Abstract
This paper describes why “device state” and “patient context” information are necessary components of device models for safe interoperability. This paper includes a discussion of the importance of describing the roles of devices with respect to interactions (including human user workflows involving devices, and device to device communication) within a system, particularly those intended for use at the point-of-care, and how this role information is communicated. In addition, it describes the importance of clinical scenarios in creating device models for interoperable devices.
Keywords: Safety, interoperability, state, context
Device state" and “patient context” information are necessary components of device models for safe and secure interoperability. It is important that the roles of devices with respect to interactions (including human user workflows involving devices, and device to device communication) within a system are characterized.
I. Introduction
It is useful to apply a model of interoperability, such as the Levels of Conceptual Interoperability Model (LCIM) [1] to better conceptualize the challenges of medical device interoperability, and help understand the information needed to achieve safety in the medical application space. Tolk’s model, consisting of five levels, was later extended to seven levels by Turnitsa [2] to characterize the types of interactions taking place both within the system and externally. Tolk and Turnitsa postulated that there are operations that need to be performed to enable safe interoperability, regardless of whether these operations were performed by human designers, human operators or interacting devices. If a device is designed to act in the place of the human, it is logical the device model would need to include at least the same information that the human considered to assure the safety of the patient. This information relates to the state of the devices and patient, the context of clinical care, and all relevant assumptions relating to the associated hazards, risks and mitigations.
Robkin et al. [3] applied Tolk’s and Turnitsa’s model of conceptual interoperability to medical devices and healthcare, and further showed that for systems to achieve an appropriate level of interoperability, the design process of the developers (of the interoperable components and interfaces) must be working at a higher level of interoperability. Current healthcare delivery systems are replete with examples of both successful and unsuccessful interoperability. Some of these examples are a failure of semantic interoperability, in which data is interpreted differently by the two parties, or of dynamic interoperability, where the two organizations don’t have a shared understanding of the clinical state, and context. We postulate that the exchange of information via a robust device model requires dynamic interoperability and is necessary to achieve safe interoperability. This paper examines the use of clinical scenarios to capture, characterize, and make this information explicitly available through an electronic data interface (EDI).
In “Solving the Interoperability Challenge” [4], Goldman highlights the challenges of interoperability,1 the ability of medical devices, clinical systems, or their components to communicate in order to safely fulfill an intended purpose [5], which is illustrated by the slow pace the medical device industry has taken to achieve plug-and-play (PnP) (i.e. seamless device interfacing based solely on configuration, not custom software development). Goldman proposes an approach in which the clinical community defines interoperable clinical scenarios where the workflow and interactions, both between the human operators and devices and between devices are appropriately specified and hazardous situations that may arise are identified. The shared use and understanding of clinical scenarios helps manufacturers, researchers, standards development organizations to achieve conceptual interoperability between themselves - that is they will have identical understanding of their mutual goals, scope, and constraints, and shared understanding of the relevant patient context. The implementation of these clinically meaningful scenarios, which are enabled by interoperable components, can be used as a metric for the adoption of interoperability. A recent AAMI publication [5] in its overview of the landscape of medical device interoperability including standards, stakeholders and issues, notes the major challenge in achieving medical device interoperability is enabling medical devices to communicate in a meaningful way. A key component of enabling meaningful device communication is the creation and use of device models that include device attributes and clinical context.
II. Medical Device Interoperability Examples
Two examples of interoperable components in current use in the medical device industry will help illustrate the benefits, challenges and importance of achieving widespread adoption of interoperability. The standardized use of the R-wave of the ECG for synchronizing the delivery of defibrillation pulses to the patient (see [6] -section 104) or the de-facto standard for the fetal ECG digital interface (with physical/data link and application layers specified) [7] are examples of the successful application of interoperability to enable a clinical application. The safety of these de-facto standards, specifically in terms of the plug-and-play interoperability (as opposed to the safety of the medical device or the connection itself), may not have been considered during the design of the components, but were accepted over time via demonstrated safe interoperability proven through widespread use, leading to adoption by numerous third parties. This could not have been achieved if the interface specifications had not been available to third party device manufacturers, and the context, intent, and use of the connectors well known to both device and the connector manufacturers. In the absence of the exchange of formal specifications, all parties must have the same understanding of context, intent, and use to prevent failures and unintended interactions.
III. Challenges in Wider Deployment of Medical Device Interoperability
The ASTM standard F2761, “Medical Devices and Medical Systems — Essential safety requirements for equipment comprising the patient-centric integrated clinical environment (ICE) ([8], Annex B) describes several clinical scenarios that lack interoperability and lead to unsafe clinical outcomes. The application of concepts used in the distributed processing field [9] can help to analyze these clinical scenarios. These concepts require knowledge of design aspects of the medical device and its associated capabilities, which F2761 refers to as a device model. It asserts that the F2761 device model includes sufficient information for communicating state and context information to enable the safe clinical use of the system comprising components, each with their associated device models. It is important to realize that the system device model (e.g. ICE device model) may have more information (e.g. physical location of device sensors and body position, care location such as ICU or home environment) than the sum of the component device models. Integrated systems can enable monitoring of device behaviors and clinical effects of nefarious activity, such as resulting from cybersecurity vulnerabilities. In this perspective, comprehensive, contextually rich data from networked devices, enables improved cybersecurity.
Platforms supporting plug and play (PnP) allow devices and applications to be connected or disconnected on-demand by supplying the underlying physical and data infrastructure to communicate information and control signals between devices and applications. PnP systems allow for the development of “apps” that reuse existing infrastructure. Goldman’s goal of creating “Lego blocks” (http://psqh.com/janfeb08/connected.html) for developing safe and interoperable healthcare applications is dependent in part on the ability of the device model to adequately represent the information required for safe use. The integrated clinical environment (ICE) standard [8] describes a system architecture and specifies the need and overall structure of a device model to represent the capabilities of the equipment and serve as the repository for this essential information including information to qualitatively and quantitatively describe, control and monitor the system. Reasons that PnP is not widely implemented in the medical device space is due in part to the absence of a formal, widely adopted definition of a device model, and an ecosystem for development and maintenance. These two pieces of the puzzle need to be resolved and clearly defined to achieve safe interoperability through PnP. The use of device models to enable reliable communication and data sharing between components within a system are well known and previously used in: (a) industrial bus standards [10] to allow data sharing between devices (e.g. controllers, data acquisition components, sensors) of different types and generations and (b) standards supporting the sharing of electronic health information (e.g. HL7 RIM) [11] based on a static reference model of health and healthcare information.
