Abstract
Multiple sclerosis (MS) is the prototypic inflammatory disease of the central nervous system (CNS) characterized by multifocal areas of demyelination, axonal damage, activation of glial cells, and immune cell infiltration. Despite intensive years of research, the etiology of this neurological disorder remains elusive. Nevertheless, the abundance of immune cells such as T lymphocytes and their products in CNS lesions of MS patients supports the notion that MS is an immune-mediated disorder. An important body of evidence gathered from MS animal models such as experimental autoimmune encephalomyelitis (EAE), points to the central contribution of CD4 T lymphocytes in disease pathogenesis. Both Th1 (producing interferon-γ) and Th17 (producing interleukin 17) CD4 T lymphocytes targeting CNS self-antigens have been implicated in MS and EAE pathobiology. Moreover, several publications suggest that CD8 T lymphocytes also participate in the development of MS lesions. The migration of activated T lymphocytes from the periphery into the CNS has been identified as a crucial step in the formation of MS lesions. Several factors promote such T cell extravasation including: molecules (e.g., cell adhesion molecules) implicated in the T cell-blood brain barrier interaction, and chemokines produced by neural cells. Finally, once in the CNS, T lymphocytes need to be reactivated by local antigen presenting cells prior to enter the parenchyma where they can initiate damage. Further investigations will be necessary to elucidate the impact of environmental factors (e.g., gut microbiota) and CNS intrinsic properties (e.g., microglial activation) on this inflammatory neurological disease.
Keywords: Central nervous system, T lymphocytes, Neuroinflammation, Demyelination, Neurodegeneration, Autoimmunity
Introduction
Multiple sclerosis (MS) is considered the prototypic inflammatory disease of the central nervous system (CNS). This neurological disorder affects over 2 million people worldwide (Multiple Sclerosis International Federation 2013).MSis clinically characterized by recurrent and transient bouts of handicap, including loss of vision, balance and mobility, and by painful sensory symptoms. An important proportion of patients with MS will experience a steady progression of handicap over several years, which can lead to extreme fatigue, cognition impairment, and paralysis. This chronic neurological disease has enormous physical, psychosocial and economic burdens not only to the patients but also to their families, as MS affects young adults at the peak of their active life. Lesions observed in the CNS of MS patients are characterized by multifocal areas of myelin sheath destruction, oligodendrocyte death, axonal and neuronal damage, and activation of glial cells. Despite decades of intense research, the etiology of this disabling disease remains unresolved. Nonetheless, the MS research community largely agrees that complex interactions between environmental factors and genes lead to the inflammatory process in the CNS. Indeed, both environmental factors and genetics have been shown to influence the susceptibility as well as the development of MS (Koch et al. 2013). A vast body of evidence gathered from both MS patients and animal models points to the fundamental role of the immune system in disease pathogenesis (Wu and Alvarez 2011). Genetic linkage analyses and more recent genome-wide association studies of MS have uncovered multiple disease associated variants in genes related to immune functions (Sawcer et al. 2014). In addition, cells of the immune system such as macrophages and T lymphocytes and their products are detected with abundance in MS lesions and in the cerebrospinal fluid (CSF) of patients with MS (Wu and Alvarez 2011). Furthermore, immunosuppressive or immunomodulatory therapies have been shown to ameliorate disease course and clinical outcomes of MS (Cross and Naismith 2014). Finally, it is well documented that activation of myelin-specific T lymphocytes is sufficient to induce experimental autoimmune encephalomyelitis (EAE), the most common animal model of MS. This review summarizes the current knowledge on the involvement of T lymphocytes in the pathobiology of both MS and its animal models.
T Lymphocytes: Key Cells of the Adaptive Immune Responses
Defense against pathogens is provided by the immune system, which comprises two well integrated and complementary arms: the innate immunity and the adaptive immunity. While the innate immune system delivers an early and rapid response, the adaptive immune system develops a response that is highly specific to the encountered infectious agents and enhanced with subsequent pathogen exposures. B and T lymphocytes mediate the adaptive immune responses and provide long-term protection. Immature T lymphocytes are subjected to stringent positive and negative selection and maturation in the thymus prior to exit in the periphery as naïve mature T cells. The main T cell subsets are: CD4 T lymphocytes, also called helper T cells (Th), and CD8 T lymphocytes, also known as cytotoxic T cells (Tc). CD4 T lymphocytes coordinate numerous immune responses and CD8 T lymphocytes play key roles in controlling intracellular pathogens but also neoplastic cells. T lymphocytes express on their surface the T cell receptor (TCR) complex composed of the highly variable antigen binding TCR and the CD3 signaling proteins. Via this TCR complex, CD4 T lymphocytes recognize antigens that are presented by the major histocompatibility complex (MHC) class II molecules, whereas CD8 T lymphocytes recognize antigens presented by MHC class I molecules. The activation and differentiation of naïve T lymphocytes into activated cells require at least two signals delivered by professional antigen presenting cells (APCs): 1- engagement of the TCR recognizing a peptide-MHC complex; 2- interaction of the coactivating receptor (e.g., CD28) with a co-activating ligand (e.g., CD80, CD86). Such efficient APC-T cell interaction induces a complex cascade of intracellular signaling that triggers the maturation (change in the expression profile of surface and intracellular molecules), proliferation and production of immune mediators (e.g., cytokines) by T lymphocytes.
Specific environmental cues such as cytokines secreted by APCs shape the differentiation of T lymphocytes into specific subsets, each characterized by distinct attributes (transcription factors, cytokines, chemokine receptors, etc.). Based on their cytokine secretion profile, three main subsets of Th and Tc lymphocytes have been described: Th1/Tc1 (e.g., interferon-γ (IFNγ), tumor necrosis factor (TNF)), Th2/Tc2 (e.g., interleukin (IL)-4, IL-5, IL-13), and Th17/Tc17 (e.g., IL-17, IL-21, and IL-22) (Mittrucker et al. 2014; Raphael et al. 2014). Th1 and Tc1 lymphocytes are key components of cell-mediated responses providing immunity to intracellular pathogens. While Th1 cells stimulate phagocyte-mediated functions, Tc1 cells can directly lyse infected cells by several mechanisms including the release of lytic enzymes (perforin, granzyme). In contrast, Th2 and Tc2 cells play an important role in humoral-mediated immunity. Cytokines (e.g., IL-4) produced by these cells promote the differentiation and maturation of B lymphocytes and the production of antibodies. Finally, Th17/Tc17 cells provide protection against certain bacterial and fungal infections; they favor the recruitment of neutrophils and the activation of innate immune cells. Other effector T cell subpopulations (e.g., Th9, Th22, etc.) as well as regulatory CD4 and CD8 T cell subsets have been documented (Mittrucker et al. 2014; Raphael et al. 2014). The diversity of T lymphocyte subsets and their associated functions illustrates the flexibility and competence of the immune system to efficiently respond to a great variety of pathogens and threats. Following their activation, T lymphocytes migrate into peripheral organs to mediate the immunosurveillance. Finally, a portion of activated T lymphocytes survives and persists as central or effector memory lymphocytes; these memory cells confer long-term protection with enhanced responses upon subsequent challenges. Unfortunately, activated T cells not only control infections and tumors but they can also contribute to pathological processes.
Auto-Reactive T Lymphocytes Recognizing CNS Antigens
Despite thymic negative selection, the mature T cell repertoire of healthy humans includes T lymphocytes recognizing self-antigens (Walker and Abbas 2002). CD4 and CD8 T lymphocytes reactive to myelin antigens [e.g., myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG)], or neuronal antigens (e.g., neurofilament light protein), were found in similar or elevated proportion in the blood and the CSF of patients with MS as compared to healthy controls (Ota et al. 1990; Liblau et al. 1991; Sun et al. 1991; Chou et al. 1992; Zhang et al. 1994; Crawford et al. 2004; Berthelot et al. 2008; Huizinga et al. 2009; Elong Ngono et al. 2012). Nonetheless, a notable number of publications advocate that myelin specific T cells obtained from MS patients display altered characteristics compared to those detected in healthy donors: an enhanced frequency of high-avidity T cells, an activated phenotype (Allegretta et al. 1990; Chou et al. 1992; Zhang et al. 1994; Vandevyver et al. 1995; Bielekova et al. 2004), and an increased production of pro-inflammatory cytokines (IL-2, IFN-γ, and TNF) (Sharief and Thompson 1993; Strunk et al. 2000; Zang et al. 2004). Injection of altered myelin peptides, which can compete with native myelin peptides and consequently alter the activation of myelin-reactive T cells, was shown to protect from EAE development (Nicholson et al. 1995; Gaur et al. 1997; Anderton et al. 1999; Genain and Zamvil 2000). Subsequently, scientists rationalized that such antigen specific immunotherapy could potentially be beneficial to MS patients (Genain and Zamvil 2000). In an attempt to decrease pro-inflammatory MBP-specific T cells, MS patients were injected with an altered MBP peptide. Regrettably, in a subgroup of patients, such treatment enhanced MBP reactive Th1 responses, triggered the development of new CNS inflammatory lesions [as detected by magnetic resonance imaging (MRI)], and caused clinical relapses (Bielekova et al. 2000). The dramatic outcome of this clinical trial provided the direct in vivo evidence that activation of human myelin reactive T lymphocytes in the periphery can lead to CNS damage in patients with MS.
Multiple lines of evidence collected from EAE models established that myelin-specific T lymphocytes that have been activated in the periphery can induce a CNS demyelinating disease. Indeed, active immunization with myelin or immunodominant myelin peptides emulsified in complete Freund’s adjuvant can trigger the development of EAE in various animal species (Rivers et al. 1933; Kabat et al. 1951; Stromnes and Goverman 2006b). Similarly, the adoptive transfer via peripheral routes (intraperitoneal or intravenous) of activated myelin specific CD4 or CD8 T lymphocytes is sufficient to induce EAE in naïve recipients (Stromnes and Goverman 2006a; Fletcher et al. 2010; Mars et al. 2011). Furthermore, activation of T lymphocytes recognizing myelin epitopes that are distinct from the first targeted antigen, a phenomenon coined as epitope spreading, has been shown to trigger EAE relapses (McRae et al. 1995; McMahon et al. 2005; Baxter 2007). Accordingly, different groups have elegantly demonstrated that induction of immune tolerance to myelin peptides can prevent the emergence of relapses in EAE models (Yu et al. 1996; Vanderlugt et al. 2000; Baxter 2007). Collectively, these results support the notion that myelin specific T cells are implicated not only in disease initiation but also in disease progression. Clinical trials in MS patients are currently ongoing in an attempt to induce tolerance to several myelin epitopes; whether such approaches will lead to clinical benefits has yet to be demonstrated (Lutterotti and Martin 2014).
