Abstract
Multiple lines of evidence suggest that CD8 T cells contribute to the pathogenesis of multiple sclerosis (MS). However, the sources and involvement of cytokines such as IL-15 in activating these cells is still unresolved. To investigate the role of IL-15 in enhancing the activation of CD8 T cells in the context of MS, we determined cell types expressing the bioactive surface IL-15 in the peripheral blood of patients and evaluated the impact of this cytokine on CD8 T cell cytotoxicity and migration. Flow cytometric analysis showed a significantly greater proportion of B cells and monocytes from MS patients expressing IL-15 relative to controls. We established that CD40L activation of B cells from healthy donors increased their IL-15 levels, reaching those of MS patients. We also demonstrated an enhanced cytotoxic profile in CD8 T cells from MS patients upon stimulation with IL-15. Furthermore, we showed that IL-15 expressed by B cells and monocytes is sufficient and functional, enhancing granzyme B production by CD8 T cells upon coculture. Exposure of CD8 T cells to this cytokine enhanced their ability to kill glial cells as well as to migrate across an in vitro inflamed human blood–brain barrier. The elevated levels of IL-15 in patients relative to controls, the greater susceptibility of CD8 T cells from patients to IL-15, in addition to the enhanced cytotoxic responses by IL-15–exposed CD8 T cells, stresses the potential of therapeutic strategies to reduce peripheral sources of IL-15 in MS.
Multiple sclerosis (MS) is an inflammatory disease of the CNS in which oligodendrocytes and neurons are subject to recurring immunological attacks (1). Although CD4 T cells are traditionally considered the main mediators of MS pathogenesis, a role for the participation of CD8 T cells has emerged (2–4), as CD8 T cells are abundantly detected in MS lesions (5). Activated CD8 T cells can secrete proinflammatory cytokines and release lytic enzymes upon recognition of specific Ag–MHC class I complexes on target cells. Oligodendrocytes and neurons display MHC class I expression in inflammatory lesions (6), and CD8 T cells with cytolytic granules have been detected in close apposition to oligodendrocytes and demyelinated axons in MS lesions (7). Furthermore, the pathogenic relevance of CD8 T cells in several animal models of demyelination and neurodegeneration has been demonstrated (8–10).
IL-15, a member of the IL-2 family, acts on the development, activation, proliferation, and survival of CD8 T cells. This cytokine maintains the memory CD8 T cell pool, even in the absence of Ag (11). Activated human monocytes/macrophages and dendritic cells are important sources of IL-15, expressing a biologically active IL-15/IL-15Rα complex on their surfaces (12). The IL-15/IL-15Rα complex stimulates cells expressing IL-15Rβ and the common γ chain, which are responsible for signal transduction (13).
Elevated levels of IL-15 have been detected in several inflammatory diseases including rheumatoid arthritis, celiac disease, psoriasis, and inflammatory bowel disease (14–16). Increased IL-15 has been reported at the mRNA and protein levels in MS patients’ blood (17–21). However, the functional significance of these latter findings needs to be elucidated.
Our group has recently demonstrated increased levels of IL-15 in MS brain lesions and that CNS-infiltrating CD8 T cells are in close proximity to IL-15–expressing cells, which include astrocytes and microglia/macrophages (22). However, as the initial activation likely takes place in the periphery, we investigated the presence and function of the IL-15/IL-15Rα complex expressed on immune cells in peripheral blood from MS patients and healthy controls. Our study shows that not only monocytes but also B cells provide elevated IL-15 in MS patients compared with controls. In addition, we examined the effects of IL-15 on enhancing the CD8 T cell cytotoxic profile and migration across the blood–brain barrier (BBB).
Materials and Methods
Patient characteristics
Ten patients with clinically definite MS characterized by a relapse-remitting disease course were recruited from the Research Center of the University of Montreal Hospital Center-Multiple Sclerosis Clinic; their ages ranged from 29 to 60 y (mean age, 42 y). Seven of the MS patients were clinically stable. The remaining three patients were in the acute phase of disease (relapse), defined by the occurrence of a new neurologic symptom lasting at least 24 h. None of the patients had received immunosuppressive, immunomodulatory, or steroid therapy for at least 6 mo prior to blood collection. Ten healthy sex-matched volunteers were included as controls; their ages ranged from 28 to 50 y (mean age, 36 y). A written informed consent was obtained from patients and healthy donors in accordance with the local ethics committee (HD 07.002 and BH 07.001).
Cell isolation procedure
PBMCs were isolated from venous blood samples collected in EDTA-coated Vacutainer tubes (BD Biosciences, Oakville, ON, Canada) using a Ficoll density gradient (Amersham Biosciences) as described previously (23). PBMCs were either analyzed by flow cytometry or put in MACS buffer (PBS containing 2 mM EDTA and 0.5% FBS) prior to proceeding to magnetic beads cell isolation. Monocytes, B cells, and CD8 T cell subsets were positively isolated using anti-human CD14, CD19, or CD8 beads, respectively, according to the manufacturer’s instructions (Miltenyi Biotec). Cell purity of each subset assessed by flow cytometry was >95%.
Flow cytometry
Cells were stained for surface and/or intracellular molecules as previously described (23, 24), acquired on a BD LSR II flow cytometer (BD Biosciences, Mississauga, ON, Canada), and analyzed using FlowJo (Tree Star, Ashland, OR). Mouse mAb directed at human protein and unconjugated or conjugated to FITC, Alexa Fluor 700, PE, PE-Cy7, Pacific Blue, or allophycocyanin were used. Surface stainings targeted were as follows: CD3, CD4, CD8, CD14, CD19, CD20, IL-15Rβ (BD Biosciences), IL-15, or IL-15Rα (goat anti-human) (R&D Systems, Minneapolis, MN). Intracellular stainings targeted were granzyme B (Invitrogen, Burlington ON, Canada) and IFN-γ (BD Biosciences). Appropriate isotype controls were used in all steps. All stainings were compared with isotype controls, and <1.5% was found acceptable in upper left, upper right, or lower right quadrants. Change in median fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence of the isotype from that of the stain.
