Abstract
Background
Combustion-generated fine particulate matter (PM2.5) is associated with cardiovascular morbidity. Both traffic-related air pollution and residential wood combustion may be important, but few studies have compared their impacts.
Objectives
To assess and compare effects of traffic-related and woodsmoke PM2.5 on endothelial function and systemic inflammation (C-reactive protein, interleukin-6, and band cells) among healthy adults in Vancouver, British Columbia, Canada using high efficiency particulate air (HEPA) filtration to introduce indoor PM2.5 exposure gradients.
Methods
We recruited 83 healthy adults from 44 homes in traffic- or woodsmoke-impacted areas to participate in this randomized, single-blind crossover intervention study. PM2.5 concentrations were measured during two consecutive 7-day periods, one with filtration and the other with “placebo filtration”. Endothelial function and biomarkers of systematic inflammation were measured at the end of each 7-day period.
Results
HEPA filtration was associated with a 40% decrease in indoor PM2.5 concentrations. There was no relationship between PM2.5 exposure and endothelial function. There was evidence of an association between indoor PM2.5 and C-reactive protein among those in traffic-impacted locations [42.1% increase in C-reactive protein per interquartile range increase in indoor PM2.5, 95% CI, 1.2 to 99.5] but not among those in woodsmoke-impacted locations. There were no associations with interleukin-6 or band cells.
Conclusions
Evidence of an association between C-reactive protein and indoor PM2.5 among healthy adults in traffic-impacted areas is consistent with the hypothesis that traffic-related particles, even at relatively low concentrations, play an important role in the cardiovascular effects of the urban PM mixture.
Trial registered at www.clinicaltrials.gov (NCT01570062)
Keywords: Air pollution, particulate matter, high-efficiency air filter, cardiovascular, intervention
INTRODUCTION
Numerous studies have linked short- and long-term exposure to airborne particulate matter (PM) with increased cardiovascular morbidity and mortality. Systematic oxidative stress and inflammation have been proposed as biological pathways underlying these effects. The promotion of pulmonary oxidative stress and inflammation by different physical and chemical components of PM can lead to the development of systematic inflammation, vascular dysfunction, and finally, plaque development and instability [1]. A number of studies have reported associations between air pollution and markers of systematic inflammation [2,3], endothelial dysfunction [2,4], and atherosclerosis[5,6].
Among other sources, it has been shown that combustion-derived air pollution plays a potentially important role in the development of adverse cardiovascular health outcomes [1]. There is a strong body of evidence linking exposure to traffic-related air pollution (TRAP) with outcomes such as coronary heart disease mortality [7,8], hypertension and stroke [9,10], and myocardial infarction [11]. Other combustion sources of PM have received less research attention. Residential wood combustion is another major source of PM in mid- and high-latitude regions [12]. With the rising costs of other fuel options, the significance of this source is expected to increase [13,14]. Despite being an important source of PM in many settings, limited evidence is available on the non-respiratory effects of woodsmoke (WS) [12,15]. Although there have been suggestions of a potential link between WS exposure and cardiovascular morbidity and mortality [2,16], the cardiovascular toxicity of woodsmoke particles remains poorly understood.
Evaluating the health effects of specific PM sources has been identified as an important research need [17], and several research methods have been utilized to date [18]. These include in vitro studies [19], controlled human exposure experiments [20], source apportionment methods [21], natural experiments [22], and semi-experimental studies that rely on “real world” exposures but attempt to isolate exposure to pollution from specific sources [23].
The current study utilized a novel semi-experimental study design to compare the effects of TRAP and WS on subclinical indicators of cardiovascular health. Our main objective was to compare the magnitude of endothelial dysfunction and systemic inflammation between WS- and TRAP-impacted individuals. While previous studies have investigated the efficacy of high efficiency particulate air (HEPA) filters in reducing the health impacts of air pollution [2,4,24], we used HEPA filters in areas with differing levels of WS- and TRAP-related PM to introduce exposure gradients that would allow us to better understand relationships between source-specific PM and cardiovascular health. Based on previous evidence of cardiovascular effects from TRAP [1,25], and the physical and chemical differences between TRAP and WS PM [26–28], we hypothesized that reductions in fine PM (PM2.5) concentrations would have a greater impact on endothelial dysfunction and systemic inflammation among those living in TRAP-impacted locations compared to those living in WS-impacted locations.
METHODS
A detailed description of the methods is available in the online supplement.
Study Design and Population
This study was conducted from December 2011 to August 2012 in greater Vancouver, British Columbia, Canada. Areas of relatively high TRAP or high WS were selected using previously developed spatial models of NO, light absorption coefficient (“absorbance”), and woodsmoke [29,30]. Postal codes were extracted from areas with relatively high TRAP and low WS concentrations and from areas with relatively high WS and low TRAP concentrations (Figure 1). WS-impacted postal codes were selected from those in the highest tertile of modeled WS concentrations [30] that also had modeled annual average PM2.5 absorbance coefficients ≤1.4 x 10−5 m−1 and NO concentrations ≤22 ppb [29]. TRAP-impacted postal codes were identified as those in the lowest two tertiles of modeled WS concentrations [30] that also had modeled annual average PM2.5 absorbance coefficients > 3.5 x 10−5 m−1 or NO concentrations > 37 ppb [29]. Thus, the 335 targeted WS-impacted postal codes had average (± SD) modeled NO and absorbance annual averages of 17.7 ± 1.7 ppb and 0.8 ± 0.2 x 10−5 m−1, respectively, and relatively high modeled WS levels. The 1,102 traffic-impacted postal codes had modeled NO and absorbance annual averages of 44.4 ± 13.5 ppb and 4.1 ± 1.0 x 10−5 m−1, respectively, and relatively low modeled WS concentrations.
