg.22907. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Suasnavas E, Xiao L, Heywood S, Qi X, Zhou1 Anhong, et al. Label-free and non-invasive monitoring of porcine trophoblast derived cells: differentiation in serum and serum-free media. 2015;8:638–645. doi: 10.1002/jbio.201400062. [DOI] [PubMed] [Google Scholar]
- 26.Schlucker S. SERS microscopy: nanoparticle probes and biomedical applications. Chemphyschem. 2009;10:1344–1354. doi: 10.1002/cphc.200900119. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Puebla RA, Liz-Marzan LM. SERS-based diagnosis and biodetection. Small. 2010;6:604–610. doi: 10.1002/smll.200901820. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L, Harihar S, Welch DR, Zhou A. Imaging of epidermal growth factor receptor on single breast cancer cells using surface-enhanced Raman spectroscopy. Anal Chim Acta. 2014;843:73–82. doi: 10.1016/j.aca.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 30.Keren S, Zavaleta C, Cheng Z, de la Zerda A, Gheysens O, Gambhir SS. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc Natl Acad Sci U S A. 2008;105:5844–5849. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indrasekara AS, Paladini BJ, Naczynski DJ, Starovoytov V, Moghe PV, Fabris L. Dimeric gold nanoparticle assemblies as tags for SERS-based cancer detection. Adv Healthc Mater. 2013;2:1370–1376. doi: 10.1002/adhm.201200370. [DOI] [PubMed] [Google Scholar]
- 32.Combs ZA, Chang S, Clark T, Singamaneni S, Anderson KD, Tsukruk VV. Label-free Raman mapping of surface distribution of protein a and IgG biomolecules. Langmuir. 2011;27:3198–3205. doi: 10.1021/la104787w. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Jiang J, Wang X, Han K, Ameen A, Khan I, et al. Large-area, uniform and low-cost dual-mode plasmonic naked-eye colorimetry and SERS sensor with handheld Raman spectrometer. Nanoscale. 2016;8:6162–6172. doi: 10.1039/c5nr08357e. [DOI] [PubMed] [Google Scholar]
- 34.Hodges MD, Kelly JG, Bentley AJ, Fogarty S, Patel II, Martin FL, et al. Combining immunolabeling and surface-enhanced Raman spectroscopy on cell membranes. ACS Nano. 2011;5:9535–9541. doi: 10.1021/nn202652h. [DOI] [PubMed] [Google Scholar]
- 35.Bohme R, Cialla D, Richter M, Rosch P, Popp J, Deckert V. Biochemical imaging below the diffraction limit--probing cellular membrane related structures by tip-enhanced Raman spectroscopy (TERS) J Biophotonics. 2010;3:455–461. doi: 10.1002/jbio.201000030. [DOI] [PubMed] [Google Scholar]
- 36.Richter M, Hedegaard M, Deckert-Gaudig T, Lampen P, Deckert V. Laterally resolved and direct spectroscopic evidence of nanometer-sized lipid and protein domains on a single cell. Small. 2011;7:209–214. doi: 10.1002/smll.201001503. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Zhang Y, Dong ZC, Jiang S, Zhang C, Chen LG, et al. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature. 2013;498:82–86. doi: 10.1038/nature12151. [DOI] [PubMed] [Google Scholar]
- 38.Carrier SL, Kownacki CM, Schultz ZD. Protein-ligand binding investigated by a single nanoparticle TERS approach. Chem Commun (Camb) 2011;47:2065–2067. doi: 10.1039/c0cc05059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander KD, Schultz ZD. Tip-enhanced Raman detection of antibody conjugated nanoparticles on cellular membranes. Anal Chem. 2012;84:7408–7414. doi: 10.1021/ac301739k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Schultz ZD. The chemical origin of enhanced signals from tip-enhanced Raman detection of functionalized nanoparticles. Analyst. 2013;138:3150–3157. doi: 10.1039/c3an36898j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Schultz ZD. TERS detection of alphaVbeta3 integrins in intact cell membranes. Chemphyschem. 2014;15:3944–3949. doi: 10.1002/cphc.201402466. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Carrier SL, Park S, Schultz ZD. Selective TERS detection and imaging through controlled plasmonics. Faraday Discuss. 2015;178:221–235. doi: 10.1039/c4fd00190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao L, Wang H, Schultz ZD. Selective Detection of RGD-Integrin Binding in Cancer Cells Using Tip Enhanced Raman Scattering Microscopy. Anal Chem. 2016;88:6547–6553. doi: 10.1021/acs.analchem.6b01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marelli UK, Rechenmacher F, Sobahi TR, Mas-Moruno C, Kessler H. Tumor Targeting via Integrin Ligands. Front Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Carrier SL, Park S, Schultz ZD. Selective TERS detection and imaging through controlled plasmonics. Faraday Discuss. 2015;178:221–235. doi: 10.1039/c4fd00190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Movasaghi Z, Rehman S, Rehman IU. Raman spectroscopy of biological tissues. 2007;42:493–541. [Google Scholar]
- 47.Zhu G, Zhu X, Fan Q, Wan X. Raman spectra of amino acids and their aqueous solutions. 2011;78:1187–1195. doi: 10.1016/j.saa.2010.12.079. [DOI] [PubMed] [Google Scholar]
- 48.Heckmann D, Meyer A, Marinelli L, Zahn G, Stragies R, Kessler H. Probing integrin selectivity: rational design of highly active and selective ligands for the alpha5beta1 and alphavbeta3 integrin receptor. Angew Chem Int Ed Engl. 2007;46:3571–3574. doi: 10.1002/anie.200700008. [DOI] [PubMed] [Google Scholar]
- 49.Mould AP, Koper EJ, Byron A, Zahn G, Humphries MJ. Mapping the ligand-binding pocket of integrin alpha 5 beta 1 using a gain-of-function approach. Biochem J. 2009;424:179–189. doi: 10.1042/BJ20090992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci U S A. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]