Device models are only one means of encapsulating the information needed to help assure reliable data sharing and, with respect to medical devices, helping assure safe interoperability. They provide the system designers content and context on how a component should be used, describe the operation of the device relative to the interactions that occur in the clinical scenario, and document the information that must be conveyed or received. When device models are shared across a community, they enable platforms and their components to be leveraged and reused. Device model re-use may lead to more rapid and efficient product development. An important caveat about the use of device models is that all parties (e.g. components) involved in an interaction has to possess the same understanding of the device model or unexpected and unintended consequences can emerge.
IV. Determining Relevant Attributes of a Device Model Related to Safety
A medical device may simultaneously function as a stand-alone device and as a component in a larger system. Therefore, it is important that this device is able to communicate the content of its device model via the EDI. The device model of a component, from a systems perspective, contains functional and non-functional specifications governing the safe operation of that component in the system. In addition to the information identifying the device hardware, software and connected accessories, various aspects of the “internal” operations of a device may be necessary to include as part of the device model. This information is identified by understanding the role the device plays in the system, the patient care environment, and what actions the human operators perform in order to maintain the safe operation of the device. The nature of the information required for a more complete and complex device model is documented in the form of clinical scenarios.
To characterize thoroughly the interactions of an interoperating device, a range of clinical scenarios should be investigated involving multiple medical disciplines, clinical environments, and including hazard information. The interactions between device and system components studied could include considerations such as: the use of legacy devices, plug and play capabilities, the ability to transfer device settings from one clinical venue to another, (physiologic) closed loop control, safety interlocks, the ability of a system to capture adverse event information, time synchronization, alarms (parameter, types), decision support, and either workflow or checklist enforcement. The clinical environments surveyed should be sufficiently broad to include the intended uses of the device or system such as the operating room, intensive care unit, post anesthesia care unit, outpatient, emergency department, transport, and home care.
Given the distributed nature of platform-based systems such as those anticipated by ASTM F2761, it is appropriate to apply the terminology and approaches outlined in the existing body of knowledge for open distributed processing of information technology to safe medical device interoperability. The standard ISO/IEC 10746-2-2009, RM-ODP Reference Model Open Distributed Processing defines a model in which the objects in the system identified from the clinical scenarios, the interfaces, their interaction points, and the behaviors of each object (a behavior is a collection of actions together contained with a set of constraints) are determined. To achieve safe plug-and-play dynamic interoperability, device and patient states, and use context must also be gathered and exchanged [3].
A contract has been described as “An agreement governing part of the collective behavior of a set of objects. A contract specifies obligations, permissions and prohibitions for the objects involved.” (ISO/IEC 10746-2 clause 13.2.1) [9]. This standard specifies two types of obligations or contractual behaviors: implicit and explicit. The current behaviors and proposed future states described in clinical scenarios may be classified based upon relationships amongst the components of an interoperable system: those relationships that assume an explicit knowledge (“resulting from actions as defined in the contract”) of the contract and those that rely on an implicit contract (transparent to at least one party). The exchange of information in a contractual manner, either explicitly during the operation of the system or implicitly with all parties having the same understanding of the model, allows higher levels of safety to be achieved. For this to occur, it is necessary a contract established during system design governs the behavior of the components. The device model is one means to represent the content of the contract. It should have sufficient information about state and context to support the safe operation of the system.
V. Examples of Shared Device Models
Two parties (e.g. devices or system components) may have a contract (e.g. “use the same device model”) and still the overall system can be considered unsafe. For example, a sensor or algorithm problem may result in spurious data generated and transmitted by a pulse oximeter, despite the fact that both parties, the sender and the receiver, are using the same data and agree on the syntax and semantics of that data. To achieve higher levels of interoperability and safety, the device model may need to include additional information such as signal quality, data constraints (e.g. parameter limits), event rules (e.g. alarms thresholds), states of devices (e.g. amplification or filter modes), pre-post conditions (e.g. remote or adaptive updates to thresholds), and temporal properties (e.g. ordering of commands, events). Interactions that rely on an implicit contract-one that is assumed to be in-place rather than verified-may be, in certain cases, acceptable; but only for systems that tolerate mismatches between the interactions of system components. In order to better assure safe and successful device interactions when changing the clinical context, or device state, explicitly shared knowledge of a device model may be needed. This distinction is presented and considered necessary because devices from different manufacturers may use different device models and may not have been designed with a device model in mind (i.e. not manufactured with the intent to be interoperable). The transmission of data from legacy devices have at times been problematic because the semantics, context, and state of the legacy device may be assumed; however, assumptions will lead to incorrect data, missed data, or misinterpretation (e.g. time synchronization, assuming there are no oxygen desaturations when in fact the filter was set to maximum smoothing thereby hiding transient desaturations).
VI. Interaction Patterns Using Device Models to Support PNP Interoperability
A series of interaction patterns are presented below illustrating the implicit versus explicit nature of the contract between “objects” and the important role that state and context, with both having static or dynamic aspects, play in defining the attributes of the underlying device model supporting safety. This illustrates that an essential component of the device model is to capture the behaviors (actions) taken by the system components as they interact. This interoperability behavior (IB) consists of two sender/receiver component interactions and the simplest type of (i.e. no behavior) component interactions. The two primary component interaction types are:
-
1)
(IB 1) the sender and receiver are using an implicit device model (i.e. the sender is broadcasting to anyone who will listen; the receiver is listening to anyone who is broadcasting). In this IB, there is no attempt by the sender to change the state of the receiver.
-
2)
(IB 2) the sender has an explicit contract with the receiver and may intend to change the state of the receiver.
