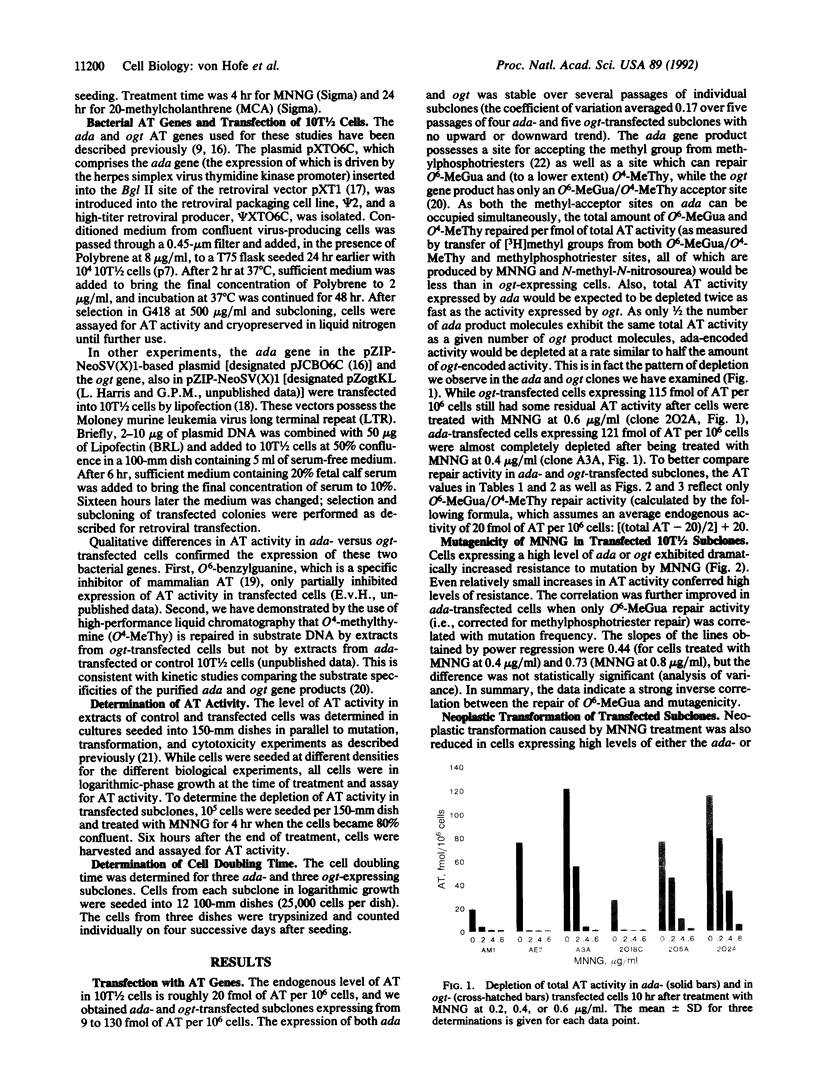
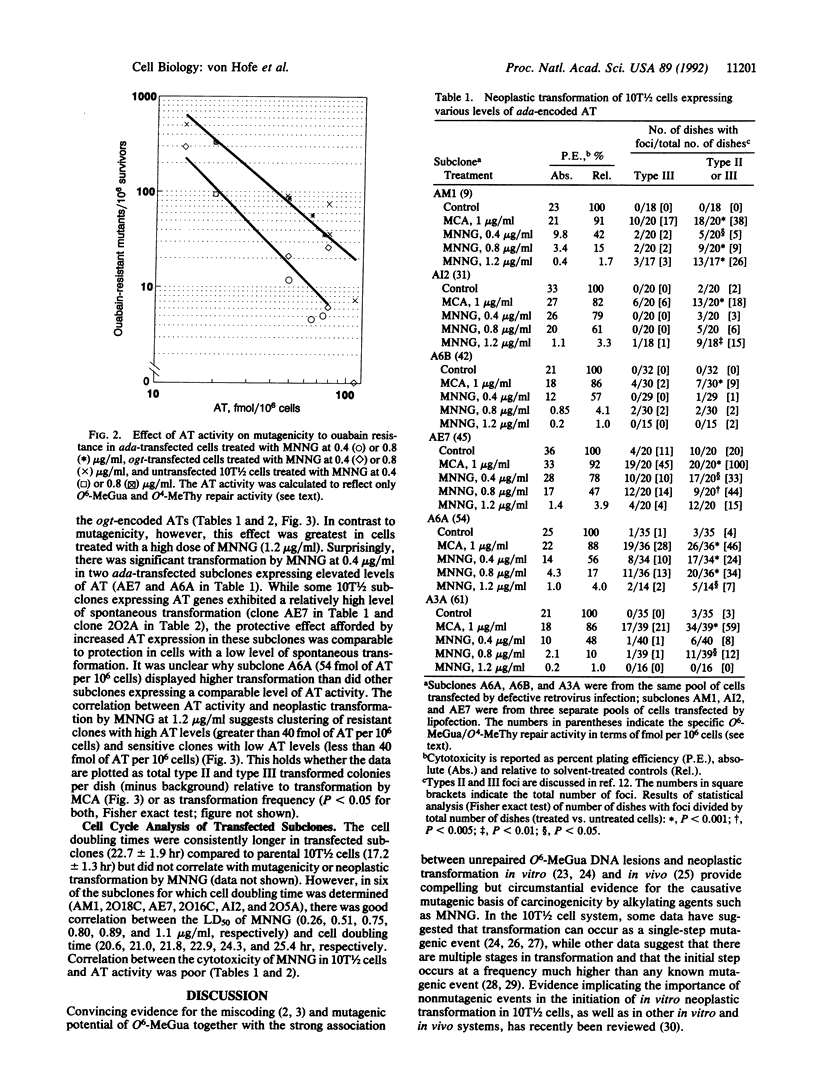
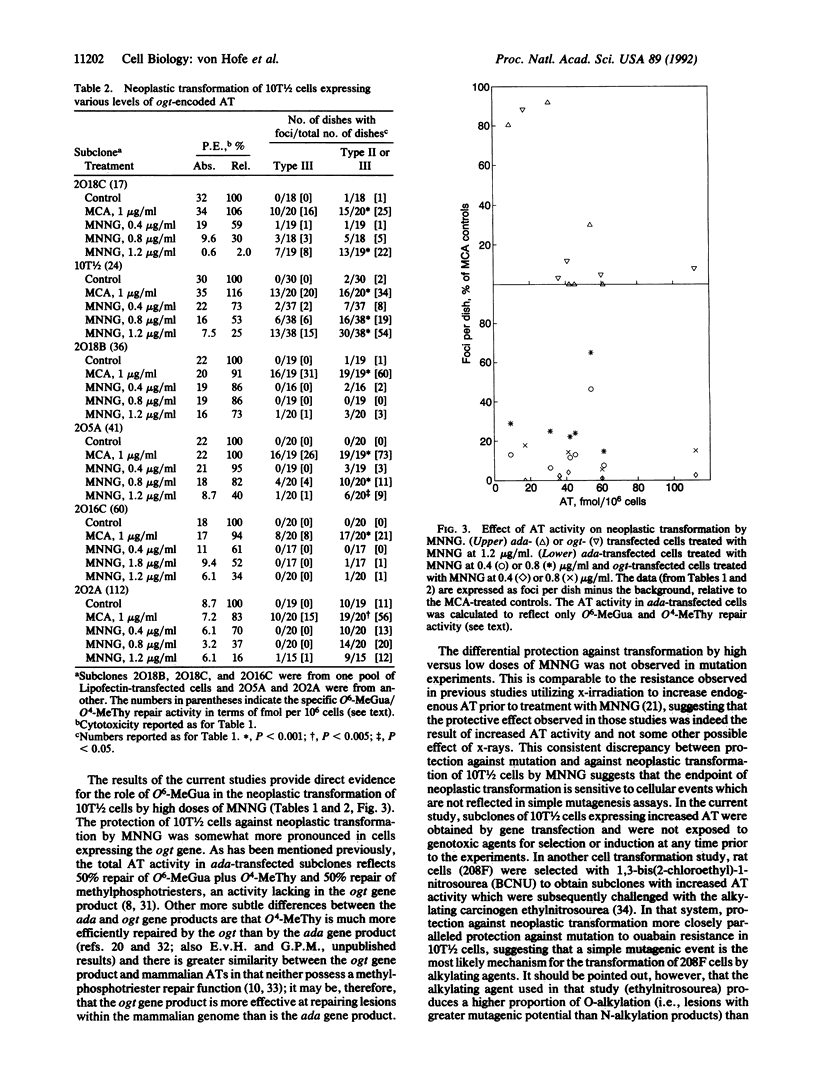
Abstract
While a great deal of evidence has directly implicated the importance of O6-alkylation of guanine in the mutagenicity of alkylating agents, evidence demonstrating the oncogenic potential of this lesion has been largely indirect. We have combined a well-studied in vitro neoplastic transformation system (using C3H/10T1/2 mouse cells) with a proven method of gene transfection for expressing the bacterial O6-alkylguanine-DNA alkyltransferase (AT; EC 2.1.1.63) repair genes ada and ogt to generate subclones which possess augmented repair capability toward specific DNA lesions. The products of these genes specifically and differentially repair O6-methylguanine (O6-MeGua), O4-methylthymine (O4-MeThy), and methylphosphotriesters. We show that the level of expression of either the ada or the ogt AT gene in C3H/10T1/2 cells directly correlates with protection against mutation to ouabain resistance by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG). Subclones expressing 70 fmol of AT per 10(6) cells exhibited a mutation frequency approximately 1/40th of that of clones expressing 15 fmol of AT per 10(6) cells when treated with MNNG at 0.4 micrograms/ml. Protection against mutagenesis by MNNG at 0.8 micrograms/ml, however, did not exceed 12-fold even in subclones expressing greater than 100 fmol of AT per 10(6) cells. As an MNNG dose of 0.6 micrograms/ml was sufficient to saturate more than 95% of the AT activity in any of the clones, the residual mutation frequency may have been caused by unrepaired O6MeGua lesions. In contrast to mutagenesis, protection against neoplastic transformation in vitro, in cells expressing high levels of AT, was most pronounced in cells treated with the highest dose of MNNG used (1.2 micrograms/ml). Low levels of transformation caused by MNNG at 0.4 and 0.8 micrograms/ml were not consistently inhibited in those clones. These data suggest that O6-MeGua formation is of major but not unique significance in the neoplastic transformation of C3H/10T1/2 cells by MNNG.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras oncogenes: their role in neoplasia. Eur J Clin Invest. 1990 Jun;20(3):225–235. doi: 10.1111/j.1365-2362.1990.