Quantitative real-time PCR
Total RNAwas extracted and transcribed into cDNA as described previously (25). Relative gene expression was determined using primers and TaqMan FAM-labeled MGB probes for IL-15 and IL-15Rα and ribosomal 18S (VIC-labeled probe, used as an endogenous control) (Applied Biosystems, Foster City, CA) and according to the manufacturer’s instruction and as described previously (25).
In vitro activation of B cells
B cells were cocultured with irradiated CD40L-expressing NIH-3T3 cells (1 × 106 B cells with 5 × 105 NIH-3T3) or control NIH-3T3 cells and IL-4 (10 ng/ml) for 3–4 d prior to being harvested as published previously (24). Alternatively, B cells were stimulated with LPS from Escherichia coli serotype 0127:B8 purified by ion exchange chromatography (Sigma-Aldrich, Oakville, ON, Canada) (100 pg/ml), CpG oligonucleotide type B (CpG; ODN2006 from InvivoGen) (18 μg/ml), or F(ab′)2 fragment goat anti-human Ig (Jackson ImmunoResearch Laboratories, West Grove, PA) (2.5 μg/ml) to stimulate the BCR. Conversely, monocytes were activated with LPS (100 pg/ml) and IFN-γ (100 U/ml).
In vitro coculture of B cells or monocytes with CD8 T cells
B cells were cocultured with irradiated CD40L-expressing NIH-3T3 cells (1 × 106 B cells with 5 × 105 NIH-3T3) or control NIH-3T3 cells and IL-4 (10 ng/ml) for 3–4 d prior to being harvested. Conversely, monocytes (1 × 105/well) were activated with LPS (100 pg/ml) and IFN-γ (100 U/ml) for 2 h. Washed B cells or monocytes were subsequently incubated with either blocking anti–IL-15 Ab (20 μg/ml) (R&D Systems) or the isotype control for 1 h as published previously (22). In proliferation assays, CFSE-labeled allogenic CD8 T cells (5 × 105 cells/well) were added in the presence of anti-CD3 (clone OKT3, 32.5 ng/ml) for 5 d, after which, cells were harvested, stained, and analyzed by FACS.
In vitro culture of CD8 T cells
CFSE-labeled CD8 T cells (1 × 106 cells/ml) were cultured on plate bound α-CD3 (32.5 ng/ml) in the presence or absence of recombinant human IL-15 (0.01–5 ng/ml) (R&D Systems) and after 5–6 d, cells were either harvested for migration or cytotoxic assays, or stimulated with PMA and ionomycin in the presence of brefeldin A prior to surface and intracellular staining as described previously (22).
Human brain-derived endothelial cells, isolation, culture, and migration
Human brain-derived endothelial cells (HBECs) were isolated from CNS tissue specimens of temporal lobe resections from young adults undergoing surgery for the treatment of intractable epilepsy, as described previously (26). Prior to surgery, ethical approval and informed consent were given (BH 07.001). HBECs were isolated from nonepileptic material to generate an in vitro model of the human BBB according to a published protocol (26). Migration assays were performed in a modified Boyden chamber as described previously (26). CD8 T cells that had been exposed to anti-CD3 in the presence or absence of IL-15 were harvested and added to the upper chamber and allowed to migrate for 18 h across HBECs preactivated for 24 h with IFN-γ (100 U/ml) and TNF (100 U/ml) (Biosource-Invitrogen, Carlsbad, CA). The ability of CD8 T cells to cross the HBEC monolayer was assessed by counting the absolute number of cells in triplicate wells that migrated to the lower chamber.
Cytotoxicity assay
Preactivated (anti-CD3 ± IL-15) CD8 T cells were preincubated with an inhibitor of V-ATPase, concanamycin A (CMA) (1 μg/ml) for 3 h. Subsequently, MO3.13 cells (a gift from Dr. P. Talbot, Institut National de la Recherche Scientifique-Institut Armand-Frappier, Laval, QC, Canada) (1 × 104/well) labeled with target fluorescent label 4 (TFL4) (OncoImmunin, Gaithersburg, MD) according to manufacturer’s instructions were added at an E:T ratio of 2:1. After 4 h, cells were harvested for FACS analysis and the same number of CD8 T cells was acquired for each condition and each experiment performed as duplicate cultures.
Statistics
Data were analyzed using GraphPad Prism software (San Diego, CA). Results are shown as mean and SEM and statistical analyses included Student t test.