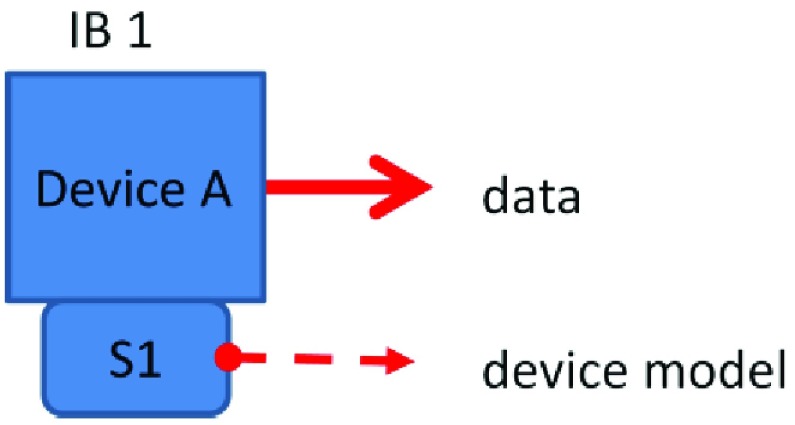
The simplest cases are where a device does nothing (doesn’t send or receive) or simply passes information from another source. A stand-alone unconnected device exhibits the simplest behavior. A human needs to perform any desired interactions between the devices. Many current devices exhibit behaviors per IB 1, transmitting data to a repository according to its own device model without regard to the capability of the connected receiving device to interpret that data. This behavior sends data with no intention of changing a receiver’s state (Figure 1). For example, devices often send data to an electronic health record, where the performance and semantics of the physiologic signals are assumed to be known by all who use the system.
FIGURE 1.
Interoperability Behavior (IB) 1 – Device A broadcasts data according to device model S1 using implicitly assumed semantics and is at least syntactically interoperable with the receivers.
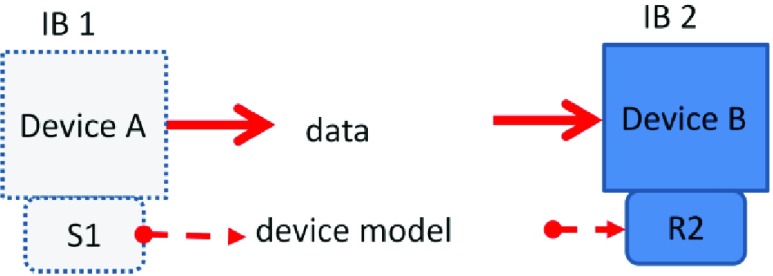
The second behavior, IB 2 (Figure 2), involves receiving data from a broadcaster. The receiving device does not know anything about the sender’s device model or if the data is valid, only that if data has appeared on its EDI. For example, some legacy medical devices may use RS232 without flow control to transmit from their functional connection. Any receiving system may not comprehend the relevance or reliability of this transmitted data.
FIGURE 2.
IB 2 – Device B receives data and interprets it as device model R2 and is unaware of the sender’s state or device model S1 (implied relationship-ISO/IEC 10746-2) Device B is at least syntactically interoperable with the senders device model S1.
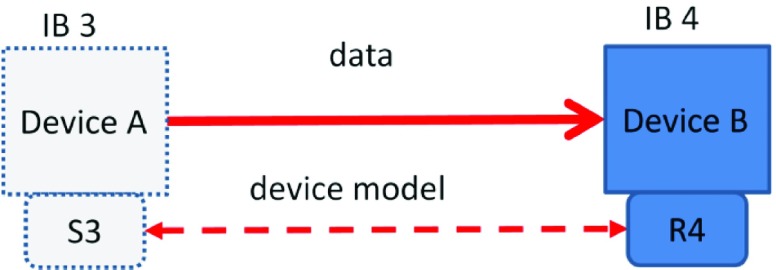
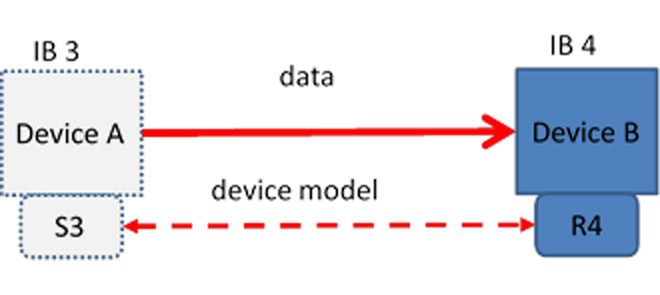
The third behavior, IB 3 (Figure 3), involves a sending device that intends to change the receiver’s state and therefore must have an explicit contract with the receiver.
FIGURE 3.
IB 3/4 – Device A sends data (IB 3) with a device model S3 with the intention of changing Device B’s state. Device B receives the information (IB 4) and acts on its content changing its own state; device model R4 is dynamically interoperable with device model S3.
IB 4 (Figure 3) is the receiving part of the exchange-it changes its state based on the incoming data and likewise must have a contract with the sender. As an example, a closed loop oxygen controller in the system, to which the pulse oximeter/controller sends a command to change the delivered oxygen concentration, is an example of this interaction. An IB 4 receiver can accept information from an IB 1 sender but will have to perform extra work to assess its validity (perhaps by getting confirmation from the operator that the information is valid).
IB3/4 adds the need for a contract between the sender and the receiver to agree upon what the device model is. IB’s 1-2 require syntactic interoperability while IB’s 3-4 require dynamic interoperability (a shared understanding of each other’s states and context).
VII. Device Model Content-Categories of Information
It is up to the manufacturer of the application who is asserting a safety or performance claim to determine the suitability of components for use in their system and specify acceptable device models. The information to be included in the device model is wide ranging and can include [8]:
-
•
Physiologic parameters and waveforms
-
•
Technical and physiologic alarm status and conditions
-
•
Device control functions
-
•
Patient information
-
•Technical information
-
•Real-time/quality of service information
-
•Security controls
-
•Data constraints
-
•
-
•
Clinical context
-
•
Device state
-
•
Role of the device (in terms of sender/receiver)
The categories of data that may be defined for and available on request from a particular device can include:
-
a.
Parameters and units of measurement: Parameters and units of measurement used within the device (e.g. measured and derived parameters, waveform values and derived indicators)
-
b.
Equipment identification: Information identifying the device (e.g. manufacturer, model, serial number, software and/or firmware versions)
-
c.
Equipment Configuration: Information identifying the particular configuration of the device. (e.g. type of accessory connected)
-
d.