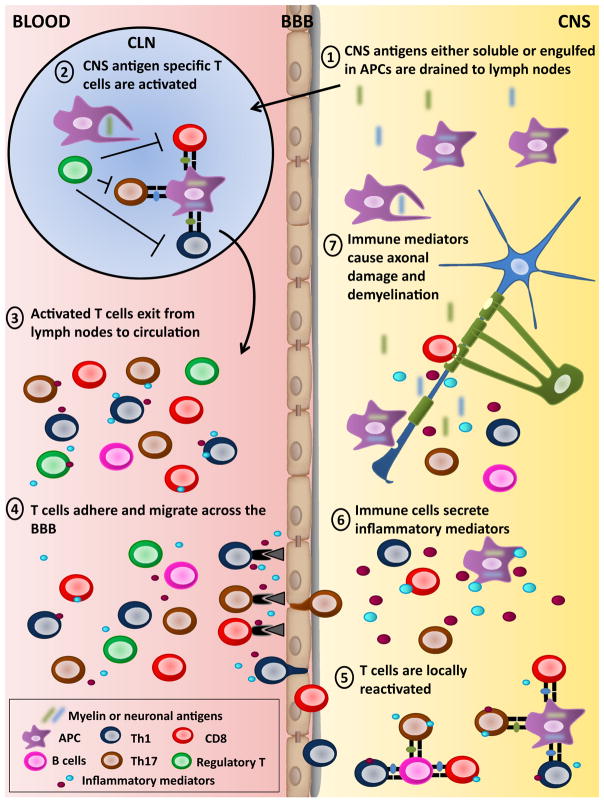
The detection of CNS-specific T lymphocytes exhibiting an activated phenotype, especially in patients with MS, suggests that these T lymphocytes had previous interactions with professional APC efficiently presenting CNS-derived antigens. In contrast to most organs, the brain and spinal cord do not contain defined lymphatic channels; nevertheless, lymphatic drainage for the CSF and the interstitial fluid of the brain parenchyma to the cervical lymph nodes does take place (Laman and Weller 2013). Professional APCs such as dendritic cells have been shown to travel from the CNS via the rostral migratory stream to the cervical lymph nodes where peripheral T cells could encounter CNS antigens (Mohammad et al. 2014). Mature APCs that have engulfed myelin or neuronal antigens were detected in cervical lymph nodes obtained from MS patients and animals (marmosets and mice) affected with EAE, implying that these APCs are positioned to efficiently activate T lymphocytes (Fig. 1) (de Vos et al. 2002; van Zwam et al. 2009b). Moreover, extracellular myelin particles were preferentially detected in the meninges and perivascular spaces of MS patients compared to controls (Kooi et al. 2009); whether these soluble antigens are eventually phagocytized and presented to myelin specific T cells is still unknown. It has been shown that transgenic mice expressing a myelin specific TCR can spontaneously develop EAE symptoms and that these self-reactive T cells were first activated in the cervical lymph nodes where some CNS antigens are continuously presented (Goverman 2009). Recently published data support the notion that under normal physiological conditions, oligodendrocyte specific antigens are presented to T cells in secondary lymphoid organs (Harris et al. 2014). Moreover, CD8 T lymphocytes that were activated within cervical lymph nodes by APCs presenting CNS antigens were shown to acquire specific integrin (e.g., CD103) that guided them back preferentially to the brain where the antigens were originally collected (Calzascia et al. 2005; Masson et al. 2007). Results obtained using intravital two-photon imaging elegantly demonstrated that activated T cells migrated from blood vessels to the CNS under inflamed conditions regardless of their antigen specificity. In contrast, T cells were licensed to enter the parenchyma only following their reactivation by local APCs presenting their cognate antigen (Bartholomaus et al. 2009). Notably, an important proportion of T cells detected in MS lesions (Kivisakk et al. 2004), or CD8 T cells observed in CSF from MS patients (Jilek et al. 2007; Ifergan et al. 2011) lacks CCR7. As loss of this chemokine receptor (CCR7) on T cells is associated with an effector memory phenotype (Sallusto et al. 2004), these observations suggest that such effector T cells are enriched in the inflamed CNS of MS patients. It is well established that several APCs present in human and mouse lesions can locally reactivate T cells (Fig. 1) including macrophages, microglia, and dendritic cells (Greter et al. 2005; Frohman et al. 2006); more recently, B cells were shown to contribute to such T cell reactivation in EAE mice (Pierson et al. 2014) (Fig. 1). Moreover, the activation of subsequent waves of CNS-reactive T lymphocytes involved in disease relapse and perpetuation could take place either in lymph nodes and/or within the inflamed CNS. In a chronic EAE model, the relapse severity was reduced following the removal of CNS draining lymph nodes (van Zwam et al. 2009a). In addition, a subpopulation of dendritic cells detected within the inflamed brain of EAE mice efficiently activated naïve myelin-specific CD8 T lymphocytes (Ji et al. 2013). Collectively, these results indicate that CNS specific T cells can be activated either locally in the CNS or in lymphoid organs.
Fig. 1.
Activation and roles of T lymphocytes in the pathogenesis of MS and EAE 1. In contrast to most organs, the brain and spinal cord do not contain defined lymphatic channels; nevertheless, lymphatic drainage for the CSF and the interstitial fluid of the brain parenchyma to the cervical lymph nodes does take place (Laman and Weller 2013). Soluble CNS antigens and professional APCs, such as dendritic cells, that have engulfed myelin or neuronal antigens can travel from the CNS to the cervical lymph nodes (CLN) (Mohammad et al. 2014). 2. Mature APCs that have engulfed myelin or neuronal antigens are detected in cervical lymph nodes obtained from MS patients and EAE animals (Laman and Weller 2013). These APCs can efficiently activate CNS-reactive CD4 and CD8 T lymphocytes. Different regulatory T lymphocyte subsets have been shown to reduce the development and severity of EAE (Kleinewietfeld and Hafler 2014; Sinha et al. 2014). Several groups reported that regulatory T cell subsets from MS patients have impaired regulatory functions compared to healthy donors (Kleinewietfeld and Hafler 2014; Sinha et al. 2014). 3. Activated myelin or neuronal-specific T lymphocytes exit into the peripheral blood to perform immunosurveillance. CNS reactive CD4 and CD8 T lymphocytes obtained from the peripheral blood of MS patients exhibit enhanced activation properties compared to those from health donors. 4. Activated autoreactive T lymphocytes have an enhanced capacity to cross the BBB given their elevated expression of mediators such as chemokine receptors, adhesion molecules, integrins, and cytokines (Goverman 2009; Larochelle et al. 2011). 5. Once in the CNS, T lymphocytes can be reactivated by local APCs (macrophages, microglia and dendritic cells, or B lymphocytes), which are present in human and mouse CNS lesions (Greter et al. 2005; Frohman et al. 2006; Pierson et al. 2014). This antigen-specific reactivation has been shown to be essential to license activated autoreactive T lymphocytes to enter the CNS parenchyma (Bartholomaus et al. 2009). 6. CNS infiltrating Th1, Th17, and CD8 T lymphocytes, and macrophages as well as inflamed microglia secrete soluble mediators (e.g., inflammatory cytokines, free radical, etc.). Moreover, cross-talk between T cells and microglia/macrophages contribute to perpetuate the inflammatory milieu within the CNS. 7. These soluble mediators can injure oligodendrocyte/myelin and axon/neuron structures. Moreover, activated microglia/macrophages can directly phagocyte oligodendrocytes. Similarly, CD8 T lymphocytes have been detected in close proximity to oligodendrocytes and demyelinated axons with polarization of their cytolytic granules (Neumann et al. 2002; Wulff et al. 2003; Lassmann 2004; Saikali et al. 2007). Activated T cells have the capacity to kill oligodendrocytes or neurons (Jurewicz et al. 1998; Sauer et al. 2013; Zaguia et al. 2013). Finally, such damage causes the release of additional CNS antigens that can be further phagocytosed and presented to new waves of CNS-specific T lymphocytes
A vast body of evidence demonstrated that infectious agents can trigger, in both humans and mice, the activation of T lymphocytes that recognize both a self-antigen such as myelin peptide and a microbial peptide, a phenomenon referred as molecular mimicry (Fujinami and Oldstone 1985; Wucherpfennig and Strominger 1995; Talbot et al. 1996; Hemmer et al. 1997; Olson et al. 2001; Chastain and Miller 2012). Moreover, self-molecular mimicry can also participate in CNS autoimmunity. Indeed, multiple clones of CD4 T lymphocytes expanded from MOG35–55 immunized mice and carrying a single TCR have been shown to recognize both the myelin antigen (MOG35–55 ) and an axonal antigen (neurofilament) (Krishnamoorthy et al. 2009; Lucca et al. 2014). Moreover, both self-antigen recognitions contributed to the observed CNS damage (Krishnamoorthy et al. 2009). Finally, the capacity of T cells to recognize multiple epitopes could also arise from dual T cells, which express two TCRs. Goverman and colleagues have demonstrated that a viral infection activated dual CD8 T cells recognizing both a viral antigen and a myelin antigen and consequently triggered CNS autoimmunity (Ji et al. 2010). A better understanding of the mechanisms implicated in the activation of myelin or neuronal reactive T lymphocytes in the context of MS will be essential to design therapies specifically targeting these detrimental cells.
Extravasation of T Lymphocytes to the CNS
The blood brain barrier (BBB) restricts the migration of cells and soluble molecules from the periphery to the CNS and consequently preserves local homeostasis and an optimal environment for neuronal functions (Larochelle et al. 2011). Under normal physiological conditions, few leukocytes, including T cells, cross the BBB to perform the immune surveillance of the CNS. In contrast, the massive infiltration of pro-inflammatory leukocytes into the CNS represents an early event in the development of MS and EAE lesions (Larochelle et al. 2011) (Fig. 1). The different steps of immune cell extravasation include: capture/rolling, activation, firm adhesion, crawling and diapedesis/transmigration (Engelhardt and Ransohoff 2012). While resting T lymphocytes have a limited capacity to invade the brain or spinal cord, activated T lymphocytes express numerous molecules including chemokine receptors, adhesion molecules, integrins, cytokines, matrix metalloproteinases, and reactive oxygen species that promote their extravasation into the CNS (Larochelle et al. 2011). An important number of studies using EAE models established that myelin-specific T lymphocytes activated in the periphery acquire these molecules and do enter the CNS (Goverman 2009).
The interaction between cell adhesion molecules (CAMs) expressed by endothelial cells of the BBB (EC-BBB) and their cognate ligands (integrins, CAMs) present on activated leukocytes plays a central role in the transmigration of immune cells into the CNS (Larochelle et al. 2011). Several inflammatory stimuli (e.g., cytokines) can induce or enhance the expression of CAMs by the EC-BBB including: intracellular cell adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), activated leukocyte cell adhesion molecule (ALCAM), and melanoma cell adhesion molecule (MCAM) (Wong and Dorovini-Zis 1992, 1995; Cayrol et al. 2008; Larochelle et al. 2012). The efficacy of an antibody (Natalizumab) targeting α4 integrin, which is part of the VCAM-1 cognate ligand, as a treatment for patients with MS underlines the crucial impact of the BBB-leukocyte interaction in the formation of MS lesions (Polman et al. 2006). Unfortunately, patients treated with biological agents widely hampering immune cell extravasation such as Natalizumab, but also Efalizumab, which targets a subunit of ICAM-1 ligand (αL integrin), have an increased risk of severe infections (e.g., JC virus induced progressive multifocal leukoencephalopathy) (Major 2010). These clinical outcomes emphasize the necessity to develop tools blocking the migration of specific lymphocyte subsets. In EAE models, blockade of either ALCAM or MCAM, which are both up-regulated in MS and EAE lesions, reduced the transmigration of CD4 T lymphocytes and decreased disease severity (Cayrol et al. 2008; Larochelle et al. 2012). Mice deficient for MCAM specifically on endothelial cells developed less severe EAE accompanied by reduced CNS T cell infiltration (Duan et al. 2013). Moreover, MCAM expression was detected on a subpopulation of activated CD4 T cells producing augmented levels of inflammatory molecules (e.g., IL-17, GM-CSF) (Larochelle et al. 2012) and preferentially migrating across the BBB compared to cells devoid of this molecule (Larochelle et al. 2012; Schneider-Hohendorf et al. 2014). Overall, these publications suggest that molecules present on subsets of inflammatory T cells rather than the widely expressed integrins (e.g., αL integrin, α4 integrin) could represent valid therapeutic targets.
