Abstract
Point-of-care (POC) technologies have proved valuable in cancer detection, diagnosis, monitoring, and treatment in the developed world, and have shown promise in low-and-middle-income countries (LMIC) as well. Despite this promise, the unique design constraints presented in low-resource settings, coupled with the variety of country-specific regulatory and institutional dynamics, have made it difficult for investigators to translate successful POC cancer interventions to the LMIC markets. In response to this need, the National Cancer Institute has partnered with the National Institute of Biomedical Imaging and Bioengineering to create the National Institutes of Health Affordable Cancer Technologies (ACTs) program. This program seeks to simplify the pathway to market by funding multidisciplinary investigative teams to adapt and validate the existing technologies for cancer detection, diagnosis, and treatment in LMIC settings. The various projects under ACTs range from microfluidic cancer diagnostic tools to novel treatment devices, each geared for successful clinical adaptation to LMIC settings. Via progression through this program, each POC innovation will be uniquely leveraged for successful clinical translation to LMICs in a way not before seen in this arena.
Keywords: Cancer, cancer detection, medical diagnosis, cryotherapy, computer aided diagnosis
This paper describes the purpose, formation, and early results of the National Institutes of Health Affordable Cancer Technologies Program, which aims to improve access to resource-appropriate technologies for cancer detection, diagnosis, monitoring and treatment in low- and middle-income countries.
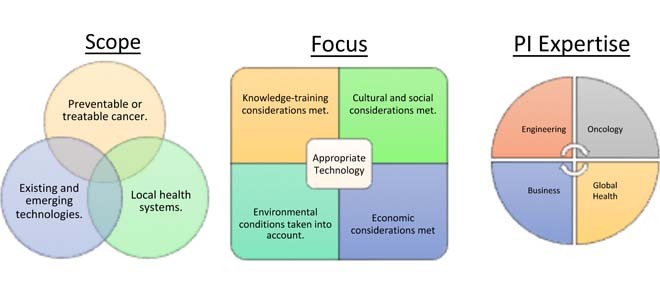
I. Introduction
In recent years, the emergence of a new generation of low-cost, point-of-care (POC) technologies has transformed the healthcare industry, and is now poised to revolutionize the field of global health. POC technologies, whether used within or beyond a traditional healthcare setting, represent a broad range of innovations whose impacts are felt in lab environments, homes, and treatment centers. In low-resource settings, such technologies have the potential to fundamentally increase access to care and open up the possibility of prevention/curative treatment for diseases traditionally thought of as death sentences in lower income parts of the world.
A world-wide epidemiologic shift has taken place. While the global community focuses on the exemplars, such as the unfortunate spread of Ebola in several West African countries and the rise of other emerging infectious diseases, the drivers of health inequity in the majority of the world are increasingly the more subtle and slow-developing diseases commonly associated with the developed world [1]. Of these chronic diseases, cancer, the “Emperor of all Maladies,” poses unique challenges to health systems. It is estimated that nearly two-thirds of the 8.2 million annual cancer deaths in the world occur in low- and middle-income countries (LMICs) [2]. Moreover, incidence rates in LMICs are on the rise, accompanied by substantial inequalities in cancer survival.
Prevention, early detection, and treatment are vital to successful cancer control. However, much of this depends on effective technologies, as many of which are not suitable for use in low resource settings due to expense, dependency on extensive medical infrastructure, or both. This situation warrants translational efforts to develop appropriate technologies that could improve treatment of cancers in resource-poor settings. To this end, the National Cancer Institute (NCI) and the National Institute for Biomedical Imaging and Bioengineering (NIBIB) have invested significant resources to create the Affordable Cancer Technologies (ACTs) program to support the development and validation of low-cost, point-of-care technologies with the potential to increase early detection, diagnosis, and non-invasive or minimally invasive treatment of cancer in LMICs.
A critical element of each project is that the work supported is fully translational in nature. ACTs supports pre-commercial technology adaptation from the bench to the bedside, with the goal of inducing a market shift towards affordable, resource-appropriate interventions in LMICs. All projects focus on preventable or treatable (i.e., with curative intent) cancers in the setting the technologies will be fielded. Each project that successfully progresses through the ACTs program will complete two distinct phases of testing. Phase I is an adaptation phase in which investigators will demonstrate analytical and clinical viability, while Phase II work will focus on clinical validation studies on human subjects in LMIC sites, with several projects electing to perform full clinical trials. Moreover, while low-cost is a goal, these technologies represent the state of the art and verify standards of care in LMIC settings are not compromised.