Results
Increased proportions of B cells and monocytes carry IL-15 in MS compared with controls
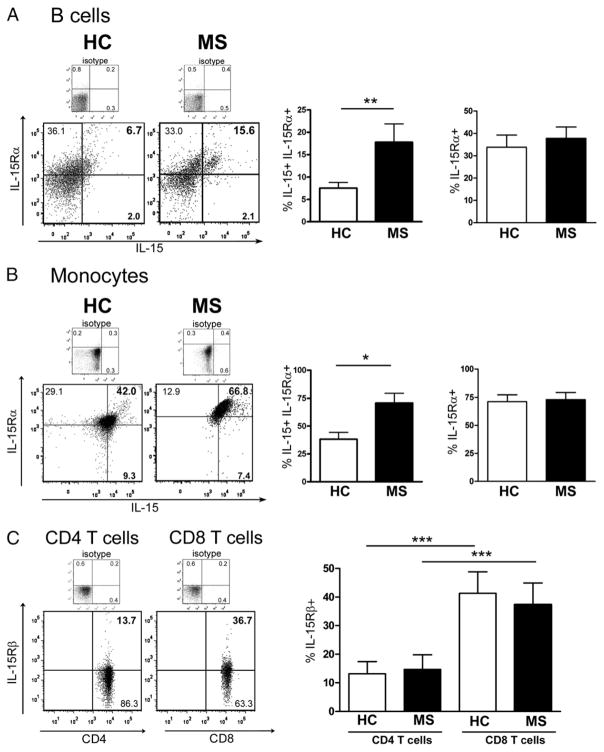
Because the surface-bound form of IL-15 is mainly responsible for cellular signaling to neighboring cells (12), we investigated surface expression of IL-15 and IL-15Rα on ex vivo PBMCs from MS patients and healthy controls by flow cytometry, using specific cell markers for B cells (CD19) and monocytes (CD14). Representative dot plots from one MS and one healthy control for IL-15 and IL-15Rα detection on either B cells or monocytes demonstrate that IL-15 was found in greater proportions on cells coexpressing IL-15Rα (Fig. 1A, 1B, left panel). Pooled data from 10 MS patients and 10 healthy controls show a significantly higher proportion of B cells (17.8 ± 4.0 versus 7.5 ± 1.2%) and monocytes (70.8 ± 8.8 versus 38.2 ± 6.2%) expressing the IL-15/IL-15Rα complex in MS patients (Fig. 1A, 1B, middle panel). These increased proportions in MS patients were observed regardless of their clinical status (relapse or stable). The ΔMFI of IL-15 on monocytes was slightly higher in MS patients as compared with controls (3676 ± 580 versus 3454 ± 312) but significantly higher on B cells from MS patients as compared with controls (326 ± 56 versus 144 ± 38; *p < 0.05). IL-15Rα expression was similar in MS patients and healthy controls on B cells (37.8 ± 5.0 versus 33.8 ± 5.4%) and monocytes (73.1 ± 6.3 versus 71.3 ± 6.0%). In addition, we assessed IL-15Rβ, the receptor chain necessary for IL-15 signaling on T cells and found increased proportions of CD8 T cells (37.4 ± 7.6%) expressing IL-15Rβ as compared with their CD4 counterparts (14.7 ± 5.1%) in both donor groups. However similar proportions of CD8 T cells express IL-15Rβ in MS and controls (37.4 ± 7.6 versus 41.4 ± 7.5%) (Fig. 1C).
FIGURE 1.
Increased proportion of B cells and monocytes carry IL-15 in MS patients compared with healthy controls. Ex vivo PBMCs of 10 MS patients and 10 healthy controls were analyzed by flow cytometry for IL-15 and IL-15Rα expression on CD19+ (B cells) and CD14+ (monocytes) cells and for IL-15Rβ expression on CD3 T cells, either CD4 or CD8. A and B, Representative flow cytometry dot plots of PBMCs from one healthy control (HC) and one MS patient gated according to isotype on either B cells (CD19; A) or monocytes (CD14; B) are illustrated for IL-15 and IL-15Rα at the cell surface. The percentage of B cells and monocytes expressing both IL-15 and IL-15Rα (A, B, middle panel) in MS patients (■) compared with HCs (□) is shown. The proportion of cells bearing IL-15Rα regardless of IL-15 presence for both groups is also illustrated (A, B, right panel). C, Representative flow cytometry dot plots of PBMCs from one MS patient gated according to isotype on CD4 or CD8 T cells for IL-15Rβ at the cell surface. The frequency of IL-15Rβ expression on CD8 T cells and CD4 T cells for both groups (MS and HC) is shown. Mean ± SEM (n = 10); *p < 0.05, **p < 0.01, ***p < 0.001 with Student t test.
CD40L-activated B cells are a source of IL-15
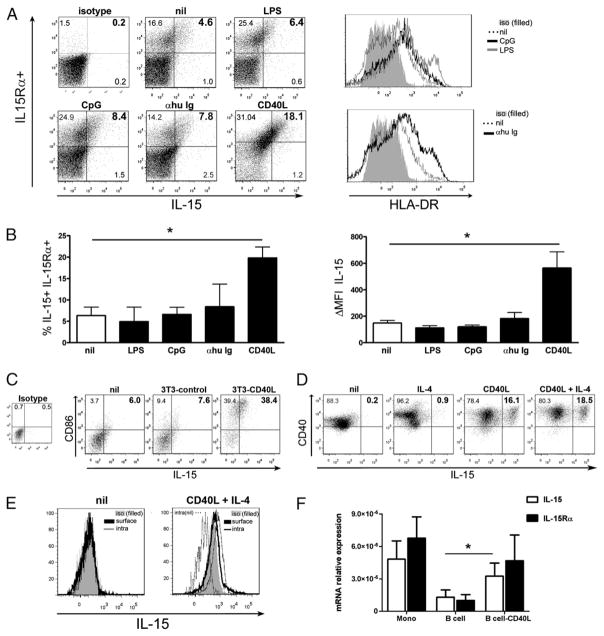
To provide further evidence that IL-15 can indeed be produced and upregulated by human B cells, we performed flow cytometry and quantitative real-time PCR (qPCR) for IL-15 and IL-15Rα on untreated and activated B cells from healthy donors (Fig. 2). Inflammatory mediators enhanced in MS patients are most likely responsible for the elevated IL-15 levels we observed in these patients compared with controls. We evaluated whether inflammatory stimuli such as LPS, known to enhance IL-15 surface expression on other cell types (13), or CpG, which stimulates B cells (27), could induce IL-15 expression by human B cells. We also determined whether B cell activation via the BCR or CD40L could trigger such expression. We observed a significant upregulation of both IL-15 and IL-15Rα in CD40L-activated B cells obtained from healthy donors at the surface protein level as assessed by flow cytometry for the percentage of cells as well as intensity of signal (Fig. 2A, 2B). However, although BCR and CpG activation efficiently boosted HLA-DR expression by human B cells (Fig. 2A, right panel), they did not lead to a significant upregulation of IL-15 (Fig. 2B, n = 4–8 tested donors). LPS did upregulate HLA-DR in some healthy donors but did not increase IL-15 (Fig. 2A). Among the potential B cell stimuli, CD40L