Figure 1.
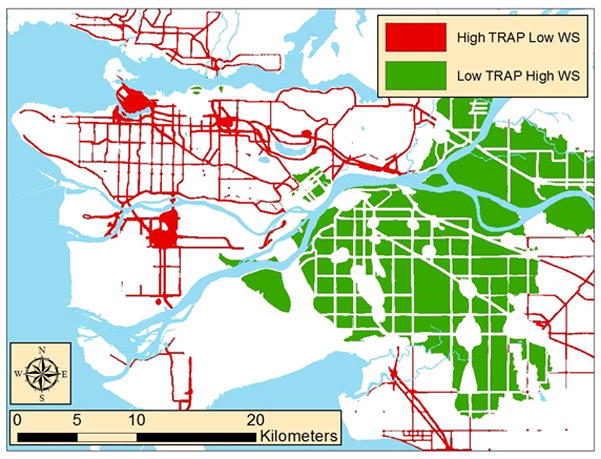
Targeted traffic- and woodmoke-impacted areas.
A total of 13,200 invitation letters were mailed out to non-smoking individuals over 19 years old residing in these targeted postal codes in greater Vancouver. Pregnant women and individuals with recent surgeries, diabetes, heart disease, hypertension, metabolic syndrome, asthma, COPD, or Reynaud’s syndrome were excluded, as were those taking anti-inflammatory medications. In addition, those expected to have high occupational exposures to air pollution (e.g., bus drivers, mechanics) were excluded. A total of 83 adult participants (54 from TRAP homes and 29 from WS homes) living in 44 different residences participated in the study. The study protocol was approved by the research ethics boards at Simon Fraser University (Approval # 2011s0431) and written informed consent was obtained from each participant prior to data collection.
Data collection in WS-impacted homes took place between December, 2011 and April, 2012 and in TRAP-impacted homes data collection occurred between December, 2011 and August, 2012. Each home was monitored during two consecutive 7-day periods with one HEPA filtration device in the living room (Model 50300; Honeywell, Morristown, NJ) and one in the bedroom (Model 18150; Honeywell, Morristown, NJ). During one 7-day period the units were operated with a HEPA filter in place and during the other 7-day period there was no HEPA filter in the unit (i.e. placebo filtration), thereby blinding participants to intervention status. The order of filtration and placebo filtration was selected randomly. Participants were asked to operate the air cleaners at the highest speed they were comfortable with. The indoor exposure sampling equipment was placed in the main living room in each residence (as far as possible from the living room HEPA filter), and the outdoor equipment was placed in the backyard or on a patio or deck.
Exposure Measurements
Indoor and outdoor PM2.5 samples were collected on 37mm Pall Teflo® membrane filters (2μm pore size) using Harvard Impactors (Air Diagnostics and Engineering Inc., Naples, Maine, USA) attached to SKC Leland Legacy pumps operated at a flow rate of 10 liter/minute for the duration of each 7-day session. Filters were analyzed for PM2.5 mass concentration, reflectance using a smoke stain reflectometer (M43D, Diffusion Systems Ltd., London, UK), and 17α(H),21β(H)-hopane (“hopanes”) and levoglucosan (LG) as markers of TRAP and WS exposure, respectively, using gas chromatography/mass spectroscopy. Reflectance values were converted to absorbance (x 10−5 m−1) [31]. Hopanes are compounds contained mainly in the hydrocarbon fractions of petroleum products (e.g. engine lubricating oil). The presence of hopanes in aerosols indicates a fossil fuel source so these tracers are used as indicators of primary particle emissions from diesel and gasoline engines [32]. LG is formed from the combustion of cellulose, one of the main polymers found in wood (50%–70% by weight) [33]. LG is a useful organic tracer for WS since it is highly stable on collected filters [34]. Indoor temperature and RH were logged continuously at one-minute intervals for both 7-day periods using HOBO data loggers (UX100, Bourne, MA). Each participant provided information on housing, health (including medications), activities (e.g., commuting, exposure to tobacco smoke) and time-location patterns.
Health Measurements
Microvascular endothelial function was measured during home visits before blood sampling via reactive hyperemia peripheral arterial tonometry at the end of each 7-day period. This test was conducted using a portable EndoPAT 2000 device (Itamar Medical Ltd, Cesari, Israel), which records digital arterial pulse wave amplitude during a period of induced reactive hyperemia and calculates a reactive hyperemia index (RHI). Blood samples were collected at baseline and at the end of each 7-day sampling period. Samples were analyzed for CRP and IL-6 using Luminex 100 fluorescent-coded bead-based immunoassay testing platform. Band cell counts (BCC) were performed under a light microscope on blood smears using Wright-Giemsa stain (Bayer HEMA-TEK 2000 Slide Stainer, Leverkusen, Germany). Laboratory staff were blinded to intervention status.
Participant Exclusions
To protect the integrity of our crossover design, only individuals with complete data for both weeks of the study (i.e. those with exposure and health measurements from both 7-day sampling periods) were included in models for each health outcome. In endothelial function models, 15 of the 83 subjects with incomplete RHI and exposure measurements were excluded. In addition, in CRP, IL-6, and BCC mixed models, 31 participants were excluded as a result of incomplete data for exposure or outcome of interest. Baseline blood samples were missing/invalid for an additional 7 participants, so our analysis focused only on the blood samples collected at the end of each 7-day period. The final analyses included 68 individuals (48 in TRAP-impacted locations and 20 in WS-impacted locations) with complete, paired data for RHI and 52 individuals (35 in TRAP-impacted locations and 17 in WS-impacted locations) with complete data on the CRP, IL-6, and BCC. Included and excluded participants were generally similar with regard to age, sex, and blood pressure. However, participants included in the analysis had slightly higher mean BMI (24.9 ± 4.0 vs. 23.4 ± 3.4) and spent more time indoors at home (74.4 ± 13.3 % vs. 69.4 ± 18.1%) compared to excluded participants (supplemental material, Table 1).