II. Supported Projects
The NCI and NIBIB are currently supporting seven exceptional research teams as part of the inaugural cohort of ACTs.
A. Cancer Prevention and Early Detection
Cancer burden is expected to double in next 2 decades and in turn severely impact both financial and human resources worldwide. Superior control of cancer depends not only on detection/diagnosis and treatment capabilities, but also on the detection of cancerous or precancerous lesions at early stage and the availability of effective affordable cancer prevention regimens or strategies. Identifying specific biomarkers and clinical factors, developing new screening and early stage cancer detection technologies based on those markers, and validating technologies that can accurately identify people at increased risk of developing cancer, as well as people harboring indolent, lethal or metastatic cancers is critical for effective cancer control. Additionally, availability of follow-up and treatment options is crucial, particularly in resources-limited LMICs. Affordable technologies that leverage on established, existing resources and infrastructure to deliver screening and early detection capabilities, and guide treatment decisions will make an immediate impact. The two technologies that are supported by the program include screen-and-treat technology for HPV detection and cervical cancer prevention; and hepatitis C viral detection and hepatocellular cancer prevention are using existing PCR machines installed in urban and rural areas in sub-Saharan Africa for multi-drug resistant TB or HIV/TB; and HIV viral load and HIV core antigen detection, respectively.
Infectious etiologies play a key role in many preventable and treatable cancers in LMICs. Moreover, cervical cancer, which is almost always the result of a chronic infection of the human papillomavirus (HPV), still represents one of the most common causes of cancer death for women in many LMICs. Cervical cancer is the third leading cause of cancer incidence and the fourth leading cause of cancer related death in women world-wide. PAP smears and cytology have controlled cervical cancer in US and Europe, but they are too expensive and laborious to perform in LMICs. There are stark global disparities in cervical cancer morbidity and mortality, most particularly in sub-Saharan Africa (SSA) where this largely-preventable cancer is one of the most common causes of cancer related deaths in women, due to the absence of affordable screening programs. Likewise, the availability of screening and treatment of HPV/cervical cancer in a single visit would likely have a greater impact in SSA and other LMIC settings where follow-up visits present a roadblock to intervention. In an attempt to address this roadblock, ACTs is supporting Dr. Louise Kuhn (Columbia) to adapt the Cepheid GeneXpert test for HPV detection across South Africa.
Dr. Kuhn has proposed a screen-and-treat (SAT) approach, comprised of 4 portions: visual inspection with acetic acid (VIA) staining where lesions are stained white; HPV genotyping using HPV16, 18, 31, 35, 39, 45, 51, 52, 56, 59, 66, and 68; HPV viral load detection; and mRNA-based biomarkers testing. In Phase I, Dr. Kuhn’s team is adapting the Cepheid multiplex qPCR test to improve the specificity of testing for use in SAT in South Africa, as well as determining whether the additional markers such as Ki67 (a cell proliferation marker) and Topoisomerase II- (overexpression of it is associated with neoplasia) can be utilized. The additions of these markers and increased specificity should reduce the number of HPV-positive women without CIN2+ receiving cryotherapy when HPV-based SAT is used. The investigators will further optimize the technology by using a new preservative to determine whether an improved HPV cut-offs can be performed in a single test. The team will also assess whether self-collected vaginal samples may be used for screening in place of clinic samples with a comparison of POC testing with Cepheid-coded testing in Switzerland. In phase II, the investigators will then perform a complete clinical validation study for their optimized test, using a cohort of 2,700 patients in South African clinics
(overexpression of it is associated with neoplasia) can be utilized. The additions of these markers and increased specificity should reduce the number of HPV-positive women without CIN2+ receiving cryotherapy when HPV-based SAT is used. The investigators will further optimize the technology by using a new preservative to determine whether an improved HPV cut-offs can be performed in a single test. The team will also assess whether self-collected vaginal samples may be used for screening in place of clinic samples with a comparison of POC testing with Cepheid-coded testing in Switzerland. In phase II, the investigators will then perform a complete clinical validation study for their optimized test, using a cohort of 2,700 patients in South African clinics
Hepatocellular carcinomas (HCCs) are one of the leading causes of cancer-related deaths worldwide. More than 700,000 new cases are diagnosed each year, with over 600,000 deaths per year. The major risk factors for HCC are hepatitis C virus (HCV) and hepatitis B virus (HBV) infections, and current estimates suggest that approximately 2 billion people are infected worldwide with hepatitis virus and 500 million more are chronic carriers of the virus. In addition, these carcinomas are often completely untreatable in advanced stages in LMICs, and thus contribute significantly to cancer morbidity and mortality in LMICs Each infected individual carries a substantially higher risk (10-100 fold) of developing liver disease such as cirrhosis and HCC. In particular, the emergence and spread of hepatitis C virus (HCV), especially among at-risk populations already facing a significant HIV/AIDS burden, has made HCV intervention a priority for the US government as a complement to our PEPFAR investments. HCV is endemic in Egypt, Nigeria, Cameron and Uganda, and in Nigeria it is the number one cause of cancer deaths.
