Abstract
For many neurological and psychiatric diseases, novel therapeutics have been elusive for decades. By focusing on attention interference in Alzheimer's disease (AD), we provide a future vision on how emerging mobile, computer, and device‐based cognitive tools are converting classically noisy, subjective, data‐poor clinical endpoints associated with neuropsychiatric disease assessment into a richer, scalable, and objective set of measurements. Incorporation of such endpoints into clinical drug trials holds promise for more quickly and efficiently developing new medicines.
Increasingly a feature of contemporary life, multitasking comes at a cognitive price. While attention can be selectively assigned to more than one task, the simultaneous management of multiple tasks presents a challenge for the brain. The increased load in neural computation when attention is divided translates into decreased performance relative to single‐task conditions. This well‐described neuropsychological phenomena is referred to as interference cost, or dual‐task decrement when studied in dual‐task paradigms.
A large body of evidence has linked attention impairment with early stages of AD.1 In addition to episodic memory, disrupted attentional control is among the first signs of disordered cognitive function in early AD. Among subdomains of attentional control, selective attention (i.e., search and orienting) and divided attention (i.e., multitask performance and task switching) are particularly disrupted in early stages of the disease. The neural origin of decreased attentional control observed in AD is complex, but is thought to include altered cortico‐cortical tract integrity and connectivity. The inability to coordinate processing across attentional networks in turn disrupts central executive components of working memory. Such executive functions represent the ability to plan and conduct goal‐oriented tasks, directly impacting activities of daily living (ADLs) such as getting dressed, cooking, or paying bills. Clinically, it is commonly reported by caregivers that patients with early AD have difficulty distributing attention, following and engaging in discussions with multiple individuals simultaneously, and planning or controlling everyday activities. A frequent clinical and experimental finding is that early AD patients perform as well as control subjects when two tasks are attempted separately, but show a disproportionate decline in performance when the tasks are performed concurrently. However, the precise stage at which AD patients exhibit dual‐task decrement remains controversial.
Relative to examination of memory processes, research in attentional impairment in AD has been modest. Recently, Festa et al. reported a robust dual‐task decrement in mild AD.2 The performance of 130 elderly participants, comprised of 89 mild AD patients (Clinical Dementia Rating, CDR, of 0.5 and 1) and 41 elderly controls (CDR of 0 and Mini Mental State Examination, MMSE, >26) was assessed under dual task conditions. In this study, participants were required to simultaneously engage in a visuomotor tracking task (i.e., maintaining car position within a simulated driving environment), at subject‐adjusted difficulty in combination with three different types of tasks (spatial orientation, Simon interference, and visual search). The results indicated a substantially greater interference cost in the AD group relative to healthy elderly controls in all three dual‐task conditions.2 Such initial findings point to the potential utility of assessing attention interference cost to detect and monitor early AD.
Many cognitive assessments exist to characterize early memory deficits associated with AD, and it is now accepted that focused deficits accumulate over several decades before frank memory symptoms appear. The International Working Group (IWG), US National Institute on Aging, and Alzheimer's Association have recently issued new criteria for the diagnosis of AD in order to better define clinical phenotypes and account for the role of biomarkers in the various stages of disease progression.3 The development of fluid biomarkers, positron emission tomography (PET) ligands, and volumetric magnetic resonance imaging (MRI) have enabled the molecular, functional, and structural staging of disease progression, shifting the research focus to prodromal or presymptomatic stages. The development of reliable endpoints to quantitatively assess cognitive domains that are subtly affected before memory is an urgent need as the emphasis shifts to detection of subjects at risk and to early therapeutic intervention.