is an important activator that has been implicated in deleterious autoimmune responses including MS (28–30). Thus, we conclude that IL-15 and its receptor cannot only be produced by human B cells but can also be upregulated in an inflammatory milieu, especially in the presence of CD40L. We confirmed the specificity of the CD40L-mediated activation because control 3T3 cells did not induce such an upregulation of IL-15 or of CD86 on human B cells (Fig. 2C). Moreover, we observed that although IL-4 alone did not affect IL-15 surface expression by B cells, it did modestly increase the impact of CD40L (Fig. 2D). IL-4 also promoted a better survival of human B cells in vitro as fewer cells were positive for a live/dead staining. To substantiate that human B cells can indeed produce IL-15, we compared surface and intracellular expression of IL-15 by these cells when brefeldin A was added for the last 16 h of incubation. Untreated cells had no detectable or very low levels of IL-15 (MFI: isotype, 86; surface stain, 84; intracellular stain, 95) (Fig. 2E), whereas the CD40L + IL-4 combination led to an increase in both compartments (one typical example in Fig. 2E; MFI: isotype, 293; surface stain, 421, intracellular stain, 623). IL-15 mRNA levels in ex vivo B cells were lower than in monocytes from the same donors; however, the CD40L activation led to a significant increased of these levels (Fig. 2F); no IL-15 was detected by qPCR using RNA isolated from 3T3-CD40L cells. In contrast, we could not detect any secreted IL-15 from B cells either untreated or CD40L-activated (n = 4 donors) using a sensitive ELISA (detection limit 4 pg/ml) (data not shown). Finally, when we added soluble rIL-15 to human B cells, we could not detect surface binding of the cytokine after 5 h of incubation at 37°C. Overall, our results support the notion that B cells synthesized IL-15, which was then translocated and expressed on the cell surface similarly seen in other cell types.
FIGURE 2.
CD40L activation increases IL-15 expression by human B cells. Purified human B cells were cultured for 3 d in the absence (nil) or presence of LPS, CpG, anti-human Ig (αhu Ig), or CD40L-expressing cells prior to staining for IL-15 and IL-15Rα. A, Representative dot plots (left panels) for IL-15 and IL-15Rα detection and representative histograms (right panels) for HLA-DR are depicted for different culture conditions. B, Pooled data of the frequency of IL-15 and IL-15Rα coexpression and IL-15 ΔMFI on B cells following different treatments are depicted; mean ± SEM (n = 4–8); *p < 0.05 with paired Student t test. C, B cells cultured for 3 d in the absence (nil) or presence of 3T3 control cells or CD40L-expressing 3T3 cells were stained for IL-15 and CD86. 3T3 control cells did not upregulate IL-15 or CD86 on human B cells. D, B cells cultured for 3 d in the absence (nil) or presence of IL-4, CD40L-expressing 3T3 cells, or both were stained for IL-15 and CD40. IL-4 alone did not affect IL-15 surface expression by B cells but did modestly increase the impact of CD40L. E, B cells cultured for 3 d in the absence (nil) or presence of CD40L-expressing 3T3 cells and IL-4 were treated with brefeldin A for 16 h and then stained for either surface or intracellular IL-15. F, qPCR analysis for IL-15 and IL-15Rα mRNA levels in CD40L-treated B cells and in ex vivo monocytes and B cells presented as relative expression compared with 18S internal control. Mean ± SEM (n = 3); *p < 0.05 with paired Student t test.
CD40L activation moderately increases IL-15 on monocytes
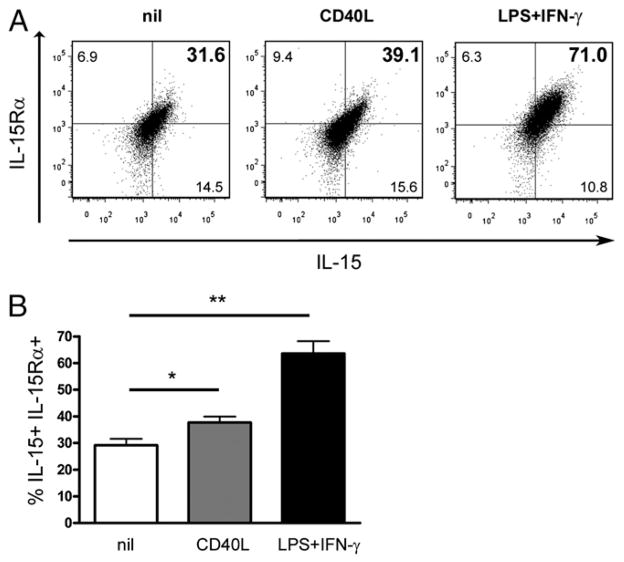
IFN-γ, which is highly elevated both in the peripheral blood and CNS of MS patients (1), has been shown to upregulate IL-15 surface expression on human monocytes (31). To investigate whether IL-15 can be upregulated by monocytes after CD40L activation similarly to B cells, we performed flow cytometry for IL-15 and IL-15Rα on untreated and activated monocytes (Fig. 3). We observed a significant upregulation of IL-15 and IL-15Rα on monocytes with either IFN-γ/LPS or CD40L activation. However, CD40L activation increased IL-15 and IL-15Rα on these cells to a lesser degree; therefore, although CD40L activation does upregulate IL-15 and its receptor on monocytes, stimulation with IFN-γ/LPS results in expression levels of IL-15 and its receptor similar to that of ex vivo monocytes from MS patients (Fig. 1B).