High-quality screening for HCV can reduce HCC incidence and mortality. If the patients with high viral load are treated early, progression of liver disease to HCC can be reduced. While the WHO, UNICEF and other NGOs are actively working with pharmaceutical industry to make hepatitis drugs affordable for controlling HCV infection, low-cost screening for HCV must also be made available to identify at risk patients, particularly in LMICs. To this end, Dr. Robert Murphy (Northwestern) is developing and fielding a low-cost test for HCV to identify patients at risk for developing hepatocellular carcinoma. The goal of Dr. Murphy’s proposal is to develop low-cost tests for HCV infections for use in LMICs to identify patients at risk of developing HCC, and to guide treatment decisions. In phase I, the investigators are adapting already deployed devices, being used for HIV load detection, to detect HCV. They are further optimizing an integrated extraction and amplification system for detection of HCV genotypes 1-6 by the RT-qPCR based assay. Additionally, they are optimizing a test strip based assay for detecting HCV viral core antigen; and subsequently they will be establishing marketing requirements for the assays. During phase II, the investigators will verify that the HCV tests meet marketing requirements, conduct clinical validation studies in Nigeria, and develop training modules.
Focusing work in Nigeria, one of the countries with highest HCV infection rates and HCC incidence, the investigators will be adapting the equipment that is already in-country to detecting HCV load, and will utilize Nigeria-based expertise of the investigators working in the for clinical validation. Therefore, the project presents special opportunities in terms of using existing infrastructure, resources, and populations with some of the high incidences; and if successful, the technology has potential to substantially reduce HCC incidence.
B. Cancer Diagnosis
Current medical technologies have been developed in response to the needs of the developed countries. As a result these technologies are expensive often requiring large infrastructures to operate. A large population of the world, however, lives in limited-resource areas and thus has limited or no access to these diagnostics tools. The increase in cancer burden around the world affects the low resource countries disproportionality, as cancer diagnosis occurs at later stages with higher morbidity, mortality and high treatment costs. This disparity has created a challenge for the biomedical engineering community to develop medical diagnostics for large portions of the world population with fewer resources and little medical infrastructure.
Non-invasive imaging technologies provide an ideal tool for the development of diagnostic devices. Development of novel imaging methods and advances in information technologies, algorithms and protocol development has opened up new possibility. Utilizing the imaging and computational capabilities of smartphones and hand held devices, “point of care” tools are being developed that provide onsite diagnosis and require little infrastructure. With the increase in the cancer burden around the world, development of such devices is critical to provide healthcare around the world. Cancers such as oral, cervical or skin are easily imaged, while algorithms can differentiate malignant and benign tumor.
Cervical cancer rates have been drastically reduced in the developed nations by the diagnostic ability of PAP smear; however, this method is not readily available in large parts of the world where cervical cancer mortality rates are high. While HPV testing can provide extremely useful negative predictive value, cervical cancer diagnosis often requires examination of the cervix itself. Due to the phenomenon of aceto whitening when 3-5% acetic acid solution is applied, the increased cellular density associated with the development of cervical cancers can often be visually detected. While this method represents the standard in many LMICs, it is highly subjective and prone to error. To address this issue, Kathleen Schmeler (MD Anderson Cancer Center) and Rebecca Richards-Kortum (Rice) have developed a tablet-based, high-resolution microendoscope (HRME) paired with a fluorescent stain for cervical cancer diagnosis. This cost-effective and compact HRME device is a fiber-optic based fluorescence microscope designed to image nuclei stained with the vital dye proflavine. The system uses a battery-powered blue LED directed through a fiber bundle to illuminate tissue of interest. Emitted light is collected through the same fiber bundle, filtered, and returned to a USB camera, and images are displayed on the tablet in real time. When an area of interest is encountered, the clinician presses a foot pedal to acquire an image of the underlying cell nuclei. The software then automatically segments nuclei, calculates nuclear area and eccentricity, and based on the percent of nuclei that show increased nuclear area and abnormal eccentricity, reports to the clinician whether the underlying tissues is likely to be neoplastic or not. The usability of the system has already been demonstrated in urban and rural settings in Brazil, and clinicians found the system easy to use and strongly preferred the incorporation of automated analysis of HRME images at the point of care. The initial performance of the system has also been evaluated in a pilot study of women referred for colposcopy to Barretos Cancer Hospital, with image quality compared to that of the standard high resolution micro-endoscope system. The investigators have incorporated the HRME in a new mobile diagnostic and treatment unit, which will travel to offer diagnostic and treatment services to women who have screened positive during a prior visit by a mobile screening unit.