Statistical Analysis
We evaluated four exposure variables in relation to our outcome measures: 1) intervention status (“HEPA”), 2) indoor PM2.5 concentration, 3) indoor LG concentration, and 4) indoor absorbance. Hopanes were not included because concentrations were less than the limit of detection on 38% of the indoor PM2.5 samples and there was a trend of decreasing hopane concentrations with increasing temperature, which complicated interpretation of the hopane measurements and limited their use as markers of traffic emissions.
Outcome variables were log-transformed before analysis. In order to compare the magnitude of the effects across different continuous exposures (PM2.5, LG, and absorbance), the exposure contrasts were scaled to the interquartile range (IQR) of indoor concentrations for each pollutant. IQR values were 4.8 μg/m3, 0.36 x 10−5 m−1, and 7.8 ng/m3 for PM2.5, absorbance, and LG, respectively. Mixed effects models with random participant and home intercepts were used to account for measurements clustered within participants and participants clustered within homes. All models were adjusted for average indoor temperature and relative humidity; in this crossover design each participant acts as his or her own control and confounding is only a concern for variables that vary between 7-day periods. Effect estimates were converted to % change in outcome variable per IQR change in exposure. We explored effect modification by age (above or below median age of 42 years), BMI (above and below 25 kg/m2), and sex.
RESULTS
Summary Statistics
The mean age for participants was 43.8 ± 12.8 years (range: 19 to 72) with an approximately equal balance between sexes (Table 1). Sixty-two of the 68 participants worked or volunteered outside the home. Participants’ homes were heated by gas furnaces, electric baseboard heaters, or hot water boilers. Only one home had any indoor wood burning reported during the study. The TRAP- and WS-exposed participants were generally similar, except TRAP-exposed participants were significantly younger (mean age 40.8 ± 12.3 years vs. 51.3 ± 10.9 years) and spent slightly less time indoors at home (73.6 ± 12.8% vs. 76.3 ± 14.3%) (Table 1). Participants’ BMI and baseline blood pressures were consistent with a healthy adult population (Table 1). None of the participants reported using any prescription cardiovascular medications (e.g., statins, ACE inhibitors, etc.).
Table 1.
Study Population Characteristics for 68 Participants with Complete RHI Data
| Variable | All Participants (mean ± SD) N = 68 |
TRAP Exposed (mean ± SD) N = 48 |
WS Exposed (mean ± SD) N = 20 |
t Test P- Value* |
|---|---|---|---|---|
| Age (yrs) | 43.8 ± 12.8 | 40.8 ± 12.3 | 51.3 ± 10.9 | <0.01 |
| % Female | 53% | 54% | 50% | |
| BMI (kg/m2) | 24.9 ± 4.0 | 24.9 ± 3.9 | 25.0 ± 4.3 | 0.73 |
| Baseline Systolic BP (mmHg) | 119.8 ± 13.3 | 119.9 ±14.1 | 119.4 ± 11.7 | 0.81 |
| Diastolic BP (mmHg) | 75.6 ± 10.5 | 75.4 ± 10.9 | 76.1 ± 9.5 | 0.83 |
| % Time Open Windows | 36.6 ± 39.4 | 45.0 ± 39.3 | 15.2 ± 31.3 | <0.01 |
| % Time at Home | 74.4 ± 13.3 | 73.6 ± 12.8 | 76.3 ± 14.3 | 0.08 |
Definition of Abbreviations: BMI = body mass index; BP = blood pressure; TRAP = traffic-related air pollution; WS = woodsmoke
2-sample t test P-value for TRAP- vs. WS-exposed participants
Average outdoor PM2.5 concentrations were low (5.4–5.7 μg/m3) and unrelated to HEPA filter status (Table 2). Outdoor temperatures ranged between −0.7 and 20.4 oC during the 14-day monitoring periods, with colder temperatures during sampling at WS-impacted homes (December to April) than at TRAP-impacted homes (December to August). As a result, participants in TRAP-exposed areas opened windows more frequently (45.0% ± 39.3% of hours) than those living in WS-exposed areas (15.2% ± 31.3%). Mean RHI, CRP, IL-6, and BCC values were not different between the HEPA and placebo periods (Table 3).
Table 2.