Equipment specifications.
-
e.
Equipment settings: Settings relating to the control and operation of the device.
-
f.
Service monitoring: Indicators relating to preventative or corrective maintenance of the device and its accessories.
VIII. Device Model in Real Life
In addition to these categories of interactions and data, manufacturers are encouraged to leverage the additional capabilities afforded by an external connection allowing data sharing and device interactions. These capabilities include the use of intelligent algorithms (e.g. closed loop control) that may reside in the device which may adjust internal algorithms or display settings based upon information received externally. Additionally, devices can have a dual nature-as stand-alone or as a component in a larger system by supplying data or therapy as a service. Standards have begun to take this approach further by considering broader clinical use cases and encouraging the availability of contextual information through the external interface. The most recent drafts of the continuous positive airway pressure delivery devices (ISO 80601-2-71:2015) and Pulse Oximeter (ISO/CD 80601-2-61) particular standards are two examples of this new direction. In the example of the pulse oximeter, this may include: location, status, and data from other sensors. Erroneous pulse oximeter values could be prevented if the pulse oximeter was aware that its sensor was on the same limb as the blood pressure cuff and of the cuff’s real-time inflation status. An example of the content of these categories of data for this particular device, the pulse oximeter, are in Table 1.
TABLE 1. Selected information in an exemplary pulse oximeter device model.
| Category | Examples |
|---|---|
| Parameters and units of measurement | SpO2 Pulse rate, Pulse Plethysmographic Waveform, Signal Quality Metric |
| Equipment identification | Manufacturer, model, serial number, software version and firmware version, unique device identifier (UDI), operating system version, anti-virus software version |
| Equipment configuration | Sensor Type Connected (reusable/single patient use; adult/pediatric; finger/ear), Sensor Model Connected |
| Equipment specifications | SpO2 accuracy, declared ranges of SpO2, Accuracy under motion and low perfusion, pulse rate accuracy, declared ranges of pulse rate |
| Equipment settings | Data Update Period, Averaging Time, Gain |
| Service monitoring | Remaining sensor life; next periodic maintenance date, time that real-time clock was last set |
One effort to capture the information necessary for a specific device’s “device model,” driven by the MD PnP research program, is the Medical Device Interface Data Sheet (MDIDS) project. The MDIDS intend to serve as an extensible reference for standards development organizations (SDOs), manufacturers, researchers, and clinical organizations. MDIDS include both “generic” and device-type sheets with all sheets including device identification and a description of the data encoding used for device-specific data elements, as well as aspects of clinical and system context. [12] (http://mdpnp.org/mdids.html) Preliminary MDIDS documents developed for devices include the pulse oximeter, critical care ventilator, anesthesia workstation, defibrillator, and dialysis machine. The data items for the MDIDS derive from existing devices capabilities and analysis of the future device capabilities necessary to support identified clinical scenarios. One device’s MDIDS, “the Anesthesia Workstation” includes, in addition to the generic list, an additional 19 measurement variables and 113 alarms (www.mdpnp.org). One standardized device model construct central to the ISO/IEEE communication standard is the domain information model, based on a device-centric paradigm, which comprises objects containing the representations of the data and their relationships [13].
It is vital to characterize device behavior, especially in regard to electronic data inputs, outputs and responses to commands. This is the information captured in the MDIDS. With increasingly complex interoperable environments, it is likely that not every potentially hazardous situation will be anticipated during the design, testing, and implementation of an interoperable medical system. It is important to minimize unintended behaviors, including those deriving from emergent properties. The MDIDS information could be used by manufacturers (including app developers) to identify information available for interoperable functions, as well as for performing risk analysis. A system data logger, a key component in an ICE compliant system [8], can be used to capture these undesirable behaviors for possible inclusion in the MDIDS.
IX. Selected Examples Illustrating the Importance of a Contract Between Components and its Content
This section describes five clinically relevant examples, primarily at the point-of-care, that highlight the importance of a complete contract and the necessity of a device model based on this contract to achieve a safe system (or to achieve safe interactions between devices). For each example, the information or interaction lacking is described in both a clinical context in the text and in a risk- based context in Table 2.
TABLE 2. Selected clinical scenarios.
| Knowledge of device settings | Physiological feedback | Location awareness | Knowledge of parameter signal processing | Device synchronization | |
|---|---|---|---|---|---|
Devices 
|
#1)Pulse oximeter used for sleep screening | #2)PCA pump* | #3)Finger pulse oximeter and BP cuff on the same arm | #4)EHR and medical device data | #5)X-Ray/Bypass and ventilation, or Oxygen and Laser devices in use * |
| Cause | Averaging time in a pulse oximeter is variable and may not be made known to the user/app | Architecture or device defect; device not capable of acknowledging, no feedback signal was architected into the platform | Inflation of cuff and monitoring of SpO2 at the same time on same limb. State of NIBP not made known to App so that it can ignore the SpO2 data | Clinically relevant events may not be captured in EHR | Failure to ventilate after X-ray procedure or resumption of cardiopulmonary bypass or failure to lower oxygen fraction during airway laser use. |
| App or System failure mode | Transient desaturation will be missed; data does not map/match to actual physiological parameter | Unknown whether command was followed or seen by pump. Unknown what state pump is in. | State of NIBP not known to App. It can ignore SpO2 data. App misinterprets SpO2 signal. | State and context may not be known | State of ventilator not known to X-ray or bypass machine. State of O2 delivery unknown to laser device. |
| Local failure effect | App underdiagnoses severity of respiratory depression due to sleep apnea. | Medication overdose; App keeps seeing physiological information that is in conflict with pump stopped. | False positive desaturation leading to stopping infusion inappropriately; false positive indication of probe unreliability | Rapidly changing clinical event may not be captured in patient record leading to failure to properly treat condition | Failure to ventilate leading to hypoxia Failure to lower oxygen levels leading to airway fires |
| Larger systemic effect | Injury or death | Injury or death | App fails to run as intended | Injury or death | Injury or death |
| System hazard/ requirement | App needs to know averaging time | pump state needs to be available to confirm stop command | NIBP should communicate the status of cuff inflation and location | State and context need to be captured by EHR-including clinical data-sufficient time resolution | Ventilation Disable should be limited in time, linked to alarms and synched to therapeutic devices |
| General class of hazard | State of the device was not conveyed to the app | Indication of state of the device not conveyed to the app; defect in command and control. | Context (device placement) and State (measurement event synchronization) of device needed to make correct decisions | Context and state not conveyed to EHR | Operational state of devices need to be shared on a timely basis |
| Patient Context | Patient in sleep lab. | Hospitalized patient in bed on PCA infusion pump. | BP cuff proximal and ipsilateral to SpO2 probe | Clinically relevant events not recorded- care may be impacted. | Ventilation Paused means patient is at risk for hypoxia. O2 concentration not lowered means risk of fire and burns |
| Device and Patient States (future) | Device: Oximeter in “fast mode” and patient not moving enables system to distinguish oxygen desaturation from noise caused by patient movement | Device: PCA pump and physiologic sensors interoperable Patient: early respiratory depression detectable | Device: Inflated BP cuff with SpO2 probe distally located Patient: artifact on SpO2 identified | Device: medical device with settings known to EHR Patient: transient events recorded | Device: Ventilator with X-ray or O2 device with Laser Patient: lack of ventilation or excess O2 detected |
| Device-device interaction | Device configuration (operational state) based on clinical status | Pump must be able to process external stop command and change its own state | SpO2 must be aware of NIBP state to avoid errors | Lack of complete data set inhibits clinical interpretation | State of O2 device must be known, exchanged, and synchronized with laser device |
These scenarios are based on content from Annex B of ASTM F2761 [8].