Breast cancer is the number one cancer killer of women globally and represents a majority of the burden of women’s cancers in LMICs. Many women present with palpable breast masses and due to a lower availability of highly trained medical personnel in many LMICs, a high throughput method is needed to enable minimally-trained health care workers to distinguish between benign and potentially malignant masses. To address this issue, the Dr. Susan Love Research Foundation has developed a low-cost, portable computer-aided detection and diagnostic (CADD) tools for non-invasive screening of breast cancer patients in remote locations in LMICs.
The main components for developing a CADD system for breast cancer triage are image processing, feature extraction, classification, fusion of classification results, and fusion of image results. However, low-cost hardware platforms tend to suffer from overall poor quality of the images produced, severely affecting the performance of clinicians and computer-aided diagnostic algorithms.
Dr. Love’s team has developed a CADD system using a combination of novel feature and classification algorithms. In order to overcome some of the shortcomings of the low-cost hardware platforms, the team is using the ClearView HD pre-processing image enhancement suite. Additionally, they use compressive re-sampling to reduce speckle noise and enhance image quality of low-cost ultrasound systems for diagnostic applications. The CADD system includes a pattern recognition algorithm that incorporates temporal information available during a live ultrasound scan. This pattern recognition algorithm is based on the AdaBoost (“adaptive boosting”) machine learning algorithm, which builds a strong classifier from the combined decisions of several weak classifiers.
Currently, the CADD software operates as a stand-alone system on a window-based computer and can accept ultrasound images from any DICOM-compatible ultrasound imaging device and outputs the enhanced images and the breast cancer triage scores. Bench testing on a database consisting of 867 benign lesions and 643 malignant lesions has shown that this system achieves 92% sensitivity for a reduction in benign biopsies by 40% compared to usual practice. These images were collected from ultrasound machines provided by several different vendors, including GE, Siemens, and Philips.
The system is currently undergoing validation tests at the Keck Medical Center of University of Southern California and Harbor-UCLA Medical Center with the goal of recruiting at least 200 women with palpable masses and determining the CAD performance on this cohort. These tests are using the GE Logiq E low-cost portable study ultrasound imaging device. Subsequently, this project is planning to extend its validation and implementation study to several clinical sites in Jalisco, Mexico.
C. Cancer Treatment
Access to treatment is the most crucial element of a POC intervention. Resource limitations often result in long travel distances to access curative care in many LMICs. To this end, technologies that enable treatment of priority cancers at the POC greatly increase the likelihood that curable cancers receive treatment and improve the quality of life of patients. Additionally, POC diagnostic technologies developed and priced for LMICs that enable assignment of targeted therapy treatments for applicable cancers can also be highly beneficial. For example, imitanib is available in many LMICs for treatment of chronic myeloid leukemia (CML) through programs like the Gleevec International Patient Assistance Program (GIPAP) [3]. It is desirable to have a POC diagnostic test suitable for LMIC use that can identify CML patients with the BCR-ABL fusion gene mutation that would benefit from Gleevec treatment.