FIGURE 3.
CD40L activation moderately increases IL-15 on monocytes. Purified monocytes were cultured for 3 d in the absence or presence CD40L-expressing cells or IFN-γ and LPS prior flow cytometry analysis. A, A representative dot plot for surface IL-15 and IL-15Rα on monocytes following different treatments is depicted. B, Percentage of monocytes coexpressing IL-15 and IL-15Rα from three different healthy donors are shown; mean ± SEM (n = 3); *p < 0.05, **p < 0.01 with paired Student t test.
IL-15 provided by B cells and monocytes increases the proportion of granzyme B-producing CD8 T cells
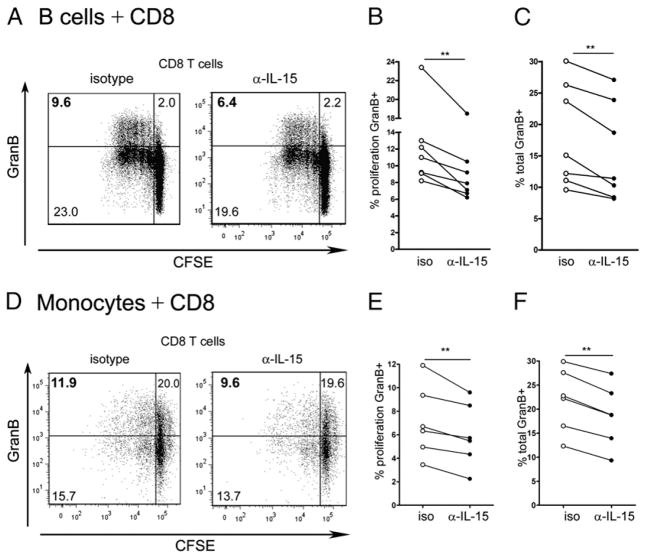
Because CD8 T cells expressing IL-15Rβ (Fig. 1) are more abundant than their CD4 counterparts, they are more likely influenced by IL-15. To determine whether the IL-15 produced by B cells and monocytes is sufficient and functional, CFSE-labeled CD8 T cells were cocultured with CD40L-activated B cells or LPS-activated monocytes obtained from healthy donors for 5 d in the presence of either an Ab against IL-15 or an isotype control. Flow cytometric data from representative CD8 T cell donors are illustrated in Fig. 4A and 4D. CD8 T cells cultured with allogeneic B cells or monocytes in the presence of the IL-15–blocking Ab displayed a significantly reduced proportion of proliferated cells expressing granzyme B as compared with cells cultured with the isotype control (Fig. 4B, 4E). Likewise, the proportion of all granzyme B-expressing CD8 T cells cultured in the presence of the IL-15–blocking Ab was significantly lower (Fig. 4C, 4F). Thus, surface IL-15 provided by both B cells and monocytes significantly elevated the expression of granzyme B by human CD8 T cells.
FIGURE 4.
IL-15 provided by B cells and monocytes increases the proportion of granzyme B-producing CD8 T cells. CFSE-labeled CD8 T cells were cocultured for 6 d with allogenic CD40L activated B cells (A–C) or allogenic monocytes pretreated with LPS and IFN-γ (D–F) in the presence of an isotype control (iso) or an anti–IL-15–blocking Ab (α-IL15). Cells were then analyzed by flow cytometry for CD8, proliferation, and granzyme B. A and D, Representative dot plots gated on CD8 T cells from one donor illustrating proliferation and detection of granzyme B after coculture with B cells (A) or monocytes (D). Percentages of CD8 T cells that proliferated and produced granzyme B (B, E) and percentages of all CD8 T cells that produce granzyme B (C, F) are shown. Each dot represents percentage of CD8 T cells from one donor. Comparison isotype versus anti–IL-15; n = 6–7; **p < 0.01 with paired Student t test.
IL-15 increases the proportion of granzyme B-producing CD8 T cells in MS
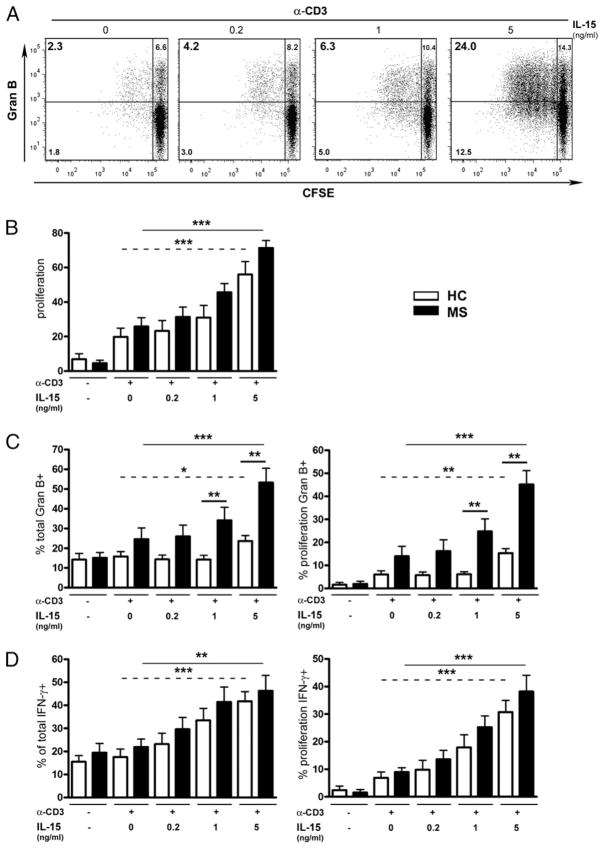
We elected to evaluate whether CD8 T cells from MS patients are more susceptible to increased IL-15 levels than their counterparts from healthy donors. CFSE-labeled CD8 T cells isolated from both donor groups were stimulated with anti-CD3 in the absence or presence of IL-15 and then assessed for proliferation and IFN-γ and granzyme B production. CD8 T cells underwent multiple divisions upon contact with anti-CD3 and the addition of IL-15 increased this proliferation in a dose-dependent manner (Fig. 5A, 5B; ***p < 0.001); CD8 T cells also expressed higher levels of IFN-γ and granzyme B in response to increasing concentrations of IL-15 (Fig. 5C, 5D; ***p < 0.001). Furthermore, not only did a significantly greater proportion of CD8 T cells from MS patients express granzyme B in response to IL-15 (1 and 5 ng/ml) as compared with healthy controls (Fig. 4C; **p < 0.01), but also the percentage of granzyme B-expressing CD8 T cells from these patients was significantly increased upon addition of IL-15 (5 ng/ml) as compared with untreated cells from the same donors. However, IL-15 had a similar impact on IFN-γ production regardless of proliferation in both donor groups (Fig. 5D). Thus, CD8 T cells from MS patients were more responsive to IL-15, specifically with regards to granzyme B production.