Example 1:
A pulse oximeter used for sleep screening. Device model is inadequate; lacks information about device model “state” (e.g. configuration settings).
Consider a clinical scenario where a pulse oximeter is supplying saturation values to another device (e.g. a decision support component) that is computing a derived index (e.g. lowest SpO2, apnea/hypopnea index, number of respective events per hour) to assess the severity of a patient’s sleep apnea. The effectiveness of these indices, such those detecting the nadir of transient desaturations, has been shown to be dependent on the SpO2 averaging time [14]. Therefore, the component computing the index needs to know the pulse oximeter SpO2 averaging time so it can determine whether it is appropriately set for the current patient state. If the device sending the information and the component receiving the information and computing the index are developed without a common understanding of those device states and the patient context (IB 1 and 2 respectively, i.e. they don’t share the device model for the specific pulse oximeter in use) then the performance of the component computing the index may not meet the sleep-analysis manufacturer’s specifications. The computing component does not determine whether the data is right, it computes using the data it receives (IB 2). It is the responsibility of the system to insure that the computing component is sent the appropriate additional parameters to ensure that the correct transmitted data is based on clinically appropriate settings. In the situation with inappropriate settings, possibly determined by querying the pulse oximeter, the operator should be alerted to set the correct averaging time on the pulse oximeter (i.e. IB 1) or if the functionality is available, the computing component can configure the settings in the oximeter, illustrating IB 3.
Example 2:
Patient controlled analgesia (PCA) pump interlock. This device model does not contain operational state of device or does not make state of device externally visible.
Consider a clinical scenario that implements a safety interlock to halt a PCA pump when respiratory depression is detected. In the case of an open medical platform, a software application or an “App” diagnoses early respiratory depression and sends a command to the pump to stop infusing prior to patient harm. If the pump interface is incompatible with the App sending the stop command, IB 1, where the pump cannot be remotely controlled, the pump cannot be stopped remotely. The App may not receive acknowledgment that the pump stopped, which is important device state information to confirm safe operation. It may be that the pump was not designed (legacy pump) or configured to acknowledge the stop command or that the pump has malfunctioned. Examples of other important state information could include: the operator may halt the pump completely instead of returning to a ready state; the pump motor may require a full revolution to stop instead of stopping immediately, thereby delaying an immediate stop request. These are examples of the interaction being dependent on the state of the component, the pump, receiving the signal [15].
Other factors to consider in a device model, related to state and context of use may be: How long does pump take to shut off? Was the pump in the correct mode of operation such that it could be shut off from another system component? Is the pump required to send a message back to the controller that it is shutoff to confirm its actions? All possible pump scenarios need to be considered in order to construct a well formed contract and device model.
Example 3:
Finger pulse oximeter and Blood Pressure (BP) cuff (same arm). The device model does not know the context (e.g. BP cuff proximal to SpO2 probe).
Consider the situation of a patient with an automated non-invasive blood pressure (NIBP) cuff placed on the arm and a pulse oximeter finger probe distal to that cuff on the ipsilateral arm. The periodic inflation of the cuff impedes both arterial and venous flow from that arm and as such causes intermittent saturation and pulse rate errors associated with cuff inflation. The impact of this cuff-induced hypoperfusion [16] on the time of the disappearance of displayed saturation values is dependent on the particular pulse oximeter (i.e. its device model). Communicating the state of the non-invasive blood pressure monitor to the pulse oximeter in order to indicate that the blood flow is being perturbed could help prevent erroneous data from being displayed and reported, thereby potentially preventing erroneous clinical diagnoses (IB 3/4).
Example 4:
EHR and medical device data. The device model is implicit on receiving end.
Consider the transfer of physiologic data from a bedside monitor to the electronic health record (EHR). What actually is recorded and how it is recorded (frequency/format) is dependent on the settings in the physiologic monitors. Relevant portions of the device model in the form of metadata, such as configuration settings for signal filters and alarm values, are rarely transmitted (IB 1). Nor is a time stamp indicating when the data was acquired. Typically, the time stamp within the record reflects time of receipt. As a result of this missing information, when the waveforms are recalled for later viewing, the absolute time and time alignment between the signals is unknown. As in Example 1, not knowing the settings of the waveform filter that was applied during the data collection may prevent or hinder definitive conclusions being drawn about certain clinical features (e.g. rapid desaturations, ST segment analysis) [17]. The data retrieval and interpretation problem is further complicated by having waveforms saved in separate data stores, and possibly not readily available, as the information in the waveform not extracted earlier as parameters may be lost. Even if the data was stored in the same data store but was collected as part of a multi-center study, the implicit device model from each center using different brands or models of the same type of monitor may not be consistent. In this case, the data cannot be reliably pooled, analyzed or compared without shared semantics.
