Abstract
AIMS/BACKGROUND--Visual symptoms are a common but not invariable feature of Alzheimer's disease (AD) and such symptoms appear to become more pronounced as the severity of the dementia increases. Pathology in both the pregeniculate and cortical parts of the visual system has been suggested to underlie the visual deficits in AD. In order to investigate the former possibility, the effect of AD on the optic nerve was investigated. METHODS--Intraorbital segments of optic nerve were taken at autopsy from nine patients with AD and seven patients with no history of psychiatric or neurological disease and no abnormal neuropathology. All patients had functional vision before death and appeared free of retinal, optic nerve, or microvascular disease. The optic nerves were processed into resin, semi-thin sections cut perpendicular to the long axis of each optic nerve, and stained with paraphenylenediamine. The sections were then investigated using an image analysis system and standard morphometric techniques. RESULTS--There was no significant difference in the mean cross sectional neural area of AD compared with control optic nerves. Neither were there any significant differences between myelinated axon surface density, total axon number, or mean cross sectional axon area in AD compared with control optic nerves. CONCLUSION--These results indicate that optic nerve degeneration is not a feature of AD and suggest that the visual deficits in the disease result from cortical dysfunction. This view is supported by the fact that visuospatial dysfunction appears to be the most common visual problem in AD.
Full text
PDF

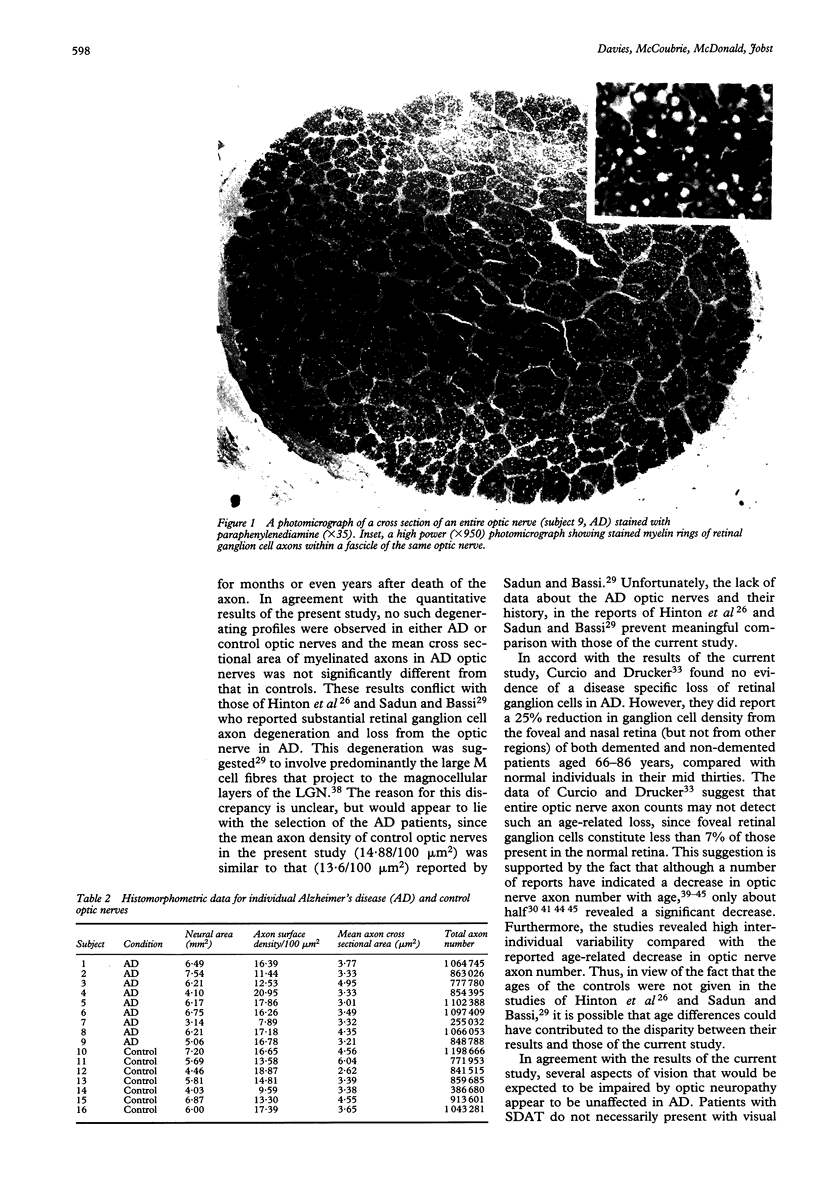


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balazsi A. G., Rootman J., Drance S. M., Schulzer M., Douglas G. R. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984 Jun;97(6):760–766. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- Beach T. G., McGeer E. G. Lamina-specific arrangement of astrocytic gliosis and senile plaques in Alzheimer's disease visual cortex. Brain Res. 1988 Nov 1;463(2):357–361. doi: 10.1016/0006-8993(88)90410-6. [DOI] [PubMed] [Google Scholar]
- Beach T. G., McGeer E. G. Senile plaques, amyloid beta-protein, and acetylcholinesterase fibres: laminar distributions in Alzheimer's disease striate cortex. Acta Neuropathol. 1992;83(3):292–299. doi: 10.1007/BF00296792. [DOI] [PubMed] [Google Scholar]
- Benson D. F., Davis R. J., Snyder B. D. Posterior cortical atrophy. Arch Neurol. 1988 Jul;45(7):789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Blanks J. C., Hinton D. R., Sadun A. A., Miller C. A. Retinal ganglion cell degeneration in Alzheimer's disease. Brain Res. 1989 Nov 6;501(2):364–372. doi: 10.1016/0006-8993(89)90653-7. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E., Kalus P. Alzheimer's disease: areal and laminar pathology in the occipital isocortex. Acta Neuropathol. 1989;77(5):494–506. doi: 10.1007/BF00687251. [DOI] [PubMed] [Google Scholar]
- Brun A., Englund E. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology. 1981 Sep;5(5):549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Cogan D. G. Visual disturbances with focal progressive dementing disease. Am J Ophthalmol. 1985 Jul 15;100(1):68–72. doi: 10.1016/s0002-9394(14)74985-2. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A., Rizzo J. F., Corkin S., Growdon J. H. Visual function in Alzheimer's disease and normal aging. Ann N Y Acad Sci. 1991;640:28–35. doi: 10.1111/j.1749-6632.1991.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Drucker D. N. Retinal ganglion cells in Alzheimer's disease and aging. Ann Neurol. 1993 Mar;33(3):248–257. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- De Lacoste M. C., White C. L., 3rd The role of cortical connectivity in Alzheimer's disease pathogenesis: a review and model system. Neurobiol Aging. 1993 Jan-Feb;14(1):1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- Dolman C. L., McCormick A. Q., Drance S. M. Aging of the optic nerve. Arch Ophthalmol. 1980 Nov;98(11):2053–2058. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- Henderson V. W., Mack W., Williams B. W. Spatial disorientation in Alzheimer's disease. Arch Neurol. 1989 Apr;46(4):391–394. doi: 10.1001/archneur.1989.00520400045018. [DOI] [PubMed] [Google Scholar]
- Hinton D. R., Sadun A. A., Blanks J. C., Miller C. A. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986 Aug 21;315(8):485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- Hof P. R., Bouras C., Constantinidis J., Morrison J. H. Balint's syndrome in Alzheimer's disease: specific disruption of the occipito-parietal visual pathway. Brain Res. 1989 Jul 31;493(2):368–375. doi: 10.1016/0006-8993(89)91173-6. [DOI] [PubMed] [Google Scholar]
- Hof P. R., Bouras C., Constantinidis J., Morrison J. H. Selective disconnection of specific visual association pathways in cases of Alzheimer's disease presenting with Balint's syndrome. J Neuropathol Exp Neurol. 1990 Mar;49(2):168–184. doi: 10.1097/00005072-199003000-00008. [DOI] [PubMed] [Google Scholar]
- Hutton J. T., Nagel J. A., Loewenson R. B. Eye tracking dysfunction in Alzheimer-type dementia. Neurology. 1984 Jan;34(1):99–102. doi: 10.1212/wnl.34.1.99. [DOI] [PubMed] [Google Scholar]
- Jonas J. B., Müller-Bergh J. A., Schlötzer-Schrehardt U. M., Naumann G. O. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990 Apr;31(4):736–744. [PubMed] [Google Scholar]
- Jonas J. B., Schmidt A. M., Müller-Bergh J. A., Schlötzer-Schrehardt U. M., Naumann G. O. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992 May;33(6):2012–2018. [PubMed] [Google Scholar]
- Katz B., Rimmer S., Iragui V., Katzman R. Abnormal pattern electroretinogram in Alzheimer's disease: evidence for retinal ganglion cell degeneration? Ann Neurol. 1989 Aug;26(2):221–225. doi: 10.1002/ana.410260207. [DOI] [PubMed] [Google Scholar]
- Kiyosawa M., Bosley T. M., Chawluk J., Jamieson D., Schatz N. J., Savino P. J., Sergott R. C., Reivich M., Alavi A. Alzheimer's disease with prominent visual symptoms. Clinical and metabolic evaluation. Ophthalmology. 1989 Jul;96(7):1077–1086. doi: 10.1016/s0161-6420(89)32769-2. [DOI] [PubMed] [Google Scholar]
- Kurylo D. D., Corkin S., Dolan R. P., Rizzo J. F., 3rd, Parker S. W., Growdon J. H. Broad-band visual capacities are not selectively impaired in Alzheimer's disease. Neurobiol Aging. 1994 May-Jun;15(3):305–311. doi: 10.1016/0197-4580(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Landis T., Cummings J. L., Benson D. F., Palmer E. P. Loss of topographic familiarity. An environmental agnosia. Arch Neurol. 1986 Feb;43(2):132–136. doi: 10.1001/archneur.1986.00520020026011. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Campbell M. J., Terry R. D., Morrison J. H. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987 Jun;7(6):1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mendez M. F., Mendez M. A., Martin R., Smyth K. A., Whitehouse P. J. Complex visual disturbances in Alzheimer's disease. Neurology. 1990 Mar;40(3 Pt 1):439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- Mendez M. F., Tomsak R. L., Remler B. Disorders of the visual system in Alzheimer's disease. J Clin Neuroophthalmol. 1990 Mar;10(1):62–69. [PubMed] [Google Scholar]