FIGURE 5.
IL-15 increases the proportion of granzyme B-producing CD8 T cells in MS. CFSE-labeled CD8 T cells isolated from MS or healthy controls (HC) were cultured in the presence of anti-CD3 with or without recombinant human IL-15 (doses indicated in nanograms per milliliter) for 6 d prior to being analyzed for IFN-γ and granzyme B expression. A, Representative flow cytometric plots for proliferation and granzyme B production by CD8 T cells are shown for one HC. B–D, Pooled data from 10 MS (■) and 10 HC (□) donors are shown. B, Percentages of CD8 T cells that proliferated are presented. C, Percentages of all CD8 T cells that produced granzyme B (C, left panel) and percentages of CD8 T cells that proliferated and produced granzyme B (C, right panel) are shown. D, Percentages of CD8 T cells that proliferated and produced IFN-γ (D, left panel) and percentages of all CD8 T cells that produced IFN-γ (D, right panel) are illustrated. Mean ± SEM (n = 10). Straight lines show comparison 0 versus 5 ng/ml for MS patients, and dotted lines show comparison 0 versus 5 ng/ml for HC; heavy lines illustrate comparison for the same dose of IL-15 MS versus HC. *p < 0.05, **p < 0.01, ***p < 0.001 with paired Student t test.
IL-15 increases the capacity of CD8 T cells to cross the BBB
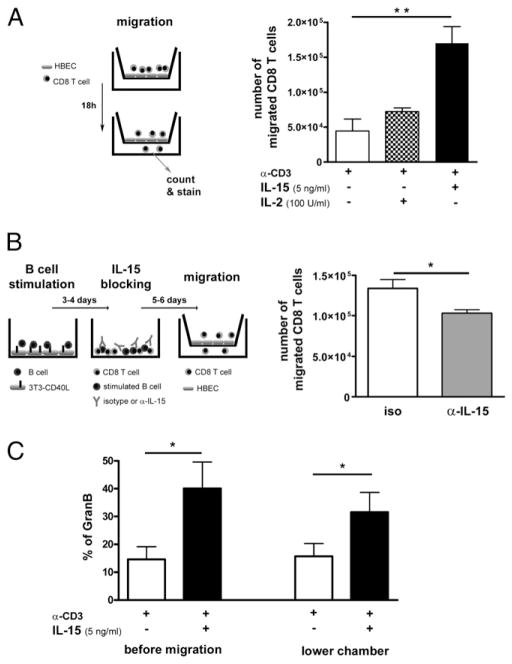
Immune cell infiltration via the BBB is considered one of the earliest immunological events leading to lesion formation in MS (32, 33). We therefore sought to determine whether IL-15 can enhance the migration of cytotoxic CD8 T cells into the CNS using an established in vitro model of the BBB (26, 34). Migration assays were performed in Boyden chambers coated with a monolayer of HBECs. To mimic the inflammation in the CNS of MS patients, HBECs were preactivated with IFN-γ and TNF. CD8 T cells that had been exposed to anti-CD3, in the presence or absence of IL-15 or IL-2, were added to the upper chamber and allowed to migrate. Significantly more CD8 T cells pretreated with IL-15 migrated through the monolayer compared with their nontreated counterparts (n = 4; **p < 0.01); pretreatment with IL-2 only moderately increased the number of migrated cells (Fig. 6A). To determine whether IL-15 provided by B cells can have a similar impact on CD8 T cell migration, CD8 T cells were cocultured with CD40L-activated B cells for 5–6 d in the presence of either an Ab against IL-15 or an isotype control before the migration assay was performed. CD8 T cells cultured with B cells in the presence of the IL-15–blocking Ab displayed a significantly reduced capacity to migrate across the HBEC monolayer compared with cells cultured with the isotype control (*p < 0.05) (Fig. 6B). Moreover, the migrated CD8 T cells maintained their cytotoxic profile (granzyme B content) as assessed by flow cytometry (Fig. 6C). To assess potential disruption of the BBB caused by activated CD8 T cells during the migration, we evaluated the permeability of the HBEC monolayer using BSA-FITC as a tracer as described previously (32). We did not observe any difference in BSA–FITC diffusion during or after the migration of CD8 T cells treated with anti-CD3 alone as compared with the presence of IL-15 or IL-2 (data not shown). Our data support the notion that the increased number of migrated CD8 T cells was not due to a non-specific disruption of the in vitro BBB. Therefore, peripheral exposure to IL-15 increases the number of CD8 T cells migrating across an in vitro BBB.
FIGURE 6.