Digital technologies offer the promise of remote monitoring of medical conditions in a natural, patient‐convenient environment.4 This “patient in the wild” approach generates personalized, real‐time, real‐world, high‐dimensional data. Useful for a variety of functional and physiological assessments, the ability of digital technologies to convert subjective assessments into more objective measurements is particularly appealing in domains of cognition. For example, tablet‐based cognitive tools have been developed that precisely assess performance on dual‐task paradigms of divided attention allowing for patient‐specific measurement of cognitive interference.5 A recent study using the NeuroRacer computerized prototype of one of these tools demonstrated a loss of performance on interference processing (“interference cost”) that increased with age, improved with training, and was associated with changes in electroencephalogram (EEG) power in the stimulus‐locked theta band. Training on the NeuroRacer game enhanced the ability of healthy elderly to resolve interference (Figure 1). Next‐generation versions of these interactive digital assessments of attention interference cost can be applied to mobile platforms, allowing for remote use, self‐administration, and training. Further, use of adaptive algorithms enables assessment tools to automatically tune the difficulty relative to the specific subject.
Figure 1.
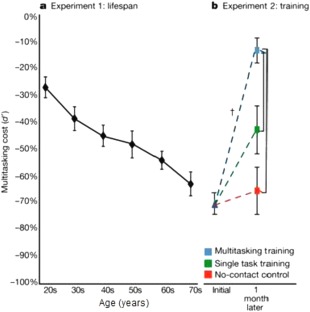
Performance on the NeuroRacer multitasking task declines progressively with age and improves with training. NeuroRacer dual‐task demonstrated a linear loss of performance on interference processing (“interference cost”) from 20 to 79 years of age. The interference cost improved with training in the older study participants (60–85 years old) in comparison to both an active control group and a no‐contact control group, attaining levels beyond those achieved by untrained 20‐year‐old participants. Adapted with permission from Macmillan Publishers: Nature, ref. 5, copyright 2013.
Will emerging digital technologies for cognitive assessment accelerate the development of new therapeutics? It will be important to determine how biochemical and pathological biomarkers linked to the risk of AD, such as brain amyloid, are associated with impaired performance in focused assessments of attention interference. Daily practice can improve attentional performance under dual‐task conditions.5 Is this learning effect itself reduced in subjects with brain amyloid or carrying other risk factors for AD? Can pharmacological intervention restore attentional performance under basal or learning paradigm conditions in early AD?
Given the explosion of interest in cognitive training, and the emergence of very large datasets that will quantitatively establish human variation across cognitive domains, the time is ripe for incorporation of novel clinical asset technologies into experimental drug trials. The future vision is one where classically slow, subjective, and data‐poor assessments of cognition are replaced by fast, objective, and data‐rich digital assessments that can be unequivocally linked to functional improvement by reference to large datasets. Achievement of this future state will require validation of emerging digital technologies through appropriate design and conduct of statistically robust clinical studies, with comparison against validated cognitive assessments and biomarkers.
In a world where the majority of the population carries a mobile digital device, where our behavioral status, cognitive capacity, and clinical function are ubiquitously and continuously tracked by the digital footprint we leave behind, the prospect for transformative efficiency and accuracy in testing novel therapeutics in neurodegenerative disease may rest in the devices we each carry in our pockets.
CONFLICT OF INTEREST
The authors are employees and shareholders of Pfizer, Inc.
References
- 1. Perry, R.J. & Hodges, J.R. Attention and executive deficits in Alzheimer's disease. A critical review. Brain 122, 383–404 (1999). [DOI] [PubMed] [Google Scholar]
- 2. Festa, E.K. , Heindel, W.C. & Ott, B.R. Dual‐task conditions modulate the efficiency of selective attention mechanisms in Alzheimer's disease. Neuropsychologia 48, 3252–3261 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubois, B. et al Advancing research diagnostic criteria for Alzheimer's disease: the IWG‐2 criteria. Lancet Neurol. 13, 614–629 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Fiordelli, M. , Diviani, N. & Schulz, P.J. Mapping mHealth research: a decade of evolution. J. Med. Internet Res. 15, e95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anguera, J.A. et al Video game training enhances cognitive control in older adults. Nature 501, 97–101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