Example 5:
X-Ray/Cardiopulmonary Bypass, and ventilation or Oxygen and Laser use. The device models do not share operational states.
Consider the situation during surgery when ventilation therapy must be temporarily interrupted for a short procedure; for example, the need to synchronize the interruption of supplemental oxygen delivery with the operation of a CO2 laser, so as to minimize the chance for an airway fire. Even with “laser safe” tubes, it is recommended to maintain the minimum inspired oxygen concentration necessary (e.g. close to room air) during the operation of laser within the airway [18]. With an interoperable system, the laser system should not turn on until it gets a message from the oxygen delivery device that its state is OFF (IB 3/4) or that inspired oxygen is at a safe level. Similarly, the oxygen concentration should not increase until its gets a message confirming that the laser state is off.
A similar level of coordination or synchronization supporting safety is required for an abdominal or chest X-ray performed during surgery on a ventilated patient. The procedure may require the temporary cessation of ventilation to prevent motion-induced image artifact or to time the exposure with the breath cycle. In the case of a cholecystectomy (gall bladder removal) with intraoperative cholangiography (x-ray), the ventilation is stopped and the cholangiogram is performed with contrast to identify internal structures. Depending on the procedure, the synchronization with the imaging can be with either expiration or inspiration. A group at the University of Florida prototyped a system to synchronize the image with the end of inspiration to ensure that the images are obtained at maximal lung inflation; thereby improving the quality of the radiograph. [19]. In order to obtain a clinically relevant image, the X-ray should not record an image until it receives a signal that ventilation is suspended. Similarly, ventilation should not resume until it receives a signal that the X-ray procedure has been completed or ventilation should resume automatically on its own if the signal is not received within a defined time interval (IB 3/4). Manually disabling ventilation for an X-ray procedure followed by unanticipated equipment problems have led to excessive delays in the resumption of patient ventilation with unfortunate outcomes [20]. An interoperable platform-based approach to this failure to ventilate has been prototyped [21]. Although not available commercially, enabling device capabilities have been incorporated in the latest Anesthesia Workstation and Critical Care Ventilator standards. This coordination requires a device model include the clinical state of the patient (mechanical pulmonary ventilation) and the state of the device (inhalation, exhalation, pause). Analogous to the X-ray-ventilation scenario is the situation with the temporary cessation of ventilation associated with the use of cardiopulmonary bypass. An excessive delay in the resumption of ventilatory support post-bypass can be life threatening or result in major organ damage [22]. In the scenarios highlighted, integration of the devices into an integrated, networked system could improve patient safety by the sharing of operational states between devices thereby minimizing the likelihood of a failure to ventilate or deliver higher levels of oxygen during airway laser procedures.
X. Discussion
Components within a system need to have an equivalent understanding of the interactions that occur and the information exchanged in order to function safely. Differences between the model for the sender and receiver can lead to hazardous situations (e.g. R2 not compatible with S1). This may or may not have clinical ramifications depending on the intended use of the system. For example, manufacturer A may choose to display vital sign trends on their front panel display using data collected from the output of a different multi-parameter monitor. Manufacturer A establishes the characteristics of the signals needed, as stated in the labeling. For some of the display “trend” parameters, there may be accepted standards or guidelines such that the signals can be used and interpreted properly. Wide spread adoption of legacy industry standards (e.g. safety/performance or informatics standards) may allow users to reach a consensus about the implicit device model. The opposite situation may exist with closed loop control, as in the case of manufacturer B controlling ventilator settings (e.g. optimizing FiO2 and/or PEEP) based on parameters derived from the pulse plethysmogram (e.g. SpO2 values) as measured with a pulse oximeter. In this case, each manufacturer has their own “private” device model hence there is no widely accepted standard for the pulse plethysmogram data, nor is there a standard model for the response time of an oximeter.
In the case of the above manufacturer A, the receiver or consumer of the data, (e.g. the trend or EHR vendor) may spend considerable effort to assure reliable communications from the physiologic monitors while assuming that the underlying signals (e.g. ECG, NIBP, SpO2) are sufficiently standardized and accurate and as such that revalidation of the clinical performance is not needed. This is an example of where the contract is implicit [21].
If the sender does not intend to change the state of the receiver (e.g. populating the EHR), a manufacturer’s risk analysis has less complexity than if the intent is to change the state, as the consequences of the state changes have to be considered for the latter. If the receiver intends to change its own state based on the received data, a more complex risk analysis is required as it needs to include the possible failure modes of the sending component.
Many medical devices fall within the scope of point-of-care (POC) technologies. These include POC laboratory tests, vital sign measuring technologies, and infusion pumps. This paper has described a conceptual framework with relevant clinical examples to emphasize the importance of and role of state and context in the design of medical devices. This is particularly crucial for devices used at the POC where context determines the requirements for the device model in terms of available parameters and device capabilities. Even though unique device identifiers and control of access to the device information would be included in a component’s device model, matters such as operator identification and authentication are related more to system-level design aspects and can serve as a means for verifying that the correct components are being used, rather than determining correct device behavior. An interesting challenge with POC technology can arise when non-experts are involved in applying medical sensors. For example, misapplication of a blood pressure cuff could cause over or under-reading of blood pressure values. Therefore, the validity of data may be influenced by the state (correct or incorrect blood pressure cuff size for that patient) and clinical context (patient active during measurement).
XI. Conclusion
In the paper, the authors have identified the different kinds of information that need to be included in a contract between system components in the form of a device model (e.g. state, context, change of either) and have discussed where this information comes from, including approaches to capture information and why the information in the device model is necessary for safety. The paper has described an approach to achieve safe medical device PnP using comprehensive device models that are “standardized”-characterized by dynamic interoperability between devices and conceptual interoperability within the organization utilizing the levels of interoperability concepts of Tolk and Muguira [1], Turnitsa [2], and Robkin et al. [3]. And lastly the paper proposes that shared clinical scenarios enable developers to be conceptually interoperable, sharing the same goals, constraints, and processes which is necessary for their work product to achieve dynamic interoperability. Future research efforts will be aimed at: developing a universal device model to enable dynamic assembly of clinical applications from well-known and characterized functional components; to enable device models to be re-used; and to architect safe communication between system components. Ranganath et al. [23] identify a collection of common communication patterns used in distributed systems including publisher-subscriber, requester-responder, sender-receiver, initiator-executor, and orchestration patterns. These patterns can be viewed as more detailed versions of the Interoperability Behaviors presented in Section IV. In addition, Ranganath et al. [23] propose quality of service contracts for each pattern that provide a basis for applications and devices to specify real-time communication constraints that be automatically checked at integration time and run-time. Publicly available research implementation frameworks illustrate how the patterns can be implemented using several widely-used middleware frameworks. The relevance and urgency of such an approach is increasing, particularly with point-of-care monitoring and therapeutic devices, given the ongoing changes to healthcare delivery models and precision medicine initiatives.