IL-15 increases the capacity of CD8 T cells to cross the BBB. A, Activated (anti-CD3 [α-CD3]) CD8 T cells in the presence or absence of IL-15 or IL-2 were allowed to migrate for 18 h across the confluent monolayers of HBECs pretreated with IFN-γ and TNF in Boyden chamber migration assays. CD8 T cells were then harvested, counted, and stained. B, CD8 T cells were cocultured with CD40L-activated B cells for 5–6 d in the presence of either an Ab against IL-15 or an isotype control before the migration assay was performed. CD8 T cells were then harvested and counted. C, Granzyme B was assessed for activated (α-CD3) CD8 T cells in the presence or absence of IL-15 following migration. There were two to five CD8 T cell donors tested for each condition in triplicates. Mean ± SEM; * p < 0.05, ** p < 0.01 with paired Student t test.
IL-15 enhances CD8 T cell-mediated cytotoxicity
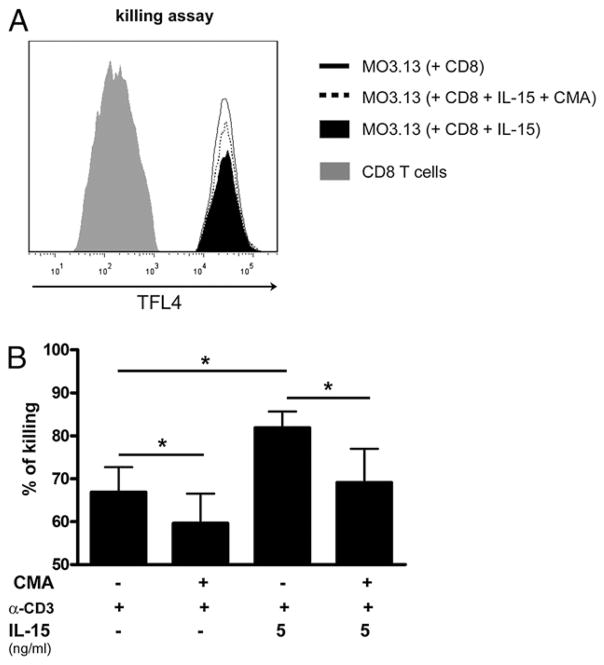
It has been shown that recombinant granzyme B can cause the death of human neurons (35). Furthermore, activated human CD8 T cells can kill oligodendrocytes and neurons (36). Thus, to investigate whether IL-15 amplifies the capacity of CD8 T cells to kill target cells via granzyme B release, we performed an in vitro assay using a human oligodendroglial cell line MO3.13 (37) as target cells. CD8 T cells were cultured in the presence of anti-CD3 with or without IL-15 (5 ng/ml) and then collected, washed, and used as effector cells. CD8 T cells were preincubated with CMA to inhibit the lytic granule-based cytolytic activity (38). Target cells were incubated with effector cells at an E:T ratio of 2:1, and after 4-h incubation, the number of CD8 T cells and target cells was analyzed by flow cytometry (Fig. 7A). CD8 T cells that had been exposed to IL-15 (5 ng/ml) were significantly more efficient at killing oligodendroglial cells than those cultured with anti-CD3 alone (pooled data n = 5 donors; *p < 0.05; Fig. 7B). Addition of CMA significantly reduced the killing of oligodendroglial cells (Fig. 7B), indicating IL-15 enhances killing via granzyme B. Thus, our results suggest that CD8 T cells pre-exposed to IL-15 demonstrate an increased ability to kill target cells such as oligodendrocytes (Fig. 8).
FIGURE 7.
IL-15 augments CD8 T cell-mediated cytotoxicity. CD8 T cells after a 5-d culture with anti-CD3 (α-CD3) in the absence or presence of IL-15 were treated with CMA or left untreated and were afterward added to TFL4-labeled MO3.13 target cells. A, Histogram illustrating detection of CD8 T cells (left gray peak) and target cell populations (black area, fine line and dotted line). Killing was assessed as the disappearance of TFL4-labeled target cells upon coculture with a constant number of CD8 T cells. The filled black area represents target cells exposed to CD8 T cells previously cultured with anti-CD3 and IL-15 (5 ng/ml), and the straight line represents target cells exposed to CD8 T cells previously cultured with anti-CD3 only. The dotted line represents target cells exposed to CD8 T cells previously cultured with anti-CD3 and IL-15 (5 ng/ml) but treated with the granzyme B inhibitor CMA. B, Pooled data of target cell killing mediated by CD8 T cells (n = 5 donors). E:T ratio of 2:1. Mean ± SEM; *p < 0.05 with paired Student t test.
FIGURE 8.
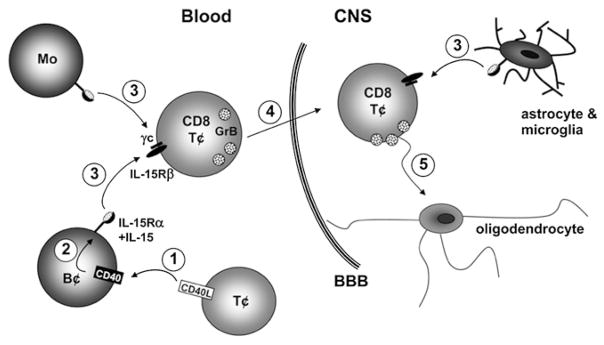
Proposed impact of elevated IL-15 on CD8 T cells in MS. 1) Increased proportions of T cells provide CD40L (30) to B cells in the peripheral blood of MS patients. 2) CD40–CD40L activation leads to an upregulation of IL-15 on B cell surface. 3) Elevated numbers of peripheral blood B cells and monocytes present the IL-15/IL-15Rα complex to IL-15Rβ–bearing CD8 T cells. In addition, IL-15 can be provided by astrocytes and microglia within the inflamed CNS (22). 4) CD8 T cells exposed to IL-15 display increased levels of granzyme B and migrate into the CNS in greater numbers. 5) Finally, CD8 T cells exposed to IL-15 display an increased capacity to kill target cells such as oligodendrocytes.