Several groups have investigated the impact of chemokines and their receptors on the capacity of T cells to invade the CNS in the context of MS and its animal models (Cheng and Chen 2014). It is well established that activated T lymphocytes up-regulate specific chemokine receptors that will influence their migratory patterns (Griffith et al. 2014). Both human and mouse Th17 cells have been shown to preferentially express CCR6, a chemokine receptor for CCL20, a chemokine present in abundance in the choroid plexus under normal physiological and MS inflamed conditions (Reboldi et al. 2009). But the role of CCR6 in CNS inflammation is still unresolved; conflicting results indicated that CCR6 deficient mice were either resistant to EAE (Reboldi et al. 2009) or exhibited more severe EAE (Elhofy et al. 2009; Villares et al. 2009). A recent publication suggests that the regional chemokine profile influences the infiltration of leukocytes within specific CNS areas and consequently shapes the clinical EAE symptoms (Stoolman et al. 2014). In EAE mice exhibiting clinical symptoms related to brainstem or cerebellar lesions, CXCL2 was indeed elevated in the brainstem. In contrast, CCL2 was increased in the spinal cord of EAE mice having typical ascending paralysis symptoms associated with spinal cord immune cell infiltration (Stoolman et al. 2014). Moreover, another group showed that following EAE induction in the absence of astrocyte-derived CCL2, the total number of CD4 T lymphocytes in the inflamed CNS was similar to controls, however, these T cells did not enter the parenchymal space and were confined to the spinal cord perivascular area (Moreno et al. 2014). These results support the notion that CNS-cell derived chemokines can shape the migratory pattern of leukocytes including activated T cells. Additional investigations will be necessary to elucidate the contribution of specific chemokines and their receptors especially given their impact on multiple cell types and their various functions.
Th1 vs. Th17 Lymphocytes in MS and EAE
Numerous groups documented that activated myelin specific CD4 T cells secreting IFNγ are sufficient to transfer disease into naïve mice (Fletcher et al. 2010; Lovett-Racke et al. 2011). Similarly, injection of IFNγ to patients with MS caused aggravated symptoms (Panitch et al. 1987a, b). These key observations were the impetus for the concept that myelin specific IFNγ-producing Th1 cells induce the demyelinating disease in both human MS patients and its animal models. But publications in the 1990s refuted the key role of IFNγ- producing cells in disease pathogenesis. Injection of antibodies blocking IFNγ augmented EAE disease severity (Lublin et al. 1993). Moreover, EAE induction into IFNγ or IFNγ receptor deficient mice caused a more severe disease course than in wild type controls (Ferber et al. 1996; Willenborg et al. 1996).A new subset of CD4 T lymphocytes was subsequently identified and named Th17 cells as these lymphocytes produce IL-17A and IL-17 F amongst many other cytokines (e.g., IL-21, IL-22). As demonstrated for Th1 cells, the adoptive transfer of activated myelin-specific Th17 lymphocytes can induce EAE in naïve recipient mice (Langrish et al. 2005; Kroenke et al. 2008; Stromnes et al. 2008). However, the signature cytokines secreted by Th17 cells are dispensable for EAE induction; indeed, mice deficient for IL-17, IL-21 or IL-22 were still susceptible to disease (Kreymborg et al. 2007; Sonderegger et al. 2008; Haak et al. 2009; Codarri et al. 2013). The more recent studies pinpoint the crucial role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in T cell-mediated autoimmune CNS inflammation (Codarri et al. 2013). This cytokine can be secreted by both myelin specific activated Th1 and Th17 lymphocytes; GM-CSF deficient mice were resistant to the induction of EAE; injection of this cytokine exacerbated disease symptoms whereas administration of blocking antibodies even after disease onset diminished disease severity (McQualter et al. 2001; Codarri et al. 2011; El-Behi et al. 2011). Notably, the adoptive transfer of not only Th1 or Th17 encephalitogenic CD4 T cells can induce EAE but Th9 myelin specific CD4 T cells, which are characterized by the secretion of IL-9 and IL-10, can also transfer disease in naïve recipients (Jager et al. 2009).
Pro-inflammatory Th1 and Th17 cytokines are present in elevated amounts in MS patients compared to controls. Indeed, IFNγ, IL-17, IL-22, and GM-CSF were detected in the CSF and CNS lesions of MS patients, especially during the active phase of the disease (Carrieri et al. 1998; Monteyne et al. 1999; Kebir et al. 2009; Mellergard et al. 2010). In fact, most T lymphocytes in acute and chronic MS lesions express IL-17 (Kebir et al. 2007; Tzartos et al. 2008). Activated CD4 T cells simultaneously producing IL-17 and IFNγ were preferentially expanded from blood samples obtained from MS patients during a relapse; these double producing cells had a greater capacity to cross the human BBB and were detectable in post-mortem MS brain tissues (Kebir et al. 2009). Moreover, IL-12 and IL-23, which are key cytokines involved in the differentiation of Th1 and/or Th17 cell subsets, are more abundant in the CSF and/or CNS of MS patients compared to controls (Link 1998; Li et al. 2007). Although the injection of an antibody targeting the shared p40 subunit of IL-12 and IL-23 provided significant benefits to patients affected with autoimmune diseases (e.g., psoriasis) (Kumar et al. 2013), such strategy was not successful in MS patients (Segal et al. 2008; Vollmer et al. 2011). Recently, a phase Ib/IIa clinical trial evaluating the impact of an antibody targeting GM-CSF in patients with rheumatoid arthritis patients has shown some efficacy (Behrens et al. 2014). Whether any therapies specifically blocking cytokines such as GM-CSF, could be beneficial in MS patients warrant further investigations.
Observations in EAE models indicate that the relative predominance of Th1 vs. Th17 immune responses influences the CNS localization of the induced inflammation (Pierson et al. 2012). Robust Th1 responses producing elevated levels of IFNγ induced an important immune cell infiltration in the spinal cord and the classical EAE symptoms (e.g., flaccid tail, hindlimb paralysis) (Stromnes et al. 2008). In contrast, encephalitogenic T cells secreting high IL-17 levels but low IFNγ levels, infiltrated preferentially the brain parenchyma and induced the atypical EAE symptoms (e.g., head tilt, spinning and axial rotation) (Stromnes et al. 2008). These distinct lesion patterns were confirmed in a different mouse strain; indeed the adoptive transfer of Th1, Th17 or Th9 encephalitogenic cells also induced CNS lesions with distinct patterns (Jager et al. 2009). Numerous factors can prompt encephalitogenic T lymphocytes to preferentially infiltrate one particular CNS area including genetic background, myelin epitope targeted, cytokines provided by professional APCs, local CNS chemokine production and cytokine receptor expression (Pierson et al. 2012). Importantly, the predominance of either Th1 or Th17 responses in MS patients has been implicated in disease heterogeneity with variations in clinical course, response to immunomodulators and localization of CNS lesions (Axtell et al. 2010, 2013; Pierson et al. 2012). Finally, an increasing body of evidence gathered from mouse models and human studies demonstrates the plasticity of activated and memory T cell subsets; the commitment of activated T cells to specific functions and characteristics (cytokines, transcription factors, etc.) associated with a particular T cell subset has been shown to be not irreversible (Geginat et al. 2014). For example, one group used a fate tracking system to reveal that during EAE, a subgroup of Th17 cells stopped producing IL-17 and secreted instead IFNγ (Hirota et al. 2011). Although the key roles of Th1 and Th17 CD4 lymphocytes in the initiation of MS and EAE are well established (Cua et al. 2003; Chen et al. 2006; Kebir et al. 2007, 2009; Stromnes et al. 2008), the mechanisms whereby these cells contribute to the pathogenesis of these demyelinating diseases are not completely resolved. Finally, other CD4 T cell subsets demonstrating cytotoxic properties can also contribute to the tissue damage. We demonstrated that the proportion of CD4 T lymphocytes expressing NKG2C as well as other markers (e.g., CD56, NKG2D, granzyme B) was elevated in the peripheral blood of MS patients compared to controls (Zaguia et al. 2013). Moreover, these NKG2C-expressing CD4 T cells were present in MS lesions and could kill human oligodendrocytes, which express HLA-E the cognate ligand of NKG2C (Zaguia et al. 2013). Additional investigations are deemed essential to elucidate the contribution of the numerous immune molecules and mechanisms used by CD4 T lymphocytes to injure neural cells (Pierson et al. 2012).
Despite the imposing body of evidence gathered from EAE models supporting the essential role of CD4 T lymphocytes in the development of MS (Fletcher et al. 2010), results from clinical trials demonstrated that the picture is far more complex. While an anti-CD4 depleting antibody therapy did not produce any clinical benefits to patients with MS (Lindsey et al. 1994; Llewellyn-Smith et al. 1997; Rep et al. 1997; van Oosten et al. 1997), a more global immunosuppressive approach such as an anti-CD52 antibody (Alemtuzumab), which dramatically reduces the number of most lymphocytes, successfully decreased the number of relapses and disease progression of MS patients (Jones et al. 2010). The emerging appreciation of the contribution of other lymphocyte subsets in MS pathogenesis warrants further investigations and will most likely provide a more accurate understanding of this multifaceted disease.
CD8 T Lymphocytes in MS and its Animal Models
An increasing body of evidence substantiates the notion that CD8 T lymphocytes actively partake in the CNS injury observed in patients with MS (Mars et al. 2011). Several groups have documented the presence of activated CD8 T lymphocytes in perivascular and parenchymal MS lesions (Fig. 1); their number reaches or surpasses that of CD4 T cells (Hauser et al. 1986; Gay et al. 1997; Babbe et al. 2000; Neumann et al. 2002; Lassmann 2004; Junker et al. 2007; Frischer et al. 2009). CD8 T lymphocytes were even detected in early stages of cortical demyelinating MS lesions (Lucchinetti et al. 2011). IL-17 producing CD8 T lymphocytes (i.e., Tc17) have been shown to be enriched especially in active MS lesions (Kebir et al. 2007; Tzartos et al. 2008). Moreover, we have recently reported that the frequency of MCAM expressing CD8 T cells is elevated in MS patients during relapses and that the proportion of MCAM+ CD8 T cells producing IL-17, IFNγ, GM-CSF and TNF is significantly greater than of their MCAM-negative counterparts (Larochelle et al. 2015).