Application developers will have a hardware agnostic platform to host their devices. Sensor and actuator manufacturers will be able to provide greater capabilities and flexibility to application developers to use their services in novel ways to improve the health and safety of patients. In order to properly implement such a system requires the necessary upfront systems engineering efforts so that proper system requirements specification, design and validation can be performed. Kim et al. [24] propose high-level requirements for the ICE Device Model that addresses requirements on information content, communication patterns, quality of service, security, safety, behavioral specifications, as well as requirements on device model authoring and compliance evaluation tools. A robust device model is key to enabling seamless device interoperability.
Acknowledgments
We like to acknowledge the contributions to this work of Steven Dain, MD from the University of Waterloo, Canada, Jennifer Jackson, formerly with the Brigham and Women’s Hospital and MD PnP Program, now with the Cedars-Sinai Medical Center, Los Angeles, CA and David Osborn, Sr. Manager of Global Regulations & Standards Philips.
Biographies

Sandy Weininger received the B.S.E.E. and M.S./B.M.E. degrees from Drexel University with a focus on the properties of the electrode-tissue interface, and the Ph.D. degree in bioengineering from the University of Pennsylvania with a specialization in signal processing and control systems related to behavioral assessment of newborns. His current scientific and regulatory focus is the performance assessment of sensors and actuators for physiologic systems, and identifying interactions within complex systems to assess safety. He works on standards development organizations, including UL, AAMI, ASTM, IEC, and ISO to construct both horizontal and vertical safety standards. He is actively involved in developing and delivering courses on achieving safety in medical devices using systems engineering principles. He is a member of AAMI/UL 2800—Interoperable Systems, AAMI Interoperability Working Group, and was the Chair of the ASTM Pulse Oximeter Committee and FDA’s liaison to IEC TC 62 and SC 62A, committees responsible for safety of electro-medical equipment, and ISO TC 121/SC3 JWG10 - oximeters. He works with academic partners to develop methods for the evaluation of interoperable systems.

Michael B. Jaffe (SM’77–M’82–S’12) received the B.S. degree from Cooper Union for the Advancement of Science, the M.S. degree from Dartmouth College, and the Ph.D. degree in biomedical engineering from the University of Southern California in 1994. He currently consults on medical device technologies and health IT. He has worked since 1981 in the respiratory field, working for such companies, such as Beckman Instruments, Sensormedics, Respironics, and Philips. He has served as a Co-Editor of Capnography: Clinical Aspects (Cambridge University Press, 2004). He has served as the Secretary of the IEC/ISO Joint Working Group for ISO 80601-2-55, respiratory gas monitors. He is also a member of several international standards committees relating to anesthesia and respiratory equipment and a member of the Anesthesia Patient Safety Foundation Committee on Technology. He has authored 18 peer-reviewed publications and holds over 30 U.S. patent families in patient monitoring.

Michael Robkin is the President of Anakena Solutions, Inc. He has been developing cutting-edge, mission-critical computer applications for more than 20 years as a Programmer, a Systems Architect, and an Executive at Hughes Aircraft, GM-Europe, and Kaiser Permanente. He was a Founding Member of the Board of Directors and a Treasurer of the Continua Health Alliance.

Tracy Rausch received the B.S. degree in biomedical engineering from Wright State University and the M.S. degree in mechanical engineer from The Catholic University of American. She is the CEO and Founder of DocBox, Inc. She is a Certified Clinical Engineer. She has been serving as an Advisor to the MDPnP program since 2006.
She has interest in the integration of technology into clinical environment and the safety and process improvements in patient care.

David Arney is the Lead Engineer for the Medical Device Plug and Play Program. He has been working on applying formal methods to medical device software since 2001 and was a Scholar in residence with the FDA’s Center for Devices and Radiological Health in the Office of Science and Engineering Laboratories. He was involved in writing the ASTM 2761-09 ICE standard for interoperable medical devices. He started at the MD PnP program in 2010, and is currently writing his dissertation for a Ph.D. in computer science under Professor Insup Lee with the University of Pennsylvania.

Julian M. Goldman is a Medical Director of Biomedical Engineering for the Partners HealthCare System, the Founder and Director of the Medical Device “Plug-and-Play” (MD PnP) Interoperability program, and an Anesthesiologist with Massachusetts General Hospital (MGH). He performed his clinical and research training at the University of Colorado, and is Board Certified in Anesthesiology and Clinical Informatics. He served as a Visiting Scholar in the FDA Medical Device Fellowship Program as well as a Chief Medical Officer of a medical device company. At MGH, he served as a Principal Anesthesiologist in the “OR of the Future.” He founded the MD PnP program in 2004 to promote innovation in patient safety and clinical care by leading the adoption of patient-centric medical device integration. He currently serves in leadership positions in several medical device standardization organizations, including the Chair of the ISO Technical Committee 121, the Co-Chair of the AAMI Interoperability Working Group, and the Co-Chair of the Healthcare Task Group of the Industrial Internet Consortium. He is the former Chair of ASTM F29 and of the ASTM subcommittee that developed the ICE standard. He is an IEEE EMBS Distinguished Lecturer.
Funding Statement
This work was supported in part by NIH under 5U01EB012470-03, NSF under IIS-1239242, and DOD under W81XWH-09-1-0705, W81XWH-12-C-0154, W81XWH-11C-077, and W81XWH-13C-0107.
Footnotes
An alternative defined per IEC: Capability of objects to collaborate, that is, the capability mutually to communicate information in order to exchange events, proposals, requests, results, commitments and flows (per ISO/IEC 10746-2: 2009).