Oral cancers, which are easily detected and treated in high-income settings, amount to the second most common cancer and third most common cause of cancer death in India [2]. Due to the widespread popularity of chewing gutka, a tobacco mixture with crushed betel nut and acacia extract, more than 80,000 new cases of oral cancer are reported every year in India. As most cases are diagnosed in later stages, treatment consists mostly of surgery and/or radiotherapy, requiring expertise and medical infrastructure that are often not available in the settings where they are most needed. Even if the disease is detected relatively early, these interventions can be disfiguring and present major quality-of-life issues including difficulties related to chewing, swallowing, speaking, and working, thus increasing the societal economic burden on an already burdened economy. While education and screening are critical, a complete solution must include new therapeutics that are effective in resource-limited settings. Treatment of oral leukoplakia is therefore a priority. To this end, Tayyaba Hasan (Massachusetts General) and Jonathan Celli (University of Massachusetts Boston) have developed a low-cost image-guided photodynamic therapy (PDT) of oral leukoplakia. Motivated by clinical studies PDT for the treatment of oral cancer using the photosensitizer precursor -aminolevulinic acid (ALA),4-9, Dr. Hasan’s and Dr. Celli’s group have introduced a strategy for adapting this light-based modality into a low-cost, portable platform for diagnosis and treatment of oral cancer in low resource settings. A prototype of a low-cost light delivery device, which utilizes high-output 635 nm LEDs coupled to a multimode fiber with a small oral insert to illuminate the affected area with the necessary power density (~50mw/cm2) for clinically feasible PDT treatments, has been developed. Leveraging the well-established tumor selectivity and multifunctional capability of ALA-PpIX to serve as a contrast agent for optical tumor imaging, a simple approach is being finalized by the investigators, using low-cost filters and the imaging capability of a smartphone to ascertain disease margins and guide the placement of the illumination fiber. Preclinical studies have already demonstrated good treatment response (DMBA) using the developed prototype. The project aims to validate clinical performance of the PDT treatment for oral cancers and dysplasia at LMIC sites. Approval to conduct a clinical study planned to start in 2016, at Aligarh Muslim University (AMU, which treats approximately 500 oral cancer patients per year) is being sought and it is expected that all clearances will be received for the study to start on time. This low cost PDT-based cancer treatment technology being developed by this group has the potential for broader impact in neighboring countries in South Asia (Pakistan, Sri-Lanka) where oral cancer is also disproportionately prevalent.
Given its prevalence, interventions targeting cervical cancers are essential. Precancerous cervical lesions, cervical intraepithelial neoplasia (CIN), can be treated and development of invasive disease thwarted. The WHO has provided evidence-based recommendations for the use of cryotherapy to treat women with CIN [4]. Unfortunately, while cryotherapy-based interventions are recommended in most LMICs, access has proven to be a challenge. The traditional cryotherapy devices have relied on clinical grade CO2 and NO2, which has proven to be difficult to obtain in the needed quantities. To this end, the NIH is supporting two projects attempting to address this issue. Dr. Jean Anderson (Johns Hopkins) is assessing the performance, safety and efficacy of a new cryotherapy device using beverage-grade liquid CO2 to produce a comparable results to traditional cryotherapy at significant cost savings, while Dr. Miriam Cremer (Basic Health International) is leading the adaptation and testing of the CryoPen cryotherapy device, which relies on electricity and a sterling compressor based refrigeration system for cooling.
Dr. Jean Anderson and her team created the “CryoPop” cryotherapy device to meet the need for an affordable, simple cervical cancer screen-and-treat modality in LMICs. The vast majority of cervical cancer deaths occur globally in LMICs that lack adequate resources for cancer prevention, screening, and treatment (5, 6). The incidence of cervical cancer in Asia is about 15.4/100,000 with a mortality rate of 8.4/100,000 in contrast to 8.0-9.8/100,000 incidence and 2.2-4.3/100,000 mortality in the United States (incidence and mortality in US is dependent on race) (7, 8). In the Philippines, cervical cancer is estimated to kill 12 women a day.
The CryoPop device is designed to have a modular components that are inexpensive to replace and easy to repair on-site by the care providers. The CryoPop device employs expanding liquid CO2 that is captured as dry ice in the CryoPop applicator using a snowhorn attached to a liquid CO2 source and requiring only 10 seconds to fill. The applicator is then capped with a thermally-conductive CryoTip once filled. With this design, the CryoPop device is not attached to the compressed gas canister during the treatment procedure, which allows for more convenient and safe operation of the device. During treatment, a manually-operated trigger mechanism continuously advances the dry ice toward the CryoTip by a spring-actuated element. The CryoTip transfers heat from the cervix to the dry ice (−78°C) to freeze the cervical lesion. When the trigger is released, it retracts the remaining dry ice and allows the tip to warm and release from the frozen tissue.
Dr. Anderson and her team have already taken the device past basic validation stages and overseen the development of an optimized CryoPop device for use in clinical trials. This device, produced by a contracted manufacturer, has cleared a device safety review performed by independent contract regulatory review board, and has been deemed ready for in-field clinical validation studies. The team has received a Certificate of Exemption has from the Philippines to allow testing of the device locally upon successful completion of performance equivalence testing in a US clinical study.