Summary Statistics for Exposure Variables by HEPA Filtration Status (Mean ± SD (Median))
| PM2.5 (μg/m3) | Absorbance (x 10−5 m−1) | Levoglucosan (ng/m3) | Outdoor Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|---|
| Indoors | Outdoors | Indoors | Outdoors | Indoors | Outdoors | |||
| ALL HOMES N = 68 | HEPA Filter Off | 7.1±6.1 (7.5) | 5.7±2.8 (5.5) | 2.3±0.3 (2.2) | 3.1±2.7 (2.3) | 13.8±36.5 (2.5) | 17.9±47.8 (3.5) | 10.7±5.2 (10.8) |
| HEPA Filter On | 4.3±2.6 (3.7) | 5.4±2.2 (5.6) | 2.1±0.2 (2.0) | 2.3±1.3 (2.3) | 10.1±16.3 (1.4) | 11.2±17.1 (3.8) | 10.5±5.4 (11.1) | |
| HOMES IN TRAFFIC- IMPACTED LOCATIONS N = 48 | HEPA Filter Off | 7.3±2.1 (7.6) | 6.0±2.9 (5.6) | 2.3±0.1 (2.3) | 3.2±2.9 (2.3) | 8.5±14.7 (2.5) | 19.5±54.3 (2.9) | 12.8±4.5 (13.2) |
| HEPA Filter On | 4.7±2.8 (4.3) | 6.1±2.0 (5.9) | 2.1±0.2 (2.1) | 2.4±0.2 (2.3) | 9.4±17.1 (1.4) | 8.4±15.3 (3.3) | 12.5±5.0 (13.5) | |
| HOMES IN WOODSMOKE- IMPACTED LOCATIONS N = 20 | HEPA Filter Off | 6.5±2.7 (6.7) | 5.0±2.5 (4.8) | 2.2±0.4 (2.0) | 2.9±2.3 (2.1) | 29.3±67.1 (3.9) | 13.2±13.5 (4.6) | 5.6±2.5 (5.7) |
| HEPA Filter On | 3.4±1.9 (2.5) | 3.9±2.1 (4.0) | 1.9±0.1 (2.0) | 2.2±0.3 (2.1) | 11.8±14.4 (5.0) | 20.6±19.9 (15.1) | 5.6±2.1 (5.2) | |
Table 3.
Summary Statistics for Outcome Variables by Filtration Status
| Variable | Non-HEPA Filtration Period mean ± SD (median) | HEPA Filtration Period mean ± SD (median) | Paired t Test P- Value |
|---|---|---|---|
| RHI (N = 68) | 2.1 ± 0.6 (2.1) | 2.1 ± 0.6 (2.1) | 0.71 |
|
|
|||
| CRP (mg/L) (N = 52) | 2.4 ± 3.3 (1.2) | 2.2 ± 3.7 (1.1) | 0.85 |
|
|
|||
| IL-6 (pg/mL) (N = 52) | 2.9 ± 5.2 (1.4) | 3.1 ± 5.3 (1.8) | 0.88 |
|
|
|||
| BCC (N = 52) | 0.8 ± 0.9 (1) | 0.8 ±0.9 (0.5) | 1.00 |
HEPA filtration caused indoor PM2.5 reductions in 85% (37 of 44) of homes. Overall, average indoor PM2.5 concentrations were reduced by 40% (7.1 to 4.3 μg/m3) with HEPA filtration, with slightly larger reductions in WS-impacted homes (6.5 to 3.4 μg/m3, 48%) than in TRAP-impacted homes (7.3 to 4.7 μg/m3, 36%). Reductions in indoor absorbance and LG were less consistent, and in woodsmoke homes the impact of HEPA filtration on indoor LG might have been offset by higher outdoor LG concentrations during periods with HEPA filtration (20.6 ± 19.9 ng/m3) than during “placebo” filtration (13.2 ± 13.5 ng/m3).
LG concentrations and roadway proximity metrics indicate that our study participants resided in areas that were correctly identified as either TRAP- or WS-impacted. For example, the average distance from TRAP homes to the nearest highway or major road was 51 ± 47 meters, compared to 363 ± 225 meters for WS homes. The median outdoor LG concentration was three times higher at WS homes (9.24 ng/m3) than at TRAP homes (3.20 ng/m3).
Mixed Model Analysis
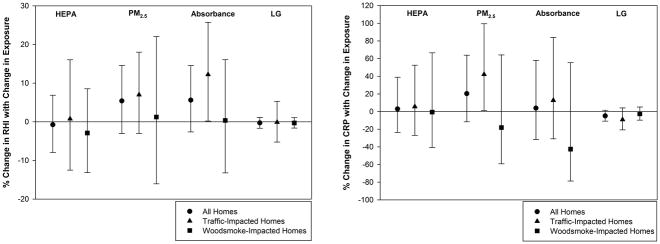
There was no association between any of the four exposure variables and RHI (Figure 2a.). CRP was also not associated with HEPA filtration. After including only homes where there was a reduction in PM2.5 concentrations with HEPA filtration (37 homes out of 44), there was still no association between HEPA filtration and CRP concentrations. There was a non-significant association between indoor PM2.5 concentration and CRP in all homes (20.4% increase per IQR; 95% CI, −11.6 to 63.8%). After stratification by home type, there was a 42.1% (95% CI, 1.25 to 99.5) increase in CRP levels per IQR increase in indoor PM2.5 in TRAP-impacted homes. No such relationship was observed for WS-impacted homes (Figure 2b). There were no associations between any exposure variables and IL-6 or BCC. There was a marginally significant (p = 0.05) association in the unexpected direction between black carbon and RHI at traffic-impacted locations.
Figure 2.
Figure 2a (Left): Percent Change in RHI with IQR Change in Exposure by home type;
Figure 2b (Right): Percent Change in CRP with Change in Exposure by home type (IQR values: PM2.5 = 4.8 μg/m3; absorbance = 0.36 x 10−5 m−1; LG = 7.8 ng/m3)
We explored effect modification by BMI (≤25 and >25), age (≤42 and >42), and sex. BMI and age did not modify any of the relationships evaluated. After stratifying by sex, a PM2.5-CRP association was observed among males (53.9% increase, 11.4% to 112.7%) but not among females (−28.7%, −51.2% to 4.2%).