- Mikelberg F. S., Drance S. M., Schulzer M., Yidegiligne H. M., Weis M. M. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989 Sep;96(9):1325–1328. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- Mirra S. S., Heyman A., McKeel D., Sumi S. M., Crain B. J., Brownlee L. M., Vogel F. S., Hughes J. P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Moore V., Wyke M. A. Drawing disability in patients with senile dementia. Psychol Med. 1984 Feb;14(1):97–105. doi: 10.1017/s003329170000310x. [DOI] [PubMed] [Google Scholar]
- Orwin A., Wright C. E., Harding G. F., Rowan D. C., Rolfe E. B. Serial visual evoked potential recordings in Alzheimer's disease. Br Med J (Clin Res Ed) 1986 Jul 5;293(6538):9–10. doi: 10.1136/bmj.293.6538.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzolo F. J., Hansch E. C. Oculomotor reaction time in dementia reflects degree of cerebral dysfunction. Science. 1981 Oct 16;214(4518):349–351. doi: 10.1126/science.7280699. [DOI] [PubMed] [Google Scholar]
- Repka M. X., Quigley H. A. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989 Jan;96(1):26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- Rizzo J. F., 3rd, Cronin-Golomb A., Growdon J. H., Corkin S., Rosen T. J., Sandberg M. A., Chiappa K. H., Lessell S. Retinocalcarine function in Alzheimer's disease. A clinical and electrophysiological study. Arch Neurol. 1992 Jan;49(1):93–101. doi: 10.1001/archneur.1992.00530250097023. [DOI] [PubMed] [Google Scholar]
- Rogers J., Morrison J. H. Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci. 1985 Oct;5(10):2801–2808. doi: 10.1523/JNEUROSCI.05-10-02801.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadun A. A., Bassi C. J. Optic nerve damage in Alzheimer's disease. Ophthalmology. 1990 Jan;97(1):9–17. doi: 10.1016/s0161-6420(90)32621-0. [DOI] [PubMed] [Google Scholar]
- Sadun A. A., Borchert M., DeVita E., Hinton D. R., Bassi C. J. Assessment of visual impairment in patients with Alzheimer's disease. Am J Ophthalmol. 1987 Aug 15;104(2):113–120. doi: 10.1016/0002-9394(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Sadun A. A., Smith L. E., Kenyon K. R. Paraphenylenediamine: a new method for tracing human visual pathways. J Neuropathol Exp Neurol. 1983 Mar;42(2):200–206. [PubMed] [Google Scholar]
- Schlotterer G., Moscovitch M., Crapper-McLachlan D. Visual processing deficits as assessed by spatial frequency contrast sensitivity and backward masking in normal ageing and Alzheimer's disease. Brain. 1984 Mar;107(Pt 1):309–325. doi: 10.1093/brain/107.1.309. [DOI] [PubMed] [Google Scholar]
- Scholtz C. L., Swettenham K., Brown A., Mann D. M. A histoquantitative study of the striate cortex and lateral geniculate body in normal, blind and demented subjects. Neuropathol Appl Neurobiol. 1981 Mar-Apr;7(2):103–114. doi: 10.1111/j.1365-2990.1981.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Strenn K., Dal-Bianco P., Weghaupt H., Koch G., Vass C., Gottlob I. Pattern electroretinogram and luminance electroretinogram in Alzheimer's disease. J Neural Transm Suppl. 1991;33:73–80. doi: 10.1007/978-3-7091-9135-4_12. [DOI] [PubMed] [Google Scholar]
- Trick G. L., Barris M. C., Bickler-Bluth M. Abnormal pattern electroretinograms in patients with senile dementia of the Alzheimer type. Ann Neurol. 1989 Aug;26(2):226–231. doi: 10.1002/ana.410260208. [DOI] [PubMed] [Google Scholar]
- Trick G. L., Silverman S. E. Visual sensitivity to motion: age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991 Sep;41(9):1437–1440. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Drasdo N., Harding G. F. Pathology of the optic nerve and visual association areas. Information given by the flash and pattern visual evoked potential, and the temporal and spatial contrast sensitivity function. Brain. 1987 Feb;110(Pt 1):107–120. doi: 10.1093/brain/110.1.107. [DOI] [PubMed] [Google Scholar]