References
- [1].Tolk A. and Muguira J. A., “The levels of conceptual interoperability model,” in Proc. Fall Simulation Interoper. Workshop, Orlando, FL, USA, 2003. [Google Scholar]
- [2].Turnitsa C. D., “Extending the levels of conceptual interoperability model,” in Proc. IEEE Summer Comput. Simulation Conf., 2005. [Google Scholar]
- [3].Robkin M., Weininger S., Preciado B., and Goldman J., “Levels of conceptual interoperability model for healthcare framework for safe medical device interoperability,” in Proc. IEEE Symp. Product Compliance Eng. (ISPCE), May 2015. [Google Scholar]
- [4].Goldman J. M., “Solving the interoperability challenge: Safe and reliable information exchange requires more from product designers,” IEEE Pulse, vol. 5, no. 6, pp. 9–37, Nov-Dec 2014. [Online]. Available: http://pulse.embs.org/november-2014/solving-interoperability-challenge/ [DOI] [PubMed] [Google Scholar]
- [5].HITI, “Medical device interoperability,” AAMI, Arlington, VA, USA, White Paper AAMI MDI/2012-03-30, 2012. [Google Scholar]
- [6].AAMI Association for the Advancement of Medical Instrumentation Medical Electrical Equipment—Part 2-4: Particular Requirements for the Safety of Cardiac Defibrillators (Including Automated External Defibrillators), document ANSI/AAMI DF80:2003, 2003. [Google Scholar]
- [7].Agilent Series 50 Fetal Monitors, Digital Interface Protocol Specifications, document M1350-9014B, Agilent Corporation, Waldbronn, Germany, Jul. 2000. [Google Scholar]
- [8].Medical Devices and Medical Systems—Essential Safety Requirements for Equipment Comprising the Patient-Centric Integrated Clinical Environment (ICE)—Part 1: General Requirements and Conceptual Model, document ASTM F2761-09, 2013. [Google Scholar]
- [9].Information Technology—Open Distributed Processing—Reference Model: Foundations, document ISO/IEC 10746-2, 2009. [Google Scholar]
- [10].Industrial Communication Networks—Fieldbus Specifications—Part 1: Overview and Guidance for the IEC 61158 and IEC 61784 Series, document IEC 61158-1, 2014. [Google Scholar]
- [11].Version 3 Standard: Reference Information Model, Release 1, document ANSI/HL7 V3 RIM, R1-2003, Ann Arbor, MI, USA, Health Level Seven International, 2003. [Google Scholar]
- [12].Dain S., Rausch T., and Goldman J. M., “Domain information model for the patient centric integrated clinical environment (ICE DIM),” in Proc. Conf. Soc. Technol. Anesthesia Annu. Meeting, 2015, pp. 1–2. [Google Scholar]
- [13].Health Informatics—Point-of-Care Medical Device Communication—Part 10201: Domain Information Model, ISO/IEEE document 11073-10201, 2004. [Google Scholar]
- [14].Zafar S., Ayappa I., Norman R. G., Krieger A. C., Walsleben J. A., and Rapoport D. M., “Choice of oximeter affects apnea-hypopnea index,” Chest, vol. 127, no. 1, pp. 80–88, Jan. 2005. [DOI] [PubMed] [Google Scholar]
- [15].Arney D., Jetley R., Jones P., Lee I., and Sokolsky O., “Formal methods based development of a PCA infusion pump reference model: Generic infusion pump (GIP) project,” in Proc. Joint Workshop High Confidence Med. Devices, Softw., Syst. Med. Device Plug-Play Interoper., Jun. 2007, pp. 23–33. [Google Scholar]
- [16].Kawagishi T., Kanaya N., Nakayama M., Kurosawa S., and Namiki A., “A comparison of the failure times of pulse oximeters during blood pressure cuff-induced hypoperfusion in volunteers,” Anesthesia Analgesia, vol. 99, no. 3, pp. 793–796, Sep. 2004. [DOI] [PubMed] [Google Scholar]
- [17].Zaleski J., Integrating Device Data into the Electronic Medical Record. Erlangen, Germany: Publicis, 2009. [Google Scholar]
- [18].Roy S. and Smith L. P., “Surgical fires in laser laryngeal surgery: Are we safe enough?” Otolaryngol. Head Neck Surg., vol. 152, pp. 67–72, Jan. 2015. [DOI] [PubMed] [Google Scholar]
- [19].Langevin P. B., Hellein V., Harms S. M., Tharp W. K., Cheung-Seekit C., and Lampotang S., “Synchronization of radiograph film exposure with the inspiratory pause. Effect on the appearance of bedside chest radiographs in mechanically ventilated patients,” Amer. J. Respirat. Crit. Care Med., vol. 160, pp. 2067–2071, Dec. 1999. [DOI] [PubMed] [Google Scholar]
- [20].Lofsky A.S, “Turn your alarms on, APSF Newsletter,” Winter, vol. 19, no. 4, p. 43, 2005. [Google Scholar]
- [21].Arney D., et al. , “Design of an X-ray/ventilator synchronization system in an integrated clinical environment,” in Proc. Conf. IEEE Eng. Med. Biol. Soc., Aug./Sep. 2011, pp. 8203–8206. [DOI] [PubMed] [Google Scholar]
- [22].Caplan R. A., Vistica M. F., Posner K. L., and Cheney F. W., “Adverse anesthetic outcomes arising from gas delivery equipment: A closed claims analysis,” Anesthesiology, vol. 87, pp. 741–748, Oct. 1997. [DOI] [PubMed] [Google Scholar]
- [23].Ranganath V.-P., Kim Y. J., Hatcliff J., and Robby, “Communication patterns for interconnecting and composing medical systems,” in Proc. 37th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. (EMBC), Aug. 2015, pp. 1711–1716. [DOI] [PubMed] [Google Scholar]
- [24].Kim Y. J., Hatcliff J., Ranganath V.-P., Robby, and Weininger S., “Integrated clinical environment device model: Stakeholders and high level requirements,” in Proc. Med. Cyber Phys. Syst. Workshop SIGBEG Rev., 2015, pp. 1–10. [Google Scholar]