DISCUSSION
In this novel study we identified locations in greater Vancouver impacted by either traffic-related or woodsmoke PM using previously developed spatial models. We used a randomized HEPA filter intervention to compare the subclinical cardiovascular impacts of these two major sources of combustion PM. Despite the overwhelming evidence on cardiovascular effects of PM, and growing evidence that combustion-generated PM plays a particularly important role, there have been very few studies evaluating potential differences between cardiovascular effects of PM from traffic and wood combustion [1]. To our knowledge, this is the first study that has attempted to directly compare these two major combustion sources of PM in a single study within a single geographical area.
This study was designed to overcome key limitations of other approaches to comparing source-specific health effects. Participants in this study were exposed to “real world” pollution mixtures at realistic concentrations over consecutive 7-day periods. In addition, our ability to observe exposure-response relationships depends, in part, on the magnitude of the exposure gradients, and those gradients can differ for different pollution sources. In this study HEPA filter air cleaners introduced similar PM2.5 concentration gradients in TRAP- and WS-impacted homes. A second challenge in observational studies is exposure assessment, and different amounts of exposure misclassification for different source emissions may limit our ability to directly compare their effect estimates in epidemiologic studies. By conducting indoor residential measurements of pollution concentrations we had comparable exposure assessment in TRAP- and WS-impacted homes, and focused on the microenvironment where participants spent the majority of their time.
HEPA filtration reduced indoor PM2.5 concentrations by approximately 36% and 48% in TRAP and WS homes, respectively. These results are in agreement with a number of other studies that have demonstrated consistent reductions in indoor PM2.5 with HEPA filtration [35,36]. Unlike some previous studies, we did not observe any relationships between PM2.5 and RHI. In our previous study among healthy adults in a woodsmoke-impacted community, HEPA filters reduced indoor PM2.5 concentrations from 11.2 ± 6.1 μg/m3 to 4.6 ± 2.6 μg/m3, and filtration was associated with a 9.4% (95% CI: 0.9–18%) increase in RHI [2]. However, after stratification, improvements in RHI with filtration were only present among those using a woodstove. Thus, the lack of woodstove use among woodsmoke-impacted participants in the present study may partly explain the lack of associations between filtration and health outcomes among this group. In a HEPA filter intervention study among healthy elderly couples in Denmark, Brauner et al. found an improvement of 8.1% (95% CI: 0.4–16%) in RHI with filtration, which reduced PM2.5 from 12.6 μg/m3 to 4.7 μg/m3 [4]. In a more recent study conducted by the same group, the authors did not find any relationship between HEPA filtration and endothelial function; however, they did report a 7.7% (95% CI: 2.3%, 13.3%) increase in RHI per 10 μg/m3 decrease in PM2.5 among participants with no medication use [37]. A study conducted in a Canadian First Nations community also did not observe an association between HEPA filtration and RHI despite an average 20.5 μg/m3 reduction in PM2.5 concentration with filtration [24]. A recent study involving 2,369 participants in the Framingham Heart Study also found no consistent associations between air pollution exposures and RHI [38]. We observed a marginally significant increase in RHI with increased absorbance at traffic-impacted homes. The lack of a reduction in indoor reflectance with HEPA filtration suggests that reflectance values were impacted by measurement error and/or indoor sources, so it is unlikely that the observed relationship with RHI is meaningful.
In this study, we used CRP as a marker of systematic inflammation. Numerous studies have shown that CRP predicts future cardiovascular events [39], but the literature is not entirely consistent regarding the relationship between short-term PM exposure and systematic inflammation [40,41]. Several previous studies of PM exposure and CRP among healthy adults have been conducted with some studies reporting associations [3,42] and others finding no relationship [43,44]. In this study, there was no significant relationship between HEPA filtration and CRP levels in either the overall population or when stratified by home type. However, we found an increase in CRP levels with increasing indoor PM2.5 concentrations in TRAP-impacted homes but not in WS-impacted homes. This result is consistent with our a priori hypothesis of a greater impact of TRAP on systematic inflammation as compared to WS, and also with recent results from a German cohort study in which long-term exposure to traffic-specific PM2.5 was more strongly associated with CRP than exposure to total PM2.5 [21].
There are at least two possible reasons why traffic particles might have greater effects on cardiovascular health than other combustion particles. First, the greater abundance of transition metals in traffic particles may lead to the generation of hydroxyl radicals, oxidative stress, and inflammation [28,45]. Second, traffic particles have been shown to have greater lung deposition than particles produced from wood combustion [26,27].
We did not find any associations between PM and cardiovascular health in participants residing in woodsmoke-impacted locations. Overall, the cardiovascular effects of WS have not been studied as extensively as general urban PM or traffic-related particles, and the available literature on WS PM and cardiovascular health is limited and inconclusive. Two recent controlled human exposure studies evaluated the effects of short-term (4 hrs) WS PM exposure levels up to 350 μg/m3 on endothelial function; neither study found any evidence of change in endothelial function after exposure [46,47]. Another recent study investigated the inflammatory effects of cross-shift (8 hrs) WS exposure among wildfire firefighters exposed to PM2.5 concentrations of 288 to 1306 μg/m3 and found borderline statistical significance increases in CRP levels with exposure [48]. Stockfelt and colleagues exposed individuals to WS concentrations of up to 295 μg/m3 for 4 hours and did not find an association with CRP [49]. In our previous study in a WS-impacted community in Canada, we found a 32.6% (95% CI, 4.4–60.9%) decrease in CRP levels with 7-days of HEPA filtration [2]. It should also be noted that outdoor LG concentrations were considerably lower in the current study compared to our previous study (613 ± 548 ng/m3 vs. 14.2 ± 34.4 ng/m3).