The clonal diversity of T lymphocytes present in a specific compartment can be assessed by spectratyping analysis of the complementarity determining region 3 of the TCR, as each clone expresses a distinct sequence. When the number of distinct T cell clones detected in a specific organ is limited, it implies that these T cells did not inadvertently move into this compartment but rather antigen-specific T lymphocytes preferentially infiltrated and locally expanded. Several groups analyzed the TCR repertoire of CD8 T lymphocytes obtained from the blood, CSF and/or the brain of MS patients. These studies found that the majority of CD8 T lymphocytes recovered from MS lesions belonged to a few clones (Babbe et al. 2000; Junker et al. 2007). Furthermore, using samples obtained from patients studied longitudinally, it has been possible to establish that some CD8 T cell clones detected in MS patients persisted over years in their CNS (CSF and/or tissue) (Jacobsen et al. 2002; Skulina et al. 2004). In sharp contrast, the repertoire of CD4 T cells recovered from the CNS of MS patients has been shown to be heterogeneous (Babbe et al. 2000; Jacobsen et al. 2002; Skulina et al. 2004; Junker et al. 2007). Overall, these reports reinforce the idea that CD8 T lymphocytes present in the CNS of MS patients are not bystander cells but rather have been engaged in active immune responses (Mars et al. 2011). However, the antigens that have been recognized by such infiltrating CD8 T cells and potentially leading to their activation and expansion in the CNS have not been identified.
CD8 T lymphocytes recognize antigens presented by MHC class I molecules. Genetic studies indicated that specific MHC class I alleles either increase (e.g., HLA-A*0301) or reduce (HLA-A*0201) the risk of developing MS (Fogdell-Hahn et al. 2000; Harbo et al. 2004; Brynedal et al. 2007; Rubio et al. 2007) suggesting that MHC class I alleles could influence the activation of self-reactive CD8 T lymphocytes (Fugger et al. 2009; Mars et al. 2011). Under normal physiological conditions, MHC class I molecules are either undetectable on most CNS cells or expressed at low levels on microglia and endothelial cells. In sharp contrast, inflammatory conditions such as those observed in the CNS of MS patients, can up-regulate these molecules on neurons, oligodendrocytes and astrocytes even in the early phases of the disease (Ransohoff and Estes 1991; Gobin et al. 2001; Hoftberger et al. 2004). Therefore, we can speculate that activated CD8 T cells can directly target these resident CNS cells. Indeed, several groups identified in MS lesions CD8 T cells in close proximity to oligodendrocytes and demyelinated axons with polarization of their cytolytic granules (Neumann et al. 2002; Wulff et al. 2003; Lassmann 2004; Saikali et al. 2007); such CD8 T cell detection positively correlated with the extent of axonal damage (Bitsch et al. 2000; Kuhlmann et al. 2002). Moreover, in primary culture systems, activated CD8 T cells were able to injure neuronal axons (Sauer et al. 2013) as well as oligodendrocytes (Jurewicz et al. 1998) in an antigen-MHC class I specific manner. Furthermore, myelin-specific CD8 T cells can induce in mice demyelinating diseases with pathological features reminiscent of the human MS disease (Huseby et al. 2001; Sun et al. 2001; Friese and Fugger 2005). As well, murine virus-induced models of demyelinating diseases (e.g., Theiler’s virus, mouse hepatitis virus) support the involvement of CD8 T cells in demyelination and axonal injury (Murray et al. 1998; Wu et al. 2000; Howe et al. 2007).
Collectively, these results emphasize the contribution of CD8 T lymphocytes to the CNS injury observed in MS patients; however, these cytotoxic T lymphocytes most likely do not act alone. Indeed, experimental data illustrated that both CD4 and CD8 T lymphocytes can work in concert to mediate the autoimmune attack observed in EAE (Huber et al. 2013). Therefore, it is deemed essential to investigate the interplay between CD4 and CD8 T lymphocytes during different stages of MS and its animal models. Such studies will most likely shed light on the complexity and heterogeneity of the immune mechanisms involved in MS pathobiology.
Regulatory CD4 and CD8 T Lymphocytes in MS and EAE
Regulatory T lymphocytes are crucial to maintain peripheral tolerance and consequently to prevent autoimmune diseases. These T cells can curtail functions of multiple immune cell subsets including CD4 and CD8 T lymphocytes, natural killer cells (NK) and APCs (e.g., monocytes, macrophages, dendritic cells) via direct contact and secreted molecules (Lowther and Hafler 2012) (Fig. 1). Both CD4 and CD8 T lymphocyte subsets with regulatory properties have been identified (Jadidi-Niaragh and Mirshafiey 2011). The most studied regulatory CD4 T cell subsets in the context of MS and its animal models are i) the naturally occurring regulatory T cells expressing the transcription factor FoxP3 (Treg) and ii) the regulatory T cells secreting IL-10 (Tr1) (Kleinewietfeld and Hafler 2014). Several investigations performed in the EAE models established that regulatory T lymphocytes can influence the development and severity of this disease and also favor the recovery phase (Olivares-Villagomez et al. 1998; Kohm et al. 2002; McGeachy et al. 2005). Indeed, the adoptive transfer of FoxP3-expressing Treg into mice conferred protection from EAE induction while depletion of these cells led to a more severe disease course (Kohm et al. 2002; Reddy et al. 2004). Others reported that depletion of T regs did not influence the first EAE remission phase in EAE mice but rather reduced the development of subsequent relapses (Gartner et al. 2006; Jadidi-Niaragh and Mirshafiey 2011). Similarly, injection of mice with IL-10 producing Tr1-like cells activated via different protocols prevented EAE induction (Barrat et al. 2002; Ding et al. 2006). Numerous groups investigated T regs or Tr1 cells in peripheral blood obtained from MS patients and found that these regulatory cells have impaired regulatory functions and migratory properties, but not necessarily altered frequencies compared to samples taken from healthy donors (Viglietta et al. 2004; Astier et al. 2006; Martinez-Forero et al. 2008; Venken et al. 2008; Schneider-Hohendorf et al. 2010). However, when activated CD4 T cells expressing high levels of both CD25 and CD127 (IL7Rα) (Liu et al. 2006; Seddiki et al. 2006) were subtracted and that T regs defined as CD4+CD25highCD127low were analyzed the suppressive capacity of T regs from MS patients was found to be either similar to healthy individuals (Michel et al. 2008; Venken et al. 2008; Baecher-Allan et al. 2011) or reduced only in early MS patients (disease duration less than 10 years) compared to healthy controls (Venken et al. 2008). The potential use of anti-CD127 antibodies, to remove activated T cells but not T regs, is currently under investigation in MS patients (www.clinicaltrails.gov). Finally, an increasing number of publications indicates that human FOXP3 expressing T regs do not represent an homogenous population; expression of additional transcription factor (e.g., Helios) and surface markers (e.g., TIGIT, FCRL3) has been associated with suppressive capacity (Shevach and Thornton 2014; Bin Dhuban et al. 2015). Further investigations will be necessary to determine whether specific T reg subsets in MS patients are altered compared to healthy controls.
Interestingly, immunomodulatory treatments for MS patients have been shown to mediate, at least in part, their positive impact by reversing the altered properties of regulatory CD4 T cells in patients (de Andres et al. 2007; Korporal et al. 2008; Haas et al. 2009; Chiarini et al. 2012); these observations suggest that restoring functions of regulatory T cells in patients represents a promising therapeutic option (Kleinewietfeld and Hafler 2014). For example, interferon-β (IFNβ), the first broadly used immunomodulatory therapy to treat MS patients, has been shown to favor the secretion of IL-27 by dendritic cells (Sweeney et al. 2011). Remarkably, monocyte-derived dendritic cells obtained from MS patients that were characterized as IFNβ responders produced significantly more IL-27 than those obtained from IFNβ non-responders (Sweeney et al. 2011). Similarly, the beneficial immunoregulatory impact of IFNβ on EAE has been shown to rely on the induced production of IL-27 (Guo et al. 2008; Shinohara et al. 2008). Notably, IL-27 can reduce EAE severity via several mechanisms including the differentiation of IL-10-producing Tr1 cells (Awasthi et al. 2007; Fitzgerald et al. 2007; Stumhofer et al. 2007; Vasanthakumar and Kallies 2013).
Subpopulations of CD8 T cells exhibiting regulatory properties have been reported in both humans and animal models although knowledge about these cells is not as extensive as for their CD4 counterparts. Unfortunately, the markers (e.g., CD25, CD122, CD56) or cytokines (IL-10 or TGFβ) that have been linked to regulatory CD8 T lymphocytes are not specific to cells with suppressive capacity (Jiang and Chess 2004; Willing and Friese 2012; Hu et al. 2013). Almost thirty years ago, impaired CD8 T cell suppressor functions were reported in MS patients compared to healthy controls (Antel et al. 1986, 1988). More recently, CD8 T lymphocytes bearing the capacity to kill myelin specific CD4 T lymphocytes in a HLA-E restricted fashion were described (Correale and Villa 2008) especially in MS patients treated with glatiramer acetate, a synthetic copolymer of four amino acids (Tennakoon et al. 2006). Similarly to the human data, the capacity of regulatory CD8 T cells to suppress auto-reactive CD4 T cells via a mechanism involving the Qa-1 molecule, which is the mouse equivalent to the human HLA-E, has been shown in EAE (Jiang et al. 1995; Lu et al. 2008). Karantikar and colleagues described CNS antigen specific CD8 T cells able to suppress the proliferation of effector CD4 T cells; this CD8 T cell-mediated suppression was reduced in samples from MS patients obtained during acute disease exacerbation compared to healthy controls or non-active MS patients (Baughman et al. 2011). Both CD28 negative and CD122-expressing CD8 T cell subpopulations have been identified as regulatory cell subsets having the capacity to protect from EAE (Najafian et al. 2003; Lee et al. 2008; Yu et al. 2014). Overall, these studies indicate that both regulatory CD4 and CD8 T cell subsets can potentially regulate detrimental autoimmune responses observed in active MS patients or in its animal models. However, the mechanisms whereby these cells accomplish such beneficial impact need to be further investigated.
Gut Microbiota and the Susceptibility to MS and EAE
Microbiota refers to the ensemble of microorganisms that resides in a given anatomical location in the body (Bhargava and Mowry 2014). Seminal publications have documented the impact of specific gut bacterial communities on the immune system including T lymphocytes. Indeed, commensal microorganisms can promote either inflammatory (Th1 or Th17) or regulatory T cell responses (Atarashi et al. 2011, 2013; Kawamoto et al. 2014). In addition to the direct impact of the gut microbiota on bowel-related disorders, a growing body of evidence suggests that these microorganisms can modulate autoimmune disorders in remote organs such as the CNS (Berer and Krishnamoorthy 2014; Bhargava and Mowry 2014). Epidemiological, genetics and biological studies have revealed that the susceptibility to MS disease is dictated by an intricate interplay between genes and environmental factors. Recently, the research community has turned the spotlight on the gut commensal microbiota as a potential environmental risk factor for MS (Berer and Krishnamoorthy 2014; Bhargava and Mowry 2014).