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Boulter C. A., Wagner E. F. A universal retroviral vector for efficient constitutive expression of exogenous genes. Nucleic Acids Res. 1987 Sep 11;15(17):7194–7194. doi: 10.1093/nar/15.17.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Sedgwick B., Robins P., Totty N., Waterfield M. D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(9):2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. E., Pegg A. E., Dumenco L. L., Moschel R. C., Gerson S. L. Comparison of the inactivation of mammalian and bacterial O6-alkylguanine-DNA alkyltransferases by O6-benzylguanine and O6-methylguanine. Carcinogenesis. 1991 Dec;12(12):2305–2309. doi: 10.1093/carcin/12.12.2305. [DOI] [PubMed] [Google Scholar]
- Doniger J., Day R. S., DiPaolo J. A. Quantitative assessment of the role of O6-methylguanine in the initiation of carcinogenesis by methylating agents. Proc Natl Acad Sci U S A. 1985 Jan;82(2):421–425. doi: 10.1073/pnas.82.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. C., Tano K., Horesovsky G. J., Preston R. J., Mitra S. The role of O6-alkylguanine in cell killing and mutagenesis in Chinese hamster ovary cells. Carcinogenesis. 1991 Jan;12(1):83–89. doi: 10.1093/carcin/12.1.83. [DOI] [PubMed] [Google Scholar]
- Farber E., Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991 Jun 1;51(11):2751–2761. [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Mondal S., Heidelberger C. Probabilistic view of the transformation of cultured C3H/10T1/2 mouse embryo fibroblasts by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7272–7276. doi: 10.1073/pnas.77.12.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Induction of foci of morphologically transformed cells in synchronized populations of 10T1/2 cells by N-methyl-N'-nitro-N-nitrosoguanidine and the effect of spontaneous transformation on calculated transformation frequency. Cancer Res. 1988 Nov 1;48(21):5977–5983. [PubMed] [Google Scholar]
- Ishizaki K., Tsujimura T., Yawata H., Fujio C., Nakabeppu Y., Sekiguchi M., Ikenaga M. Transfer of the E. coli O6-methylguanine methyltransferase gene into repair-deficient human cells and restoration of cellular resistance to N-methyl-N'-nitro-N-nitrosoguanidine. Mutat Res. 1986 Sep;166(2):135–141. doi: 10.1016/0167-8817(86)90011-8. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Telfer N., Heidelberger C. Further evidence that ouabain-resistant variants induced by chemical carcinogens in transformable C3H/10T1/2 Cl 8 mouse fibroblasts are mutants. Mutat Res. 1980 Sep;72(2):295–310. doi: 10.1016/0027-5107(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Brennand J. Cloning of the E. coli O6-methylguanine and methylphosphotriester methyltransferase gene using a functional DNA repair assay. Nucleic Acids Res. 1985 Mar 25;13(6):1939–1952. doi: 10.1093/nar/13.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Potter P. M. The E. coli ogt gene. Mutat Res. 1990 Nov-Dec;233(1-2):15–21. doi: 10.1016/0027-5107(90)90146-u. [DOI] [PubMed] [Google Scholar]
- McCarthy J. G., Edington B. V., Schendel P. F. Inducible repair of phosphotriesters in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7380–7384. doi: 10.1073/pnas.80.24.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesnow S., Garland H., Curtis G. Improved transformation of C3H10T1/2CL8 cells by direct- and indirect-acting carcinogens. Carcinogenesis. 1982;3(4):377–380. doi: 10.1093/carcin/3.4.377. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Lehman N. L., Henderson B. E., Landolph J. R. Study of the ability of phenacetin, acetaminophen, and aspirin to induce cytotoxicity, mutation, and morphological transformation in C3H/10T1/2 clone 8 mouse embryo cells. Cancer Res. 1989 Feb 15;49(4):1038–1044. [PubMed] [Google Scholar]
- Pegg A. E. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- Potter P. M., Rafferty J. A., Cawkwell L., Wilkinson M. C., Cooper D. P., O'Connor P. J., Margison G. P. Isolation and cDNA cloning of a rat O6-alkylguanine-DNA-alkyltransferase gene, molecular analysis of expression in rat liver. Carcinogenesis. 1991 Apr;12(4):727–733. doi: 10.1093/carcin/12.4.727. [DOI] [PubMed] [Google Scholar]
- Potter P. M., Wilkinson M. C., Fitton J., Carr F. J., Brennand J., Cooper D. P., Margison G. P. Characterisation and nucleotide sequence of ogt, the O6-alkylguanine-DNA-alkyltransferase gene of E. coli. Nucleic Acids Res. 1987 Nov 25;15(22):9177–9193. doi: 10.1093/nar/15.22.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Sassanfar M., Dosanjh M. K., Essigmann J. M., Samson L. Relative efficiencies of the bacterial, yeast, and human DNA methyltransferases for the repair of O6-methylguanine and O4-methylthymine. Suggestive evidence for O4-methylthymine repair by eukaryotic methyltransferases. J Biol Chem. 1991 Feb 15;266(5):2767–2771. [PubMed] [Google Scholar]