Our secondary markers of inflammation, IL-6 and BCC, were not associated with exposure. IL-6 is one cytokine involved in initiating acute phase inflammatory response by promoting the synthesis of various proteins including CRP [50,51]. Our results are in agreement with a number of other studies that did not observe effects of TRAP and WS PM on IL-6 [4,49,52]. Band cells are immature polymorphonuclear leukocytes (PMN) produced in the bone marrow. Increases in BCC are an indication of stimulation of the immune system leading to an increased release of granulocytes in the bone marrow [53]. In a study of healthy nonsmoking wildfire firefighters, cross-shift exposure to PM levels as high as 2,000 μg/m3 was associated with significant increases in BCC [54]. Sakai and colleagues also found that the geographical relocation of 39 research expedition members from Japan to Antarctica was associated with significant reduction in BCC [55]. In our previous study we reported an association between indoor LG concentrations and BCC [2].
Our study had some important limitations. To compare with the findings of our previous study in Smithers, BC and other available literature indicating a potential relationship between RHI and air pollution, we used the EndoPAT to measure RHI. However, the literature comparing EndoPAT results with conventional methods (e.g. endothelium-dependent brachial artery flow-mediated dilation) has not been consistent. Of the nine available studies comparing the two methods, six found statistically significant weak to moderate correlations [56–61], while three found no significant correlation between EndoPAT results and flow-mediated dilation [62–64]. Despite these differences, there have been suggestions that RHI is able to predict adverse cardiovascular health outcomes [65]. It should also be noted that many different mixed models were used in this study. This might have increased the chances of finding an association due to chance. In addition, the relatively low baseline pollution concentrations and small exposure gradients may have limited our ability to detect relationships between exposure and these health outcomes. In their recent follow-up study, Karottki et al. (2013) also suggested low PM2.5 levels and a relatively small gradient (8 μg/m3 with filtration vs. 4 μg/m3 without filtration) as possible explanations for the lack of association between HEPA filtration and RHI or inflammatory markers. It is important to study environments with low air pollution levels in order to better understand effects at concentrations below current air quality standards and the shape of the concentration-response functions at the low end of the concentration range. Participants were asked to operate the air cleaners at the highest comfortable speed, but we cannot rule out the possibility that some participants turned off the air cleaners. This may explain why indoor PM2.5 concentrations did not decrease in seven of 44 homes during the week with HEPA filters in place. Another limitation of our study is that woodsmoke-impacted homes were sampled during the heating season, while the majority of valid data in TRAP-impacted homes was collected in the non-heating season. Thus, window-opening behavior differed between the groups and it is likely that the average PM2.5 infiltration efficiency was higher for TRAP-impacted homes. This may be another reason why the PM2.5 CRP – relationship was seen only in TRAP-impacted homes. We only measured indoor concentrations in the main activity rooms of participants’ homes, which may not accurately represent concentrations in other areas of some homes. Karrotki et al. found that bedroom PM2.5 concentrations were more closely linked with RHI than living room concentrations [37]. Finally, it was not possible to measure air pollution concentrations away from the home, where the participants spent about 25% of their time.
Despite the benefits of the crossover design in minimizing the influence of differences between participants on the analysis, treatment carryover effects are a concern when using this study design. We minimized the carryover effect between HEPA and control periods by using a 7-day period for each treatment. The half-life of CRP is approximately 19 hours [50] and there is a lag time of 2 days for observing the effects of HEPA filtration on microvascular endothelial function [4,37]. Considering these findings, it is expected that there were minimal carryover effects at the end of each 7-day treatment session.
In conclusion, the use of HEPA filters was associated with a 40% reduction in PM2.5 concentrations. This study suggests an association between relatively low levels of indoor PM2.5 exposure in traffic-impacted locations and CRP among healthy adults, which is consistent with the hypothesis that traffic-generated particles play an important role in the inflammatory effects of the urban air pollution mixture. No associations were observed among participants living in woodsmoke-impacted locations. These results add to a growing body of literature suggesting a particularly important role for traffic-related air pollution on cardiovascular health.
Supplementary Material
WHAT THIS PAPER ADDS.
Previous studies have linked exposure to particulate matter (PM) air pollution with cardiovascular morbidity and mortality, and combustion-generated PM is thought to play an important role.
Few direct comparisons of the health impacts of specific PM sources have been conducted.
This study suggests an association between relatively low levels of indoor PM exposure in traffic-impacted locations and inflammation among healthy adults.
We found no evidence of cardiovascular effects of indoor PM among participants residing in woodsmoke-impacted locations.
These results add to a growing body of literature suggesting a particularly important role for traffic-related air pollution on cardiovascular health.
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (MOP 111042). We are grateful to the study participants and to the field technicians, Paul Adam and Malgosia Zapala.