Modulation of the gut microbiota in mice has been shown to influence the susceptibility to EAE. Following an oral antibiotic regimen, which dramatically reduced gastro-intestinal bacterial populations, mice were found to be significantly less susceptible to EAE induction with delayed onset and attenuated disease severity compared to controls (Ochoa-Reparaz et al. 2009; Lee et al. 2011). Similarly, an oral antibiotic treatment given to mice that generally spontaneously develop EAE, due to the transgenic expression of a TCR recognizing a myelin epitope, abolished the development of the CNS disease (Berer et al. 2011). The protection induced by eradicating the gut microbiota has been linked to mechanisms such as: increased number of FoxP3 regulatory T cells, reduced populations of Th1 (IFNγ- producing) and Th17 (IL-17-producing) cells, and impaired ability of dendritic cells to activate Th1 and Th17 responses (Ochoa-Reparaz et al. 2009; Berer et al. 2011; Lee et al. 2011). Moreover, the adoptive transfer of CD25+ CD4 T lymphocytes from antibiotic-treated mice to naïve recipients before EAE induction decreased disease severity while CD25+CD4 T cells from non-treated donors did not alter EAE susceptibility (Ochoa-Reparaz et al. 2009). The re-colonisation of germ-free mice with segmented filamentous bacteria, that have been shown to promote Th17 cell activation, was sufficient to restore their susceptibility to EAE induction (Lee et al. 2011). In contrast, oral administration of capsular polysaccharide A of bacteroides fragilis or Pediococcus acidilactici (strain R037), both known to reduce intestinal inflammation by inducing IL-10-producing CD4 T cells, reduced EAE disease severity (Ochoa-Reparaz et al. 2010; Takata et al. 2011). Overall, these reports indicate that, at least in rodent models, specific gut bacteria or bacterial products can skew T cell responses and thus influence whether or not a detrimental autoimmune response targeting the distant CNS will take place.
Few studies investigated the impact of gut microorganisms on MS pathobiology. Correale and Farez showed that MS patients infected with intestinal parasites, which alter the gut microbiota but also induce a robust Th2 response, had a significantly reduced number of relapses compared to uninfected MS patients (Correale and Farez 2007, 2011). Clostridium perfringens type B, usually associated with gastro-intestinal diseases in ruminants, was isolated from a patient with MS at her first clinical presentation (Rumah et al. 2013). Interestingly, these bacteria produce the epsilon toxin, which is neurotoxic in animals (Bokori-Brown et al. 2011). Antibodies targeting this toxin were more prevalent in sera from MS patients than in those from healthy controls (Rumah et al. 2013); whether bacteria of the Clostridium genus can modulate immune responses in MS patients is still unknown. It has been recently uncovered that diet patterns such as high salt diet can increase EAE severity (Kleinewietfeld et al. 2013). Moreover, long-term as well as short-term changes in the human diet have been shown to modify the human gut microbiota (David et al. 2014). As diets shape the gut microbiota, which can influence immune cell responses, we can speculate that some diet patterns favoring specific microbial populations could lead to dampened Th1 and/or Th17 responses while other diets could support the growth of microbial populations promoting such inflammatory responses.
The commensal gut flora has been shown to be essential for the expansion of Mucosal-associated invariant T cells (MAITs) (Treiner et al. 2003). These innate-like cells express an invariant TCRα chain (Vα7.2 Jα33 in humans) and are found in mucosal tissues but also in other organs (e.g., liver) and in peripheral blood (Treiner et al. 2003; Dusseaux et al. 2011; Le Bourhis et al. 2011; Gapin 2014). Human MAITs express high levels of CD161, CCR6 and cytokine receptors (IL-12, IL-18 and IL-23) and can produce inflammatory cytokines including IFN-γ and IL-17 (Gapin 2014). Annibali and colleagues observed an elevated proportion of CD161hi CD8 T cells in the peripheral blood of MS patients compared to controls (Annibali et al. 2011). In contrast, other groups detected significantly reduced frequency of CD161hi Vα7.2 expressing T cells (Miyazaki et al. 2011) or CD161hi Vα7.2 CD8 T cells (Willing et al. 2014) in the blood of MS patients compared to healthy controls. Willing and colleagues suggested that such inconsistencies between studies could be due to variation in donors’ age as they observed a diminished proportion of MAITs with age in healthy donors (Willing et al. 2014). Notably, the frequency of MAITs was significantly reduced in MS patients following immunosuppressive therapies (Abrahamsson et al. 2013). Moreover, CD8 T cells expressing CD161 and producing IFNγ (Annibali et al. 2011) or expressing the TCRVα7.2 chain (Abrahamsson et al. 2013; Willing et al. 2014) were detected in post-mortem CNS tissues from MS patients. Whether MAITs contribute to pathological processes observed in MS patients is still unresolved. Finally, whether the impact of the gut microbiota on this specific immune cell subset or other immune cell subsets modulates the development of MS is unknown. Further investigations are deemed essential to determine whether the gut microbiota is indeed a key factor in MS pathogenesis and could be modulated to alter disease (Berer and Krishnamoorthy 2014; Bhargava and Mowry 2014).
Crosstalk Between T Lymphocytes and Glial Cells
Glial cells, which include astrocytes, oligodendrocytes and microglia, perform highly complex and complementary functions with the ultimate goal of providing support, protection and optimal conditions for neurons. Microglia are the resident innate immune cells of the CNS and consequently provide the first line of defense against both endogenous and exogenous insults (Giunti et al. 2014). Under normal physiological conditions, resting microglia constantly scrutinize their surroundings, remove cell debris, and sense potential threats via a plethora of receptors such as toll-like receptors and scavenger receptors (Goldmann and Prinz 2013; Giunti et al. 2014; Strachan-Whaley et al. 2014). A growing body of evidence suggests that the activation of microglia is one of the earliest stages in the development of MS lesions even prior the infiltration of monocytes/macrophages and T lymphocytes (van Noort et al. 2011; Strachan-Whaley et al. 2014). Using a biomarker of activated microglia, investigators performed PET imaging on MS patients as well as on clinically isolated syndrome patients, whom had a first episode of CNS demyelination. They observed increased microglial activation not only in lesions but also in normal appearing white matter in these patients compared to healthy subjects (Politis et al. 2012; Giannetti et al. 2015). Similarly, using multiple techniques (immunohistochemistry, flow cytometry and two-photon microscopy), several groups reported that the activation of microglia precedes the infiltration of macrophages and T cells in EAE models (Ponomarev et al. 2005; Davalos et al. 2012; Giunti et al. 2014). Given their immune properties, such as expression of MHC class I and class II molecules and production of cytokines, as well as their distribution throughout the CNS, microglia are uniquely positioned to influence and modulate the responses of CNS infiltrating immune cells, especially T lymphocytes (Almolda et al. 2011; Strachan-Whaley et al. 2014). In the inflamed CNS, activated microglia and infiltrating macrophages exhibit very similar properties and functions, and are thus often undistinguishable by techniques such as immunohistochemical analysis. Other authors reviewed the numerous protective and beneficial effects of microglia in MS and its animal models (Goldmann and Prinz 2013; Giunti et al. 2014; Strachan-Whaley et al. 2014). Below, we will briefly discuss the cross-talk between T lymphocytes and microglia/infiltrating macrophages in the context of these demyelinating diseases.
Microglia and macrophages are abundantly present in white matter and cortical demyelinating lesions of MS and EAE; these cells are often detected in close proximity to T lymphocytes (Lucchinetti et al. 2011; Hucke et al. 2012; Lassmann 2014) supporting the likelihood of active interactions between these cell subsets. An important number of studies documented that both human and rodent activated microglia are immune competent cells and can efficiently present antigens to both CD4 and CD8 T lymphocytes (Aloisi et al. 1999; Carson et al. 1999; Almolda et al. 2011; Jarry et al. 2013; Strachan-Whaley et al. 2014; Wlodarczyk et al. 2014). Therefore, microglia can contribute to the initial activation of naïve T cells but also the local re-stimulation of infiltrating activated T cells. Activated microglia express elevated levels of MHC class I and class II molecules and several costimulatory molecules including CD80, CD86, CD40 and OX40 (Williams et al. 1994; Raivich and Banati 2004; Almolda et al. 2010; Goldmann and Prinz 2013; Giunti et al. 2014; Strachan-Whaley et al. 2014; Wlodarczyk et al. 2014). These molecules are engaged in the physical contact between T cells and microglia and can trigger activating signaling pathways in T cells. Moreover, specific subsets of activated microglia have been shown to secrete cytokines that favor either Th1 or Th17 cell subsets (e.g., IL-6, IL-12, IL-23, TNF) (Jack et al. 2005; Strachan-Whaley et al. 2014); although the cytokine levels produced by activated microglia can be lower than those produced by macrophages (Wlodarczyk et al. 2014). In contrast, specific in vitro culture conditions of microglia promoted the development of regulatory T lymphocytes expressing FoxP3, which had the capacity to protect from EAE upon adoptive transfer in mice (Ebner et al. 2013). Finally, microglia can acquire the same pro-inflammatory M1 phenotype (producing IL-1, IL-12, TNF) or anti-inflammatory M2 phenotype (producing IL-10 and showing enhanced myelin phagocytosis capacity) that have been originally described for macrophages (Durafourt et al. 2012; Nakagawa and Chiba 2014). Published reports suggest that M2 microglia or macrophages can favor repair and recovery in EAE models (Mikita et al. 2011; Strachan-Whaley et al. 2014). Overall, these results suggest that activated microglia can locally either establish a local inflammatory milieu sustaining the activation of inflammatory T lymphocytes (Matyszak et al. 1999; Giunti et al. 2014), or favor regulatory and anti-inflammatory T lymphocytes (Strachan-Whaley et al. 2014).
The cross-talk between microglia and T cells can also shape microglial properties (Strachan-Whaley et al. 2014). Whereas murine myelin auto-reactive Th1 cells triggered the secretion of proinflammatory molecules such as TNF, IL-1β and nitrite by microglia, myelin auto-reactive Th2 cells induced the expression of neurotrophic factors such as brain-derived neurotrophic factor and neurotrophin-3 by the same cells (Roy et al. 2007). Moreover, infiltration of IFNγ and IL-17 producing CD4 T cells during EAE concurred with elevated production of inflammatory cytokines (IL-1β, IL-6 and TNF) most likely by microglia. Furthermore, Th1 or Th17 cells caused elevated MHC class I and II expression by microglia in vitro (Murphy et al. 2010). Collectively, these results illustrate that the cross-talk between T lymphocytes and microglia can lead to both detrimental and beneficial effects and contribute to the pathobiology of MS and its animal models. Further studies are deemed essential to understand this intricate T cell-microglia dialogue, especially since several activated T cell subsets (Th1, Th2, Th17, T regs) have the capacity to migrate into the CNS.
An increasing number of publications have underlined the roles of astrocytes in modulating T cell responses especially in the context of MS and its animal models (Mayo et al. 2012).A detailed description of this literature is beyond the scope of the current review. However, we should at least mention that astrocytes can produce cytokines and chemokines, or express molecules that alter T cell responses (Mayo et al. 2012). For example, we have shown that astrocytes in MS lesions expressed elevated levels of PD-L1 and IL-15 and that these mediators can either inhibit or enhance CD8 T cell responses using in vitro assays (Saikali et al. 2010; Pittet et al. 2011). Although, there is no convincing data demonstrating that astrocytes can efficiently present antigen to T cells, these very abundant glial cells can significantly alter CNS inflammation.
