TO THE EDITOR
The immune reconstitution inflammatory syndrome (IRIS) can be a serious complication in immunocompromised patients in whom progressive multifocal leukoencephalopathy (PML) has developed. IRIS occurs in such patients when their immunocompromised state reverses.1 Although restored immune surveillance is vital for clearing the JC virus that causes PML, improved management strategies for IRIS are needed because of its potential for considerable complications and death. The common, though unproven, use of glucocorticoids to treat IRIS may limit JC viral clearance.2 Because of the indirect implication of chemokine receptor 5–positive (CCR5+) T cells in IRIS pathophysiology,3 we used the small-molecule CCR5 antagonist maraviroc, which has been approved for the treatment of human immunodeficiency virus (HIV) infection to inhibit CCR5-dependent viral docking, in a patient with multiple sclerosis who presented with natalizumab-associated PML and features that were expected to predict the development of IRIS.4,5
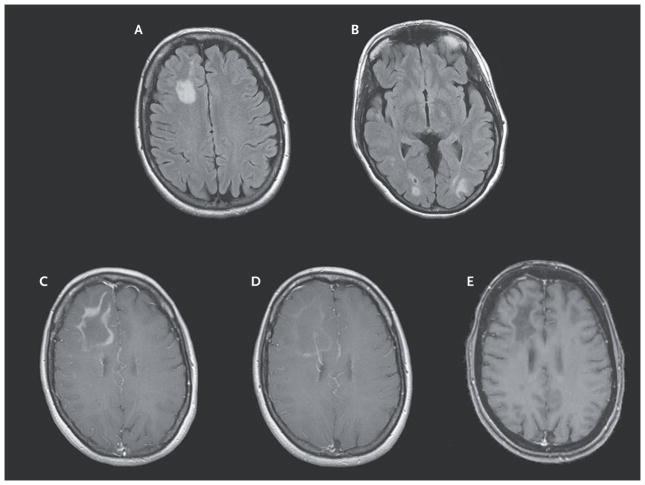
A 49-year-old, HIV-negative woman with relapsing–remitting multiple sclerosis and a score of 2.5 on the Expanded Disability Status Scale (EDSS, which ranges from 0 to 10, with higher scores indicating more severe disability) had received 43 infusions of natalizumab monotherapy over a period of 3.6 years. She received a diagnosis of PML after presenting with an episode of aphasia and seizure. She was considered at risk for severe IRIS because of the multilobar PML (Fig. 1A and 1B) and an elevated JC viral load in the cerebrospinal fluid (130,000 copies per milliliter) as measured by means of a polymerase-chain-reaction assay (Focus Diagnostics). Oral maraviroc at a dose of 300 mg twice daily was initiated shortly after plasmapheresis. No glucocorticoids or other immunomodulating therapy was given throughout the course of the PML illness. The patient was monitored closely with frequent clinical and imaging evaluations. Serial cerebrospinal fluid analyses showed that maraviroc treatment was associated with a selective decrease in CCR5+ immune cells in the cerebrospinal fluid (see the Figure in the Supplementary Appendix, available with the full text of this letter at NEJM.org). These findings suggest that maraviroc functionally inhibits CCR5-dependent immune-cell trafficking into the central nervous system (CNS). The patient’s condition remained stable, without clinical or imaging evidence of overt IRIS. Approximately 2 months after plasmapheresis, she inadvertently stopped taking maraviroc for 5 days and presented with cognitive and behavioral changes. Features on brain magnetic resonance imaging (MRI) were consistent with IRIS that involved all visible PML lesions (Fig. 1C). Oral maraviroc at a dose of 300 mg twice daily was reintroduced (without glucocorticoids), with close clinical and imaging follow-up. The patient’s cognitive and behavioral abnormalities steadily improved, though mild deficits persisted, and features of IRIS on imaging attenuated over weeks (Fig. 1D). The dose of maraviroc was reduced to 150 mg twice daily because of imaging evidence of IRIS regression, and the dose was eventually tapered off after 7 months once the lesions on MRI were stable and no longer showed gadolinium enhancement (Fig. 1E). Repeat cerebrospinal fluid analysis at 10 months was negative for JC virus.
Figure 1. Sequential Magnetic Resonance Images of the Brain.
When the patient presented for treatment, axial fluid-attenuated inversion recovery images showed multilobar progressive multifocal leukoencephalopathy (Panels A and B). A T1-weighted image after gadolinium infusion showed frank immune reconstitution inflammatory syndrome (IRIS) after the patient discontinued maraviroc (Panel C).
After the patient began to receive maraviroc again, a T1-weighted image after gadolinium infusion showed regression of IRIS (Panel D). A T1-weighted image after gadolinium infusion obtained 2 months after gradual discontinuation of maraviroc showed stable lesions with no enhancement (Panel E).
The patient survived both her PML infection and IRIS with few persisting sequelae; at 1 year after the initiation of maraviroc, she had an EDSS score of 3.0 and she continued to have mild cognitive deficits. No new multiple sclerosis disease activity was noted, and no new therapy for multiple sclerosis was introduced.
Our findings, which implicate CCR5+ immune cells as mediators of IRIS, suggest that without the use of glucocorticoids, maraviroc contributed both to initial prevention of IRIS and to active treatment of IRIS once it was established. Our data on selective reduction in the proportions of CCR5+ immune-cell subsets in the cerebrospinal fluid show that maraviroc may selectively limit trafficking into the CNS compartment in vivo and highlight the potential for selective targeting of immune-cell chemotaxis and trafficking in the management of complex immunologic disorders.
Supplementary Material
Footnotes
Members of the MIMSAPI Group are listed in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Paul S. Giacomini, McGill University, Montreal, QC, Canada
Ayal Rozenberg, McGill University, Montreal, QC, Canada
Imke Metz, Georg-August University, Göttingen, Germany
David Araujo, McGill University, Montreal, QC, Canada
Nathalie Arbour, University of Montreal, Montreal, QC, Canada
Amit Bar-Or, McGill University, Montreal, QC, Canada
References
- 1.Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77:1061–7. doi: 10.1212/WNL.0b013e31822e55e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniol C, Jilek S, Schluep M, et al. Impairment of JCV-specific T-cell response by corticotherapy: effect on PML-IRIS management? Neurology. 2012;79:2258–64. doi: 10.1212/WNL.0b013e3182768983. [DOI] [PubMed] [Google Scholar]
- 3.Schwab N, Höhn KG, Schneider-Hohendorf T, et al. Immunological and clinical consequences of treating a patient with natalizumab. Mult Scler. 2012;18:335–44. doi: 10.1177/1352458511421919. [DOI] [PubMed] [Google Scholar]
- 4.Delbue S, Elia F, Carloni C, et al. JC virus load in cerebrospinal fluid and transcriptional control region rearrangements may predict the clinical course of progressive multifocal leukoencephalopathy. J Cell Physiol. 2012;227:3511–7. doi: 10.1002/jcp.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72:779–87. doi: 10.1002/ana.23676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.