References
- 1.Brook RD, Rajagopalan S, Pope CA, et al. Particulate Matter Air Pollution and Cardiovascular Disease An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Allen RW, Carlsten C, Karlen B, et al. An Air Filter Intervention Study of Endothelial Function Among Healthy Adults in a Woodsmoke-Impacted Community. Am J Respir Crit Care Med. 2011;183:1222–30. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- 3.Riediker M, Cascio WE, Griggs TR, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169:934–40. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 4.Bräuner E, Forchhammer L, Møller P, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177:419–25. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 5.Adar SD, Sheppard L, Vedal S, et al. Fine Particulate Air Pollution and the Progression of Carotid Intima-Medial Thickness: A Prospective Cohort Study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiol Camb Mass. 2009;20:254–64. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan WQ, Tamburic L, Davies HW, et al. Changes in residential proximity to road traffic and the risk of death from coronary heart disease. Epidemiol Camb Mass. 2010;21:642–9. doi: 10.1097/EDE.0b013e3181e89f19. [DOI] [PubMed] [Google Scholar]
- 8.Gan WQ, Koehoorn M, Davies HW, et al. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect. 2011;119:501–7. doi: 10.1289/ehp.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccarelli A, Martinelli I, Pegoraro V, et al. Living near major traffic roads and risk of deep vein thrombosis. Circulation. 2009;119:3118–24. doi: 10.1161/CIRCULATIONAHA.108.836163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuks K, Moebus S, Hertel S, et al. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ Health Perspect. 2011;119:1706–11. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Klot S, Cyrys J, Hoek G, et al. Estimated personal soot exposure is associated with acute myocardial infarction onset in a case-crossover study. Prog Cardiovasc Dis. 2011;53:361–8. doi: 10.1016/j.pcad.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 13.de Richter BD, Jenkins DH, Karakash JT, et al. Wood Energy in America Science. 2009;323:1432–3. doi: 10.1126/science.1166214. [DOI] [PubMed] [Google Scholar]
- 14.Zezima K. With Oil Prices Rising, Wood Makes a Comeback. N. Y. Times; 2008. [accessed 16 Feb2012]. http://www.nytimes.com/2008/02/19/us/19woodstove.html. [Google Scholar]
- 15.Kocbach Bølling A, Pagels J, Yttri KE, et al. Health effects of residential wood smoke particles: the importance of combustion conditions and physicochemical particle properties. Part Fibre Toxicol. 2009;6:29. doi: 10.1186/1743-8977-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanhueza PA, Torreblanca MA, Diaz-Robles LA, et al. Particulate air pollution and health effects for cardiovascular and respiratory causes in Temuco, Chile: a wood-smoke-polluted urban area. J Air Waste Manag Assoc. 1995–2009;59:1481–8. doi: 10.3155/1047-3289.59.12.1481. [DOI] [PubMed] [Google Scholar]
- 17.WHO. [accessed 21 Jan2014];Health relevance of particulate matter from various sources. 2007 http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/health-relevance-of-particulate-matter-from-various-sources.
- 18.Ayala A, Brauer M, Mauderly JL, et al. Air pollutants and sources associated with health effects. Air Qual Atmosphere Health. 2012;5:151–67. doi: 10.1007/s11869-011-0155-2. [DOI] [Google Scholar]
- 19.Baulig A, Garlatti M, Bonvallot V, et al. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L671–9. doi: 10.1152/ajplung.00419.2002. [DOI] [PubMed] [Google Scholar]
- 20.Mills NL, Törnqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–82. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 21.Hennig F, Fuks K, Moebus S, et al. Association between source-specific particulate matter air pollution and hs-CRP: local traffic and industrial emissions. Environ Health Perspect. 2014;122:703–10. doi: 10.1289/ehp.1307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy L, Goodman P, Sinclair H, et al. Effect of air-pollution control on death rates in Dublin, Ireland: an intervention study. Lancet. 2002;360:1210–4. doi: 10.1016/S0140-6736(02)11281-5. [DOI] [PubMed] [Google Scholar]
- 23.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory Effects of Exposure to Diesel Traffic in Persons with Asthma. N Engl J Med. 2007;357:2348–58. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 24.Weichenthal S, Mallach G, Kulka R, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 2013;23:175–84. doi: 10.1111/ina.12019. [DOI] [PubMed] [Google Scholar]
- 25.Health Effects Institute. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. 2010 http://pubs.healtheffects.org/view.php?id=334.
- 26.Löndahl J, Pagels J, Boman C, et al. Deposition of biomass combustion aerosol particles in the human respiratory tract. Inhal Toxicol. 2008;20:923–33. doi: 10.1080/08958370802087124. [DOI] [PubMed] [Google Scholar]
- 27.Löndahl J, Massling A, Swietlicki E, et al. Experimentally Determined Human Respiratory Tract Deposition of Airborne Particles at a Busy Street. Env Sci Technol. 2009;43:4659–64. doi: 10.1021/es803029b. [DOI] [PubMed] [Google Scholar]
- 28.Verma V, Polidori A, Schauer JJ, et al. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ Sci Technol. 2009;43:954–60. doi: 10.1021/es8021667. [DOI] [PubMed] [Google Scholar]
- 29.Henderson SB, Beckerman B, Jerrett M, et al. Application of land use regression to estimate long-term concentrations of traffic-related nitrogen oxides and fine particulate matter. Environ Sci Technol. 2007;41:2422–8. doi: 10.1021/es0606780. [DOI] [PubMed] [Google Scholar]
- 30.Larson T, Su J, Baribeau A-M, et al. A spatial model of urban winter woodsmoke concentrations. Environ Sci Technol. 2007;41:2429–36. doi: 10.1021/es0614060. [DOI] [PubMed] [Google Scholar]
- 31.American National Standards Institution. ISO 9835:1993, Ambient air - Determination of a black smoke index. Washington, D.C: Multiple. Distributed through American National Standards Institute; 1993. [Google Scholar]