Conclusions
In the last decades, the progress achieved by the scientific community to elucidate the complex immune mechanisms involved in the pathobiology of MS is remarkable. Major advances include the identification and characterization of T cell subsets (e.g., Th1, Th17, CD8, regulatory T cells), molecules (e.g., cytokines, chemokines, cell adhesion molecules), and interactions with CNS cells (e.g., BBB-EC, microglia, astrocytes), that contribute to the prototypic inflammatory disease of the CNS and potentially to some extent to other neurological diseases. Moreover, the development of additional rodent models to mirror the intricacies of the multifaceted human disease have provided and will continue to provide invaluable insights to examine the causes underlying disease heterogeneity in patients. This review presented an overview of the impressive body of evidence advocating that both CD4 and CD8 T lymphocytes participate in the development of MS and its animal models. We should highlight that an increasing number of publications have substantiated the contribution of other immune factors such as B lymphocytes and antibodies to the development of these demyelinating diseases (Krumbholz et al. 2012). In spite of the great number of milestones reached in elucidating MS immunopathobiology, the exact etiology of MS has yet to be defined. Moreover, despite an increasing number of available immunomodulatory or immunosuppressive therapies altering MS progression, there is still no cure available. Therefore, the scientific community should continue hunting for potential immune mechanisms that can be targeted to prevent but also eradicate MS.
Acknowledgments
L.L. is supported by a doctoral studentship from the Multiple Sclerosis Society of Canada. N.A. holds a New Investigator Salary Award from the Canadian Institutes of Health Research.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, Carassiti D, Reynolds R, Salvetti M, Calabresi PA, Coles AJ, Battistini L, Martin R, Burt RK, Muraro PA. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136:2888–2903. doi: 10.1093/brain/awt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Almolda B, Gonzalez B, Castellano B. Activated microglial cells acquire an immature dendritic cell phenotype and may terminate the immune response in an acute model of EAE. J Neuroimmunol. 2010;223:39–54. doi: 10.1016/j.jneuroim.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci (Landmark Ed) 2011;16:1157–1171. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Polazzi E, Minghetti L, Adorini L. CD40–CD154 interaction and IFN-gamma are required for IL-12 but not prostaglandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol. 1999;162:1384–1391. [PubMed] [Google Scholar]
- Anderton SM, Kissler S, Lamont AG, Wraith DC. Therapeutic potential of TCR antagonists is determined by their ability to modulate a diverse repertoire of autoreactive T cells. Eur J Immunol. 1999;29:1850–1857. doi: 10.1002/(SICI)1521-4141(199906)29:06<1850::AID-IMMU1850>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Annibali V, Ristori G, Angelini DF, Serafini B, Mechelli R, Cannoni S, Romano S, Paolillo A, Abderrahim H, Diamantini A, Borsellino G, Aloisi F, Battistini L, Salvetti M. CD161(high) CD8+ T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–554. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- Antel JP, Bania MB, Reder A, Cashman N. Activated suppressor cell dysfunction in progressive multiple sclerosis. J Immunol. 1986;137:137–141. [PubMed] [Google Scholar]
- Antel J, Brown M, Nicholas MK, Blain M, Noronha A, Reder A. Activated suppressor cell function in multiple sclerosis–clinical correlations. J Neuroimmunol. 1988;17:323–330. doi: 10.1016/0165-5728(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal MR, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell RC, Raman C, Steinman L. Type I interferons: beneficial in Th1 and detrimental in Th17 autoimmunity. Clin Rev Allergy Immunol. 2013;44:114–120. doi: 10.1007/s12016-011-8296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by microma-nipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan CM, Costantino CM, Cvetanovich GL, Ashley CW, Beriou G, Dominguez-Villar M, Hafler DA. CD2 costimulation reveals defective activity by human CD4+CD25(hi) regulatory cells in patients with multiple sclerosis. J Immunol. 2011;186:3317–3326. doi: 10.4049/jimmunol.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Baughman EJ, Mendoza JP, Ortega SB, Ayers CL, Greenberg BM, Frohman EM, Karandikar NJ. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J Autoimmun. 2011;36:115–124. doi: 10.1016/j.jaut.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Behrens F, Tak PP, Ostergaard M, Stoilov R, Wiland P, Huizinga TW, Berenfus VY, Vladeva S, Rech J, Rubbert-Roth A, Korkosz M, Rekalov D, Zupanets IA, Ejbjerg BJ, Geiseler J, Fresenius J, Korolkiewicz RP, Schottelius AJ, Burkhardt H. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer K, Krishnamoorthy G. Microbial view of central nervous system autoimmunity. FEBS Lett. 2014;588:4207–4213. doi: 10.1016/j.febslet.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Berthelot L, Laplaud DA, Pettre S, Ballet C, Michel L, Hillion S, Braudeau C, Connan F, Lefrere F, Wiertlewski S, Guillet JG, Brouard S, Choppin J, Soulillou JP. Blood CD8+ T cell responses against myelin determinants in multiple sclerosis and healthy individuals. Eur J Immunol. 2008;38:1889–1899. doi: 10.1002/eji.200838023. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14:492. doi: 10.1007/s11910-014-0492-2. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Sung MH, Kadom N, Simon R, McFarland H, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol. 2004;172:3893–3904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- Bin Dhuban K, d’Hennezel E, Nashi E, Bar-Or A, Rieder S, Shevach EM, Nagata S, Piccirillo CA. Coexpression of TIGIT and FCRL3 Identifies Helios+Human Memory Regulatory T Cells. J Immunol. 2015;194:3687–3696. doi: 10.4049/jimmunol.1401803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Bokori-Brown M, Savva CG, Fernandes da Costa SP, Naylor CE, Basak AK, Titball RW. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J. 2011;278:4589–4601. doi: 10.1111/j.1742-4658.2011.08140.x. [DOI] [PubMed] [Google Scholar]
- Brynedal B, Duvefelt K, Jonasdottir G, Roos IM, Akesson E, Palmgren J, Hillert J. HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS ONE. 2007;2:e664. doi: 10.1371/journal.pone.0000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by cross presenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Carrieri PB, Provitera V, De Rosa T, Tartaglia G, Gorga F, Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol. 1998;20:373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55:127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Chastain EM, Miller SD. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol Rev. 2012;245:227–238. doi: 10.1111/j.1600-065X.2011.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Chen G. Chemokines and chemokine receptors in multiple sclerosis. Mediators Inflamm. 2014;2014:659206. doi: 10.1155/2014/659206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini M, Serana F, Zanotti C, Capra R, Rasia S, Rottoli M, Rovaris M, Caputo D, Cavaletti G, Frigo M, Frigeni B, Clerici R, Rezzonico M, Caimi L, Imberti L. Modulation of the central memory and Tr1-like regulatory T cells in multiple sclerosis patients responsive to interferon-beta therapy. Mult Scler. 2012;18:788–798. doi: 10.1177/1352458511427720. [DOI] [PubMed] [Google Scholar]
- Chou YK, Bourdette DN, Offner H, Whitham R, Wang RY, Hashim GA, Vandenbark AA. Frequency of T cells specific for myelin basic protein and myelin proteolipid protein in blood and cerebrospinal fluid in multiple sclerosis. J Neuroimmunol. 1992;38:105–113. doi: 10.1016/0165-5728(92)90095-3. [DOI] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Codarri L, Greter M, Becher B. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol. 2013;34:114–119. doi: 10.1016/j.it.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–134. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- Cross AH, Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med. 2014;275:350–363. doi: 10.1111/joim.12203. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andres C, Aristimuno C, de Las HV, Martinez-Gines ML, Bartolome M, Arroyo R, Navarro J, Gimenez-Roldan S, Fernandez-Cruz E, Sanchez-Ramon S. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;182:204–211. doi: 10.1016/j.jneuroim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- de Vos AF, van Meurs M, Brok HP, Boven LA, Hintzen RQ, van der Valk P, Ravid R, Rensing S, Boon L, t Hart BA, Laman JD. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol. 2002;169:5415–5423. doi: 10.4049/jimmunol.169.10.5415. [DOI] [PubMed] [Google Scholar]
- Ding Q, Lu L, Wang B, Zhou Y, Jiang Y, Zhou X, Xin L, Jiao Z, Chou KY. B7H1-Ig fusion protein activates the CD4+ IFN-gamma receptor+type 1 T regulatory subset through IFN-gamma-secreting Th1 cells. J Immunol. 2006;177:3606–3614. doi: 10.4049/jimmunol.177.6.3606. [DOI] [PubMed] [Google Scholar]
- Duan H, Xing S, Luo Y, Feng L, Gramaglia I, Zhang Y, Lu D, Zeng Q, Fan K, Feng J, Yang D, Qin Z, Couraud PO, Romero IA, Weksler B, Yan X. Targeting endothelial CD146 attenuates neuroinflammation by limiting lymphocyte extravasation to the CNS. Sci Rep. 2013;3:1687. doi: 10.1038/srep01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- Ebner F, Brandt C, Thiele P, Richter D, Schliesser U, Siffrin V, Schueler J, Stubbe T, Ellinghaus A, Meisel C, Sawitzki B, Nitsch R. Microglial activation milieu controls regulatory T cell responses. J Immunol. 2013;191:5594–5602. doi: 10.4049/jimmunol.1203331. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhofy A, Depaolo RW, Lira SA, Lukacs NW, Karpus WJ. Mice deficient for CCR6 fail to control chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:91–99. doi: 10.1016/j.jneuroim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A, Pettre S, Salou M, Bahbouhi B, Soulillou JP, Brouard S, Laplaud DA. Frequency of circulating autoreactive T cells committed to myelin determinants in relapsing-remitting multiple sclerosis patients. Clin Immunol. 2012;144:117–126. doi: 10.1016/j.clim.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33:579– 589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55:140–148. doi: 10.1034/j.1399-0039.2000.550205.x. [DOI] [PubMed] [Google Scholar]
- Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis–the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Fugger L, Friese MA, Bell JI. From genes to function: the next challenge to understanding multiple sclerosis. Nat Rev Immunol. 2009;9:408–417. doi: 10.1038/nri2554. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gapin L. Check MAIT. J Immunol. 2014;192:4475–4480. doi: 10.4049/jimmunol.1400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner D, Hoff H, Gimsa U, Burmester GR, Brunner-Weinzierl MC. CD25 regulatory T cells determine secondary but not primary remission in EAE: impact on long-term disease progression. J Neuroimmunol. 2006;172:73–84. doi: 10.1016/j.jneuroim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gaur A, Boehme SA, Chalmers D, Crowe PD, Pahuja A, Ling N, Brocke S, Steinman L, Conlon PJ. Amelioration of relapsing experimental autoimmune encephalomyelitis with altered myelin basic protein peptides involves different cellular mechanisms. J Neuroimmunol. 1997;74:149–158. doi: 10.1016/s0165-5728(96)00220-2. [DOI] [PubMed] [Google Scholar]
- Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. identification and characterization of the primary demyelinating lesion. Brain. 1997;120:1461–1483. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain CP, Zamvil SS. Specific immunotherapy: one size does not fit all. Nat Med. 2000;6:1098–1100. doi: 10.1038/80424. [DOI] [PubMed] [Google Scholar]
- Giannetti P, Politis M, Su P, Turkheimer FE, Malik O, Keihaninejad S, Wu K, Waldman A, Reynolds R, Nicholas R, Piccini P. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain. 2015;138:110–119. doi: 10.1093/brain/awu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunti D, Parodi B, Cordano C, Uccelli A, Kerlero de Rosbo N. Can we switch microglia’s phenotype to foster neuroprotection? Focus on multiple sclerosis. Immunology. 2014;141:328–339. doi: 10.1111/imm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin SJ, Montagne L, Van Zutphen M, Van Der Valk P, Van Den Elsen PJ, De Groot CJ. Upregulation of transcription factors controlling MHC expression in multiple sclerosis lesions. Glia. 2001;36:68– 77. doi: 10.1002/glia.1096. [DOI] [PubMed] [Google Scholar]