The CryoPen (Cryosurgical systems) is a device that is traditionally used for dermatology applications, but in 2011 the CryoPen was approved by the FDA to treat cervical precancer lesions. Since this development, the CryoPen has been proposed to overcome the challenges of cervical cancer treatment in LMICs. This is due to the fact that CryoPen achieves cold temperature by the use of compression cooling technology, eliminating the need for compressed gas. Similar to the CryoPop device, when the CryoPen probe is applied to cervical tissue, it draws heat from the cervix, freezing the cervical lesion and producing necrosis of the cells.
Dr. Miriam Cremer and her team have created a LMIC version of the CryoPen for field use, with a built-in handle to facilitate transport, and future adaptability to be powered through car batteries. This new LMIC-adapted CryoPen, developed with consultation with potential users from LMICs, showed to be non-inferior to traditional CO2 Cryotherapy (compared to Wallach LL100 CO2 system) in a pilot study (data unpublished). The end-user identified effectiveness (not inferior than traditional CO2 cryotherapy), ease of use (e.g. ease to apply and ease of cleaning), durability (the CryoPen was tested in harsh temperatures and difficult field settings) and safety (safety will be evaluated in future bench testing) as the most important characteristics of an ideal device. The LMIC-CryoPen prototype was also evaluated in St. Luke’s Hospital in Haiti, where local midwives were trained in its use using ballistic gelatin. Currently with NCI funding the LMIC-CryoPen is being evaluated in Peru and Colombia.
III. Discussion and Conclusion
Point-of-care technologies show great potential in addressing the gaps seen in cancer detection, treatment, and diagnosis in LMICs. In the current 7-project portfolio in the first round of the ACTs program, one can see a broad spectrum of innovative POC interventions in these settings. The portfolio of technologies discussed in this paper include two molecular diagnostic tests, two imaging devices, and three ablative treatment technologies.
In the area of molecular diagnostics we see two projects with closely aligned approaches. Dr. Murphy’s work on HCV detection and diagnosis relies on an RNA-based viral load test with low-cost consumables, while Dr. Kuhn performs a DNA-based viral load test for HPV and relies on the presence of the lab-based GenXpert systems across South Africa with moderately more costly disposables. Both tests rely on PCR to amplify nucleic acid and detect specific viral subtypes. Because they seek to intervene in the natural history of these two cancers by detecting and then treating a necessary condition for the development of disease, these projects both represent global health applications of the trend toward precision medicine in the cancer prevention space.
Moreover, a recurring theme within the ACTs portfolio that is representative of the broader field as a whole is the use of microfluidics in in vitro tests in LMIC settings. The concept of micro-scale testing has always had promise in low-resource settings, as the reduction of testing scale from both a physical and infrastructural point of view links the design criteria for these devices in the developed and developing world [9]. With the advent of paper-based microfluidic POC tests, the cost constraints of low-resource settings can more easily be met. There has already been success with microfluidics in the cancer diagnostics field panning biomarker types, with oncogene biomarkers like E6/E7 (HPV diagnosis) [10] showing strong diagnostic potential, and other biomarkers, including the marker seen in Dr. Kuhn’s HPV diagnostic tool, represent the future of the microfluidic diagnostics in both the developed and developing world. The ability to develop these technologies in parallel despite the constraints provided by low-resource settings justify the work of focused translation LMIC POC research such as that supported by ACTs.
Medical imaging, to many, is a field of cumbersome, expensive, sophisticated machines that take up whole rooms in anything but austere clinical settings. Both of the imaging technologies discussed in this paper operate under a different paradigm. In particular, the technologies are portable, low-cost, and easy to use at the point-of-care by minimally trained healthcare workers. Moreover, Dr. Hasan’s and Dr. Celli’s PDT device relies on image-guidance using CCD camera obtained images froma smart phone.
While the levels of sophistication vary greatly, all of these technologies rely on the application of image analysis algorithms. The impact of these types of tools is potentially high in the global cancer sphere, as many LMICs often suffer from shortages in highly trained clinical staff. In this setting, the use of technologies can often ease the burden of the health care professionals, simplifying the diagnostic and treatment process and allowing duties to shift down the chain of command, optimizing their existing health infrastructures. All of the technologies within ACTs reduce this burden to great degree, but the shift towards computer-assisted detection, diagnosis, and treatment planning as well as image-guided interventions is a particularly powerful example, as it promises to allow clinicians to circumvent the low-resourced pathology infrastructures in many of their countries, potentially providing a true point-of-care diagnosis with minimal training and lowered risk of false positive results
An extension of this computational aspect is the use of mobile devices in the imaging sphere. The m-health revolution has had a profound effect on the global community, as many of the lowest-resource populations still have widespread access to mobile devices. While m-health in global cancer control is commonly seen as a medium for preventative interventions or to reach out isolated patient populations, recently the imaging community has shown great promise in the use of mobile-based imaging platforms. By providing image capture, processing, analysis in the compact and commonplace package of a smartphone or tablet device, these devices can more easily be translated into the infrastructure and practices of clinicians in LMIC settings, reducing cost, size, and training burden. Drs. Hasan and Celli and Drs. Schmeler and Richards-Kortum, through their respective imaging devices, are capitalizing on this movement, one which will only continue to shape the POC technological field, both domestically and globally.