- 32.Cass GR. Organic molecular tracers for particulate air pollution sources. TrAC Trends Anal Chem. 1998;17:356–66. doi: 10.1016/S0165-9936(98)00040-5. [DOI] [Google Scholar]
- 33.Simoneit B. A review of biomarker compounds as source indicators and tracers for air pollution. Environ Sci Pollut Res. 1999;6:159–69. doi: 10.1007/BF02987621. [DOI] [PubMed] [Google Scholar]
- 34.Fraser MP, Lakshmanan K. Using Levoglucosan as a Molecular Marker for the Long-Range Transport of Biomass Combustion Aerosols. Env Sci Technol. 2000;34:4560–4. doi: 10.1021/es991229l. [DOI] [Google Scholar]
- 35.Fisk WJ. Health benefits of particle filtration. Indoor Air. 2013;23:357–68. doi: 10.1111/ina.12036. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler AJ, Gibson MD, MacNeill M, et al. Impacts of Air Cleaners on Indoor Air Quality in Residences Impacted by Wood Smoke. Environ Sci Technol. 2014;48:12157–63. doi: 10.1021/es503144h. [DOI] [PubMed] [Google Scholar]
- 37.Karottki DG, Spilak M, Frederiksen M, et al. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environ Health. 2013;12:116. doi: 10.1186/1476-069X-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ljungman PL, Wilker EH, Rice MB, et al. Short-Term Exposure to Air Pollution and Digital Vascular Function. Am J Epi. 2014;180:480–489. doi: 10.1093/aje/kwu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Rittenhouse-Olson K, Scheider WL, et al. Effect of particulate matter air pollution on C-reactive protein: a review of epidemiologic studies. Rev Environ Health. 2012;27:133–49. doi: 10.1515/reveh-2012-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rückerl R, Schneider A, Breitner S, et al. Health effects of particulate air pollution: A review of epidemiological evidence. Inhal Toxicol. 2011;23:555–92. doi: 10.3109/08958378.2011.593587. [DOI] [PubMed] [Google Scholar]
- 42.Chuang K-J, Chan C-C, Su T-C, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 43.O’Toole TE, Hellmann J, Wheat L, et al. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010;107:200–3. doi: 10.1161/CIRCRESAHA.110.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudez G, Janssen NAH, Kilinc E, et al. Effects of Ambient Air Pollution on Hemostasis and Inflammation. Environ Health Perspect. 2009;117:995–1001. doi: 10.1289/ehp.0800437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghio AJ, Stonehuerner J, Dailey LA, et al. Metals associated with both the water-soluble and insoluble fractions of an ambient air pollution particle catalyze an oxidative stress. Inhal Toxicol. 1999;11:37–49. doi: 10.1080/089583799197258. [DOI] [PubMed] [Google Scholar]
- 46.Forchhammer L, Møller P, Riddervold IS, et al. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 2012;9:7. doi: 10.1186/1743-8977-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pope C, Hansen JC, Kuprov R, et al. Vascular Function and Short-Term Exposure to Fine Particulate Air Pollution. J Air Waste Manag Assoc. 2011;61:858–63. doi: 10.3155/1047-3289.61.8.858. [DOI] [PubMed] [Google Scholar]
- 48.Hejl AM, Adetona O, Diaz-Sanchez D, et al. Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. J Occup Environ Hyg. 2013;10:173–80. doi: 10.1080/15459624.2012.760064. [DOI] [PubMed] [Google Scholar]
- 49.Stockfelt L, Sallsten G, Almerud P, et al. Short-term chamber exposure to low doses of two kinds of wood smoke does not induce systemic inflammation, coagulation or oxidative stress in healthy humans. Inhal Toxicol. 2013;25:417–25. doi: 10.3109/08958378.2013.798387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 51.Van Eeden SF, Yeung A, Quinlam K, et al. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:61–7. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs L, Nawrot TS, de Geus B, et al. Subclinical responses in healthy cyclists briefly exposed to traffic-related air pollution: an intervention study. Environ Health. 2010;9:64. doi: 10.1186/1476-069X-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan WC, Qiu D, Liam BL, et al. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med. 2000;161:1213–7. doi: 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- 54.Swiston JR, Davidson W, Attridge S, et al. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32:129–38. doi: 10.1183/09031936.00097707. [DOI] [PubMed] [Google Scholar]
- 55.Sakai M, Sato Y, Sato S, et al. Effect of relocating to areas of reduced atmospheric particulate matter levels on the human circulating leukocyte count. J Appl Physiol Bethesda Md. 1985–2004;97:1774–80. doi: 10.1152/japplphysiol.00024.2004. [DOI] [PubMed] [Google Scholar]
- 56.Dhindsa M, Sommerlad SM, DeVan AE, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol Bethesda Md. 1985–2008;105:427–32. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heffernan KS, Karas RH, Mooney PJ, et al. Pulse wave amplitude is associated with brachial artery diameter: implications for gender differences in microvascular function. Vasc Med Lond Engl. 2010;15:39–45. doi: 10.1177/1358863X09349523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 59.Kuvin JT, Mammen A, Mooney P, et al. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med Lond Engl. 2007;12:13–6. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 60.Onkelinx S, Cornelissen V, Goetschalckx K, et al. Reproducibility of different methods to measure the endothelial function. Vasc Med Lond Engl. 2012;17:79–84. doi: 10.1177/1358863X12436708. [DOI] [PubMed] [Google Scholar]
- 61.Schnabel RB, Schulz A, Wild PS, et al. Noninvasive Vascular Function Measurement in the Community Cross-Sectional Relations and Comparison of Methods. Circ Cardiovasc Imaging. 2011;4:371–80. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 62.Aizer J, Karlson E, Chibnik L, et al. A controlled comparison of brachial artery flow mediated dilation (FMD) and digital pulse amplitude tonometry (PAT) in the assessment of endothelial function in systemic lupus erythematosus. Lupus. 2009;18:235–42. doi: 10.1177/0961203308096663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr. 2011;93:500–5. doi: 10.3945/ajcn.110.006155. [DOI] [PubMed] [Google Scholar]
- 64.Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–6. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.