- Goldmann T, Prinz M. Role of microglia in CNS autoimmunity. Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation inmice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17 F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J Neuroimmunol. 2009;216:113–117. doi: 10.1016/j.jneuroim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Harbo HF, et al. Genes in the HLA class I region may contribute to the HLA class II-associated genetic susceptibility to multiple sclerosis. Tissue Antigens. 2004;63:237–247. doi: 10.1111/j.0001-2815.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- Harris MG, Hulseberg P, Ling C, Karman J, Clarkson BD, Harding JS, Zhang M, Sandor A, Christensen K, Nagy A, Sandor M, Fabry Z. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci Rep. 2014;4:4422. doi: 10.1038/srep04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19:578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Fleckenstein BT, Vergelli M, Jung G, McFarland H, Martin R, Wiesmuller KH. Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J Exp Med. 1997;185:1651–1659. doi: 10.1084/jem.185.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Ure D, Adelson JD, LaFrance-Corey R, Johnson A, Rodriguez M. CD8+ T cells directed against a viral peptide contribute to loss of motor function by disrupting axonal transport in a viral model of fulminant demyelination. J Neuroimmunol. 2007;188:13–21. doi: 10.1016/j.jneuroim.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Weiner HL, Ritz J. Identification of cytolytic CD161– CD56+ regulatory CD8 T cells in human peripheral blood. PLoS One. 2013;8:e59545. doi: 10.1371/journal.pone.0059545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, Guralnik A, Bollig N, Jeltsch K, Heinemann C, Wittmann E, Buch T, Prazeres da Costa O, Brustle A, Brenner D, Mak TW, Mittrucker HW, Tackenberg B, Kamradt T, Lohoff M. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucke S, Flossdorf J, Grutzke B, Dunay IR, Frenzel K, Jungverdorben J, Linnartz B, Mack M, Peitz M, Brustle O, Kurts C, Klockgether T, Neumann H, Prinz M, Wiendl H, Knolle P, Klotz L. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator-activated receptor gamma. Brain. 2012;135:1586–1605. doi: 10.1093/brain/aws058. [DOI] [PubMed] [Google Scholar]
- Huizinga R, Hintzen RQ, Assink K, van Meurs M, Amor S. T-cell responses to neurofilament light protein are part of the normal immune repertoire. Int Immunol. 2009;21:433–441. doi: 10.1093/intimm/dxp011. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific cd8(+) t cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifergan I, Kebir H, Alvarez JI, Marceau G, Bernard M, Bourbonniere L, Poirier J, Duquette P, Talbot PJ, Arbour N, Prat A. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain. 2011;134:3560–3577. doi: 10.1093/brain/awr268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320– 4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jacobsen M, Cepok S, Quak E, Happel M, Gaber R, Ziegler A, Schock S, Oertel WH, Sommer N, Hemmer B. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–550. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- Jadidi-Niaragh F, Mirshafiey A. Regulatory T-cell as orchestra leader in immunosuppression process of multiple sclerosis. Immunopharmacol Immunotoxicol. 2011;33:545–567. doi: 10.3109/08923973.2010.513391. [DOI] [PubMed] [Google Scholar]
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry U, Jeannin P, Pineau L, Donnou S, Delneste Y, Couez D. Efficiently stimulated adult microglia cross-prime naive CD8(+) T cells injected in the brain. Eur J Immunol. 2013;43:1173–1184. doi: 10.1002/eji.201243040. [DOI] [PubMed] [Google Scholar]
- Ji Q, Perchellet A, Goverman JM. Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs. Nat Immunol. 2010;11:628–634. doi: 10.1038/ni.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol. 2013;14:254–261. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ware R, Stall A, Flaherty L, Chess L, Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing V beta 8 TCR: a role of the Qa-1 molecule. Immunity. 1995;2:185–194. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]
- Jilek S, Schluep M, Rossetti AO, Guignard L, Le Goff G, Pantaleo G, Du Pasquier RA. CSF enrichment of highly differentiated CD8+ T cells in early multiple sclerosis. Clin Immunol. 2007;123:105–113. doi: 10.1016/j.clim.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jones JL, Anderson JM, Phuah CL, Fox EJ, Selmaj K, Margolin D, Lake SL, Palmer J, Thompson SJ, Wilkins A, Webber DJ, Compston DA, Coles AJ. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain. 2010;133:2232–2247. doi: 10.1093/brain/awq176. [DOI] [PubMed] [Google Scholar]
- Junker A, Ivanidze J, Malotka J, Eiglmeier I, Lassmann H, Wekerle H, Meinl E, Hohlfeld R, Dornmair K. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789–2799. doi: 10.1093/brain/awm214. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–3059. [PubMed] [Google Scholar]
- Kabat EA, Wolf A, Bezer AE, Murray JP. Studies on acute disseminated encephalomyelitis produced experimentally in rhesus monkeys. J Exp Med. 1951;93:615–633. doi: 10.1084/jem.93.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, Hattori M, Fagarasan S. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152–165. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390– 402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518– 522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MW, Metz LM, Agrawal SM, Yong VW. Environmental factors and their regulation of immunity in multiple sclerosis. J Neurol Sci. 2013;324:10–16. doi: 10.1016/j.jns.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Kooi EJ, van Horssen J, Witte ME, Amor S, Bo L, Dijkstra CD, van der Valk P, Geurts JJ. Abundant extracellular myelin in the meninges of patients with multiple sclerosis. Neuropathol Appl Neurobiol. 2009;35:283–295. doi: 10.1111/j.1365-2990.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- Korporal M, Haas J, Balint B, Fritzsching B, Schwarz A, Moeller S, Fritz B, Suri-Payer E, Wildemann B. Interferon beta-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells. Arch Neurol. 2008;65:1434–1439. doi: 10.1001/archneur.65.11.1434. [DOI] [PubMed] [Google Scholar]
- Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Saxena A, Mars LT, Domingues HS, Mentele R, Ben-Nun A, Lassmann H, Dornmair K, Kurschus FC, Liblau RS, Wekerle H. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat Med. 2009;15:626–632. doi: 10.1038/nm.1975. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8:613–623. doi: 10.1038/nrneurol.2012.203. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Kumar N, Narang K, Cressey BD, Gottlieb AB. Long-term safety of ustekinumab for psoriasis. Expert Opin Drug Saf. 2013;12:757–765. doi: 10.1517/14740338.2013.808330. [DOI] [PubMed] [Google Scholar]
- Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8:840–856. doi: 10.1007/s11481-013-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770– 3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lecuyer MA, Ifergan I, Viel E, Bourbonniere L, Beauseigle D, Terouz S, Hachehouche L, Gendron S, Poirier J, Jobin C, Duquette P, Flanagan K, Yednock T, Arbour N, Prat A. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 2012;135:2906–2924. doi: 10.1093/brain/aws212. [DOI] [PubMed] [Google Scholar]
- Larochelle C, Lecuyer MA, Alvarez JI, Charabati M, Saint-Laurent O, Ghannam S, Kebir H, Flanagan K, Yednock T, Duquette P, Arbour N, Prat A. MCAM CD8 T lymphocytes mediate CNS inflammation. Ann Neurol. 2015 doi: 10.1002/ana.24415. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Recent neuropathological findings in MS–implications for diagnosis and therapy. J Neurol. 2004;251(Suppl 4):IV2–IV5. doi: 10.1007/s00415-004-1402-3. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Mechanisms of white matter damage in multiple sclerosis. Glia. 2014;62:1816–1830. doi: 10.1002/glia.22597. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Lee YH, Ishida Y, Rifa’i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825– 832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- Liblau R, Tournier-Lasserve E, Maciazek J, Dumas G, Siffert O, Hashim G, Bach MA. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991;21:1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Lindsey JW, Hodgkinson S, Mehta R, Mitchell D, Enzmann D, Steinman L. Repeated treatment with chimeric anti-CD4 antibody in multiple sclerosis. Ann Neurol. 1994;36:183–189. doi: 10.1002/ana.410360210. [DOI] [PubMed] [Google Scholar]
- Link H. The cytokine storm in multiple sclerosis. Mult Scler. 1998;4:12– 15. doi: 10.1177/135245859800400104. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St F, Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith N, Lai M, Miller DH, Rudge P, Thompson AJ, Cuzner ML. Effects of anti-CD4 antibody treatment on lymphocyte subsets and stimulated tumor necrosis factor alpha production: a study of 29 multiple sclerosis patients entered into a clinical trial of cM-T412. Neurology. 1997;48:810–816. doi: 10.1212/wnl.48.4.810. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunol Rev. 2012;248:156–169. doi: 10.1111/j.1600-065X.2012.01130.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin FD, Knobler RL, Kalman B, Goldhaber M, Marini J, Perrault M, D’Imperio C, Joseph J, Alkan SS, Korngold R. Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity. 1993;16:267–274. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- Lucca LE, Desbois S, Ramadan A, Ben-Nun A, Eisenstein M, Carrie N, Guery JC, Sette A, Nguyen P, Geiger TL, Mars LT, Liblau RS. Bispecificity for myelin and neuronal self-antigens is a common feature of CD4 T cells in C57BL/6mice. J Immunol. 2014;193:3267– 3277. doi: 10.4049/jimmunol.1400523. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterotti A, Martin R. Antigen-specific tolerization approaches in multiple sclerosis. Expert Opin Investig Drugs. 2014;23:9–20. doi: 10.1517/13543784.2014.844788. [DOI] [PubMed] [Google Scholar]
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- Mars LT, Saikali P, Liblau RS, Arbour N. Contribution of CD8 T lymphocytes to the immuno-pathogenesis of multiple sclerosis and its animal models. Biochim Biophys Acta. 2011;1812:151–161. doi: 10.1016/j.bbadis.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, Inoges S, Lopez-Diaz de Cerio A, Palacios R, Sepulcre J, Moreno B, Gonzalez Z, Fernandez-Diez B, Melero I, Bendandi M, Villoslada P. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–586. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179:845–853. doi: 10.4049/jimmunol.179.2.845. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P. Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. Eur J Immunol. 1999;29:3063–3076. doi: 10.1002/(SICI)1521-4141(199910)29:10<3063::AID-IMMU3063>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mayo L, Quintana FJ, Weiner HL. The innate immune system in demyelinating disease. Immunol Rev. 2012;248:170–187. doi: 10.1111/j.1600-065X.2012.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellergard J, Edstrom M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler. 2010;16:208–217. doi: 10.1177/1352458509355068. [DOI] [PubMed] [Google Scholar]
- Michel L, Berthelot L, Pettre S, Wiertlewski S, Lefrere F, Braudeau C, Brouard S, Soulillou JP, Laplaud DA. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Mittrucker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8(+) T cells. Arch Immunol Ther Exp (Warsz) 2014;62:449–458. doi: 10.1007/s00005-014-0293-y. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23:529–535. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- Mohammad MG, Tsai VW, Ruitenberg MJ, Hassanpour M, Li H, Hart PH, Breit SN, Sawchenko PE, Brown DA. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124:1228–1241. doi: 10.1172/JCI71544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteyne P, Van Antwerpen MP, Sindic CJ. Expression of costimulatory molecules and cytokines in CSF and peripheral blood mononuclear cells from multiple sclerosis patients. Acta Neurol Belg. 1999;99:11–20. [PubMed] [Google Scholar]
- Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci. 2014;34:8175–8185. doi: 10.1523/JNEUROSCI.1137-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multiple Sclerosis International Federation. Atlas of MS 2013. 2013 http://www.msif.org/includes/documents/cm_docs/2013/m/msif-atlas-of-ms-2013-report.pdf?f=1 Edition (Federation MSI, ed). http://www.msif.org/includes/documents/cm_docs/2013/m/msif-atlas-of-ms-2013-report.pdf?f=1.
- Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viralmodel of multiple sclerosis. J Virol. 1998;72:7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, Clarkson MR, Sayegh MH, Khoury SJ. Regulatory functions of CD8+ CD28-T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals. 2014;7:1028–1048. doi: 10.3390/ph7121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Croxford JL, Calenoff MA, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. J Clin Invest. 2001;108:311–318. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987a;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987b;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev. 2012;248:205–215. doi: 10.1111/j.1600-065X.2012.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ER, Stromnes IM, Goverman JM. B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J Immunol. 2014;192:929–939. doi: 10.4049/jimmunol.1302171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet CL, Newcombe J, Antel JP, Arbour N. The majority of infiltrating CD8 T lymphocytes in multiple sclerosis lesions is insensitive to enhanced PD-L1 levels on CNS cells. Glia. 2011;59:841–856. doi: 10.1002/glia.21158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M, Giannetti P, Su P, Turkheimer F, Keihaninejad S, Wu K, Waldman A, Malik O, Matthews PM, Reynolds R, Nicholas R, Piccini P. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology. 2012;79:523–530. doi: 10.1212/WNL.0b013e3182635645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW Investigators A. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Estes ML. Astrocyte expression of major histocompatibility complex gene products in multiple sclerosis brain tissue obtained by stereotactic biopsy. Arch Neurol. 1991;48:1244–1246. doi: 10.1001/archneur.1991.00530240048017. [DOI] [PubMed] [Google Scholar]
- Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2014 doi: 10.1016/j.cyto.2014.09.011. S1043–4666(14):00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep MH, van Oosten BW, Roos MT, Ader HJ, Polman CH, van Lier RA. Treatment with depleting CD4 monoclonal antibody results in a preferential loss of circulating naive T cells but does not affect IFN-gamma secreting TH1 cells in humans. J Clin Invest. 1997;99:2225– 2231. doi: 10.1172/JCI119396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers TM, Sprunt DH, Berry GP. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J Exp Med. 1933;58:39–53. doi: 10.1084/jem.58.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Liu X, Pahan K. Myelin basic protein-primed T cells induce neurotrophins in glial cells via alphavbeta3 [corrected] integrin. J Biol Chem. 2007;282:32222–32232. doi: 10.1074/jbc.M702899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio JP, Bahlo M, Stankovich J, Burfoot RK, Johnson LJ, Huxtable S, Butzkueven H, Lin L, Taylor BV, Speed TP, Kilpatrick TJ, Mignot E, Foote SJ. Analysis of extended HLA haplotypes in multiple sclerosis and narcolepsy families confirms a predisposing effect for the class I region in Tasmanian MS patients. Immunogenetics. 2007;59:177–186. doi: 10.1007/s00251-006-0183-5. [DOI] [PubMed] [Google Scholar]
- Rumah KR, Linden J, Fischetti VA, Vartanian T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One. 2013;8:e76359. doi: 10.1371/journal.pone.0076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, Cayrol R, Prat A, Hall JA, Arbour N. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci. 2007;27:1220–1228. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185:5693–5703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sauer BM, Schmalstieg WF, Howe CL. Axons are injured by antigen-specific CD8(+) T cells through a MHC class I- and granzyme B-dependent mechanism. Neurobiol Dis. 2013;59:194–205. doi: 10.1016/j.nbd.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S, Franklin RJ, Ban M. Multiple sclerosis genetics. Lancet Neurol. 2014;13:700–709. doi: 10.1016/S1474-4422(14)70041-9. [DOI] [PubMed] [Google Scholar]
- Schneider-Hohendorf T, Stenner MP, Weidenfeller C, Zozulya AL, Simon OJ, Schwab N, Wiendl H. Regulatory T cells exhibit enhanced migratory characteristics, a feature impaired in patients with multiple sclerosis. Eur J Immunol. 2010;40:3581–3590. doi: 10.1002/eji.201040558. [DOI] [PubMed] [Google Scholar]
- Schneider-Hohendorf T, Rossaint J, Mohan H, Boning D, Breuer J, Kuhlmann T, Gross CC, Flanagan K, Sorokin L, Vestweber D, Zarbock A, Schwab N, Wiendl H. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211:1833– 1846. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, de St F, Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH, Ustekinumab MSI. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Thompson EJ. Correlation of interleukin-2 and soluble interleukin-2 receptor with clinical activity of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:169–174. doi: 10.1136/jnnp.56.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Itani FR, Karandikar NJ. Immune regulation of multiple sclerosis by CD8+ T cells. Immunol Res. 2014;59:254–265. doi: 10.1007/s12026-014-8529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101:2428– 2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- Stoolman JS, Duncker PC, Huber AK, Segal BM. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J Immunol. 2014;193:564–570. doi: 10.4049/jimmunol.1400825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan-Whaley M, Rivest S, Yong VW. Interactions between microglia and T cells in multiple sclerosis pathobiology. J Interferon Cytokine Res. 2014;34:615–622. doi: 10.1089/jir.2014.0019. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006a;1:1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006b;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T, Bubel S, Mascher B, Schlenke P, Kirchner H, Wandinger KP. Increased numbers of CCR5+ interferon-gamma- and tumor necrosis factor-alpha-secreting T lymphocytes in multiple sclerosis patients. Ann Neurol. 2000;47:269–273. [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Sun JB, Olsson T, Wang WZ, Xiao BG, Kostulas V, Fredrikson S, Ekre HP, Link H. Autoreactive T and B cells responding to myelin proteolipid protein inmultiple sclerosis and controls. Eur J Immunol. 1991;21:1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;25:1170– 1181. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA, Sugimoto T, Kumanogoh A, Kayama H, Takeda K, Sakoda S, Nakatsuji Y. The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PLoS One. 2011;6:e27644. doi: 10.1371/journal.pone.0027644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot PJ, Paquette JS, Ciurli C, Antel JP, Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol. 1996;39:233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–7129. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort JM, van den Elsen PJ, van Horssen J, Geurts JJ, van der Valk P, Amor S. Preactive multiple sclerosis lesions offer novel clues for neuroprotective therapeutic strategies. CNS Neurol Disord Drug Targets. 2011;10:68–81. doi: 10.2174/187152711794488566. [DOI] [PubMed] [Google Scholar]
- van Oosten BW, Lai M, Hodgkinson S, Barkhof F, Miller DH, Moseley IF, Thompson AJ, Rudge P, McDougall A, McLeod JG, Ader HJ, Polman CH. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–357. doi: 10.1212/wnl.49.2.351. [DOI] [PubMed] [Google Scholar]
- van Zwam M, Huizinga R, Heijmans N, van Meurs M, Wierenga-Wolf AF, Melief MJ, Hintzen RQ, t Hart BA, Amor S, Boven LA, Laman JD. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol. 2009a;217:543–551. doi: 10.1002/path.2476. [DOI] [PubMed] [Google Scholar]
- van Zwam M, Huizinga R, Melief MJ, Wierenga-Wolf AF, van Meurs M, Voerman JS, Biber KP, Boddeke HW, Hopken UE, Meisel C, Meisel A, Bechmann I, Hintzen RQ, t Hart BA, Amor S, Laman JD, Boven LA. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med (Berl) 2009b;87:273–286. doi: 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Neville KL, Nikcevich KM, Eagar TN, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- Vandevyver C, Mertens N, van den Elsen P, Medaer R, Raus J, Zhang J. Clonal expansion of myelin basic protein-reactive T cells in patients with multiple sclerosis: restricted T cell receptor V gene rearrangements and CDR3 sequence. Eur J Immunol. 1995;25:958–968. doi: 10.1002/eji.1830250416. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar A, Kallies A. IL-27 paves different roads to Tr1. Eur J Immunol. 2013;43:882–885. doi: 10.1002/eji.201343479. [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127 low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez AC, Varona R. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39:1671–1681. doi: 10.1002/eji.200839123. [DOI] [PubMed] [Google Scholar]
- Vollmer TL, Wynn DR, Alam MS, Valdes J. A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis. Mult Scler. 2011;17:181–191. doi: 10.1177/1352458510384496. [DOI] [PubMed] [Google Scholar]
- Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223– 3227. [PubMed] [Google Scholar]
- Williams K, Ulvestad E, Antel JP. B7/BB-1 antigen expression on adult human microglia studied in vitro and in situ. Eur J Immunol. 1994;24:3031–3037. doi: 10.1002/eji.1830241217. [DOI] [PubMed] [Google Scholar]
- Willing A, Friese MA. CD8-mediated inflammatory central nervous system disorders. Curr Opin Neurol. 2012;25:316–321. doi: 10.1097/WCO.0b013e328352ea8b. [DOI] [PubMed] [Google Scholar]
- Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, Piedavent M, Friese MA. CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL- 18 serum levels in multiple sclerosis. Eur J Immunol. 2014;44:3119–3128. doi: 10.1002/eji.201344160. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk A, Lobner M, Cedile O, Owens T. Comparison of microglia and infiltrating CD11c(+) cells as antigen presenting cells for T cell proliferation and cytokine response. J Neuroinflammation. 2014;11:57. doi: 10.1186/1742-2094-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K. Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J Neuroimmunol. 1992;39:11–21. doi: 10.1016/0165-5728(92)90170-p. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K. Expression of vascular cell adhesion molecule-1 (VCAM-1) by human brain microvessel endothelial cells in primary culture. Microvasc Res. 1995;49:325–339. doi: 10.1006/mvre.1995.1028. [DOI] [PubMed] [Google Scholar]
- Wu GF, Alvarez E. The immunopathophysiology of multiple sclerosis. Neurol Clin. 2011;29:257–278. doi: 10.1016/j.ncl.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Dandekar AA, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Johnson JM, Tuohy VK. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J Exp Med. 1996;183:1777–1788. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Bamford RN, Waldmann TA. IL-15-dependent CD8+ CD122+ T cells ameliorate experimental autoimmune encephalomyelitis by modulating IL-17 production by CD4+ T cells. Eur J Immunol. 2014;44:3330–3341. doi: 10.1002/eji.201444675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaguia F, Saikali P, Ludwin S, Newcombe J, Beauseigle D, McCrea E, Duquette P, Prat A, Antel JP, Arbour N. Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis. J Immunol. 2013;190:2510–2518. doi: 10.4049/jimmunol.1202725. [DOI] [PubMed] [Google Scholar]
- Zang YC, Li S, Rivera VM, Hong J, Robinson RR, Breitbach WT, Killian J, Zhang JZ. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J Immunol. 2004;172:5120– 5127. doi: 10.4049/jimmunol.172.8.5120. [DOI] [PubMed] [Google Scholar]
- Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]