- Singer B., Essigmann J. M. Site-specific mutagenesis: retrospective and prospective. Carcinogenesis. 1991 Jun;12(6):949–955. doi: 10.1093/carcin/12.6.949. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Grisham J. W. Activation of the Ha-ras gene in C3H 10T1/2 cells transformed by exposure to N-methyl-N'-nitro-N-nitrosoguanidine. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1194–1199. doi: 10.1016/s0006-291x(87)80196-1. [DOI] [PubMed] [Google Scholar]
- Snow E. T., Foote R. S., Mitra S. Base-pairing properties of O6-methylguanine in template DNA during in vitro DNA replication. J Biol Chem. 1984 Jul 10;259(13):8095–8100. [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tano K., Shiota S., Collier J., Foote R. S., Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc Natl Acad Sci U S A. 1990 Jan;87(2):686–690. doi: 10.1073/pnas.87.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomale J., Huh N. H., Nehls P., Eberle G., Rajewsky M. F. Repair of O6-ethylguanine in DNA protects rat 208F cells from tumorigenic conversion by N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9883–9887. doi: 10.1073/pnas.87.24.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M. C., Potter P. M., Cawkwell L., Georgiadis P., Patel D., Swann P. F., Margison G. P. Purification of the E. coli ogt gene product to homogeneity and its rate of action on O6-methylguanine, O6-ethylguanine and O4-methylthymine in dodecadeoxyribonucleotides. Nucleic Acids Res. 1989 Nov 11;17(21):8475–8484. doi: 10.1093/nar/17.21.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok A. J., Sips H., den Engelse L., Simons J. W. Transformation of C3H10T1/2 cells by N-ethyl-N-nitrosourea occurs as a single, low frequency, mutation-like event. Carcinogenesis. 1986 Aug;7(8):1387–1392. doi: 10.1093/carcin/7.8.1387. [DOI] [PubMed] [Google Scholar]
- von Hofe E., Kennedy A. R. X-ray induction of O6-alkylguanine-DNA alkyltransferase protects against some of the biological effects of N-methyl-N'-nitro-N-nitrosoguanidine in C3H 10T1/2 cells. Radiat Res. 1991 Aug;127(2):220–225. [PubMed] [Google Scholar]