Three ablative treatment technologies were discussed in this paper. Two of these technologies tackle the issue of providing access to cryotherapy in areas where access to large quantities of clinical-grade liquid nitrogen or carbon-dioxide may be unrealistic. These approaches are substantially different. The CryoPen approach relies the elimination of most disposables in favor of a moderately-priced closed system, while the CryoPop relies on the fabrication of a very low-cost delivery vehicle that more efficiently uses a lower grade (beverage grade) of carbon-dioxide. Either of these devices may be appropriate in different clinical settings.
The CryoPop as well as Dr. Hasan’s and Dr. Celli’s PDT device also share an interesting similarity that is indicative of changes in the field. In particular, both devices are fabricated in large part with 3D printers. This type of design may ultimately allow for a much larger paradigm shift in technology development than is within the scope of this article, that is to say, the move towards open source hardware development.
As discussed, the portfolio of the ACTs program contains elements that are and will continue to define the global health POC technology space as it develops and begins to truly shape clinical work in LMICs. However, the program is continually expanding, and as more projects are funded under the umbrella of ACTs, there will be many more concepts in POC technologies that will be explored, including multiplexing point of care technologies. For example, as the cost and size of image capturing and image-displaying devices continues to drop, it can be expected that more surgical monitoring devices could be adapted for use at POC in LMICs, even within treatments already covered by the program such as cervical cancer coagulation/ablation and cryotherapy. Another area of interest would be the translation of treatment beyond the techniques currently being supported.
While the cancer control in LMICs is challenging, early detection and minimally invasive treatments are more likely to yield successful outcomes. In recent years the NIH has demonstrated its commitment to global health research. Resource appropriate medical technologies play an important role in shaping that broader landscape. In the coming years, the NCI and NIBIB will continue to support this area of research by funding the development of low-cost, portable technologies, which have the potential to increase early detection, diagnosis, and non- or minimally-invasive treatment of cancer, for LMICs.
Biographies

Paul C. Pearlman (M’02) received the M.S., M.Phil., and Ph.D. degrees in electrical engineering from Yale University in 2011. He was involved in post-doctoral research with the University Medical Center Utrecht. He has been with the U.S. National Cancer Institute’s Center for Global Health since 2013, where he is currently a Program Director.

Rao Divi received the M.S. degree in biochemistry from Andhra University, and the Ph.D. degree in biochemistry from Osmania University, India, in 1993. He was involved in post-doctoral research with the National Center for Toxicological Research, the Center for Cancer Research, U.S. FDA, and the National Cancer Institute (NCI) from 1993 to 2008. He has been with the Division of Cancer Control and Population Sciences, NCI, as a Program Director.
Michael Gwede, photograph and biography not available at the time of publication.

Pushpa Tandon received the M.Sc. and Ph.D. degrees in biochemistry from the Industrial Toxicological Research Institute, University of Lucknow. He was with the NIH as a Fogarty Fellow. She was involved in research with the U.S. Environmental Protection Agency, Boston College. She was with Wellstat Therapeutics as a Scientific Review Officer and a Program Director.

Brian S. Sorg (SM’07) received the B.S. degree in electrical engineering from the University of Maryland, the M.S. degree in biomedical engineering from Johns Hopkins University, and the Ph.D. degree in biomedical engineering from the University of Texas at Austin. He was a Post-Doctoral Research Associate with the Duke University Medical Center, and an Assistant Professor with the J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida. He is currently a Program Director with the Cancer Diagnosis Program, National Cancer Institute.

Miguel R. Ossandon received the B.S. degree in medical technology from the University of Tarapaca, Chile, the bachelor’s degree in computer science from Georgetown University Washington, DC, and the M.S. degree in computer science from George Washington University in 2006. He is currently pursuing the Ph.D. degree with the University of Maryland, Baltimore County. He is a Program Director with the National Cancer Institute in the Technology Development, Branch. His research interests focus in technology development for low resources settings.

Lokesh Agrawal received the B.S. and M.S. degrees from different institutions in India, and the Ph.D. degree from the All India Institute of Medical Sciences, India. He was with Rapid Pharmaceuticals, Inc. He came to NCI from MedImmune Inc. He directs and leads Biospecimen Research Network-PI-led projects on human biospecimen integrity and biomarker development by studying preanalytical variables using proteomics and molecular approaches.

Vinay Pai received the Ph.D. degree in mechanical engineering from Florida State University in 1997. He was involved in post-doctoral research with the National Heart, Lung, and Blood Institute (NHLBI). He had faculty appointments with New York University, NY and Upstate Medical University, NY. He was a Staff Scientist with NHLBI from 2008 to 2012. Since 2012, he has been with the U.S. National Institute of Biomedical Imaging and Bioengineering (NIBIB), where he is currently directing the Division of Health Information Technology.

Houston Baker received the B.A. degree in physical sciences from Harvard University, and the Ph.D. degree in physiology and biophysics from The Ohio State University. He was a Post-Doctoral Fellow with the Universitat des Saarlandes in electrophysiology and biophysics of excitable tissues, where he was involved in post-doctoral work with the Red Cross Blood Research Laboratory in cryobiology. He is currently with the National Cancer Institute as a Program Director of the NCI Cancer Imaging Program. He served as an Executive Officer of the American Society of Plant Physiologists, and the American Society for Pharmacology and Experimental Therapeutics, and a Health Scientist Administrator with the Walter Reed Army Institute of Research, and the National Center of Research Resources, Center for Scientific Review, National Institutes of Health’s Division of Research Grants.

Tiffani Bailey Lash received the Ph.D. degree in chemistry from North Carolina State University. She was selected as a Policy Fellow with the American Association for the Advancement of Science and the National Academy of Sciences. She serves as a Health Scientist Administrator with the National Institutes of Health. She manages the research portfolios for the Biosensors, Platform Technologies, and mHealth programs with the National Institute of Biomedical Imaging and Bioengineering (NIBIB). She is also the Program Director for the NIBIB Point of Care Technologies Research Network consisting of three centers charged with developing point-of-care diagnostic technologies through collaborative efforts that merge scientific and technological capabilities with clinical need.
References
- [1].Council on Foreign Relations, “The Emerging Global Health Crisis,” CFR.org., Sep. 2016. [Google Scholar]
- [2].International Agency for Research on Cancer. (2012). Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. [Online]. Available: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- [3].Tekinturhan E., et al. , “Improving access to care in low and middle-income countries: Institutional factors related to enrollment and patient outcome in a cancer drug access program,” BMC Health Services Res., vol. 13, p. 304, Aug. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Use of Cryotherapy for Cervical Intraepithelial Neoplasia, WHO Guidelines, World Health Org, Geneva, Switzerland, 2011. [PubMed] [Google Scholar]
- [5].Lancet T., “Moving cancer up the global health agenda,” Lancet, vol. 375, no. , p. 2051, Jun. 2010. [DOI] [PubMed] [Google Scholar]
- [6].Jacob M., Broekhuizen F. F., Castro W., and Sellors J., “Experience using cryotherapy for treatment of cervical precancerous lesions in low-resource settings,” Int. J. Gynaecol. Obstetrics, vol. 89, pp. S13–S20, May 2005. [DOI] [PubMed] [Google Scholar]
- [7].Sawaya G. F. and Grimes D. A., “New technologies in cervical cytology screening: A word of caution,” Obstetrics Gynecol., vol. 94, no. 2, pp. 307–310, Aug. 1999. [DOI] [PubMed] [Google Scholar]
- [8].Collins Y., Holcomb K., Chapman-Davis E., Khabele D., and Farley J. H., “Gynecologic cancer disparities: A report from the health disparities taskforce of the society of gynecologic oncology,” Gynecol. Oncol., vol. 133, no. 2, pp. 353–361, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chin C. D., Chin S. Y., Laksanasopin T., and Sia S. K., “Low-cost microdevices for point-of-care testing,” in Point-of-Care Diagnostics on a Chip, 1st ed. Heidelberg, Germany, Springer, 2013, ch. 1, sec. 2, p. 4. [Google Scholar]
- [10].Kraus I., et al. , “Presence of E6 and E7 mRNA from human papillomavirus types 16, 18, 31, 33, and 45 in the majority of cervical carcinomas,” J. Clin. Microbiol., vol. 44, no. 4, pp. 1310–1317, Apr. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