Discussion
Our study demonstrates that multiple immune cells in MS patients can modulate CD8 T cell responses by providing IL-15 (Fig. 8). We observed a significantly greater proportion of B cells and monocytes expressing the IL-15/IL-15Rα complex in MS patients as compared with controls. Both B cells and monocytes can supply functional IL-15 to CD8 T cells, enhancing granzyme B production (Fig. 8). Furthermore, CD8 T cells from MS patients are more responsive to IL-15, a significantly greater proportion producing granzyme B in response to this cytokine as compared with controls. Last, not only does exposure to IL-15 enhance the cytotoxic capacity of CD8 T cells via granzyme B production but it also promotes their migration across the BBB (Fig. 8).
We observed elevated levels of B cells and monocytes expressing the surface IL-15/IL-15Rα complex, the biologically active form, in MS patients. Another group previously reported increased levels of surface IL-15 on MS patients’ monocytes as compared with controls (20) without assessing IL-15Rα. Murine B cells (39, 40) and human B cell lines (40, 41) have been shown to produce IL-15. To our knowledge, we are the first to describe elevated levels of B cells expressing IL-15 and IL-15Rα in MS patients’ blood (Fig. 1A) and human primary B cells as a source of IL-15. To provide further evidence concerning the upregulation and a functional effect of the IL-15 expressed by B cells, we designed in vitro experiments to mimic the characteristic inflammation of MS. The increased IL-15 levels on B cells we observed upon CD40L-mediated activation (Fig. 2) could be provided by elevated numbers of T cells expressing CD40L found in MS patients (30). Therefore, the higher levels of surface IL-15 on B cells from MS patients could be due to enhanced B cell production, such as following CD40L-mediated activation, and/or due to the acquisition of this cytokine from other still unknown sources. Stimulation with TLR ligands (CpG, LPS) or BCR activation did not lead to a significant upregulation of IL-15 or its receptor on B cells (Fig. 2). Human B cell expression of TLR4, the TRL ligand triggered by LPS, has been reported to be very low but upregulated upon activation or inflammation (42–44). We observed a modest LPS impact, observing upregulated HLA-DR expression without IL-15 increased, on B cells from a subset of healthy donors, which might be due to different TLR4 expression levels. Thus, we conclude that human B cells are a potential source of IL-15, especially in the context of enhanced CD40L stimulation, as observed in MS patients. Furthermore, the IL-15 presented by activated B cells is sufficient and functional in that it induces granzyme B in CD8 T cells in a similar fashion to monocytes (Fig. 4D–F). B cell depletion with a CD20 mAb has been shown to be effective in reducing new gadolinium-enhancing lesions and the occurrence of relapses in MS patients (45). We reported a rapid and almost complete depletion of CD19 B cells in the peripheral blood of treated patients. Interestingly, Ab-producing plasma cells are not directly targeted by this treatment, and a previous study reported only modest reduction of Abs against myelin peptides in some treated MS patients (46). The long-term effects of B cell depletion on Ab levels are still unknown. It is possible that the abolishment of B cells in the peripheral blood hampers the activation of T cells by proinflammatory cytokines and costimulatory molecules (e.g., CD86) provided by B cells (47); we demonstrate in this study that IL-15 could be such a cytokine. Hence, B cell depletion could result in a decrease of functional IL-15 presented to T cells, consequently leading to an attenuation of the cytotoxic T cell response in MS.
Our in vitro results demonstrate that IL-15 has the capacity to enhance CD8 T cell effector functions in the context of MS. Using rIL-15, we studied the direct impact of this cytokine on CD8 T cells from MS patients. As the addition of soluble human IL-15Rα to recombinant human IL-15 neither enhanced nor inhibited the CD8 T cell response in a previous study (48), we did not add soluble IL-15Rα to our assays. In both donor groups, there is an increase in the proportion of granzyme B-producing CD8 T cells when activated in the presence of IL-15 (Fig. 5), but CD8 T cells from MS patients show a significantly higher production of granzyme B as compared with either untreated cells from the same patients or healthy controls. These results are in agreement with a previous study showing IL-15 to be more important for the upregulation of granzyme B than of IFN-γ in CD8 T cells (49). We observed similar proportions of T cells from MS patients and healthy controls expressing IL-15Rβ, the receptor chain recognized to be required for IL-15 signaling (13). Thus, the T cells’ enhanced susceptibility to the boosting effects of IL-15 observed in MS patients is likely due to other factors such as downstream signaling mediators. Another group reported increased expression of the IL-15R on CD4 T cells of MS patients compared with healthy controls (20); however, CD8 T cells were not assayed, and it was not specified which IL-15R chain was examined.
Furthermore, we have shown that exposure of activated CD8 T cells to IL-15 favors their migration across inflamed HBECs (Fig. 6), demonstrating a possible mechanism as to how these effectors reach their target cells in the CNS. The IL-15–mediated enhanced T cell migration is most likely due to a combination of effects induced by this cytokine on human T cells such as the increased expression of chemokines and their receptors (50) and binding capacity of LFA-1 (51) as well as the acquisition of motile cell morphology (52). Once in the CNS, CD8 T cells could be re-exposed to IL-15 in MS lesions as we have demonstrated (22). IL-15 has been well characterized for its crucial role in the generation of efficient cytotoxic CD8 T cells (53, 54). CD8 T cells have been described to directly kill CNS cell subsets including oligodendrocytes and neurons in vitro (36, 55) most likely through granzyme B production (56–58), even in the absence of perforin (35). In addition, the delivery of granzyme B to target cells induces apoptosis (59). We have shown that IL-15–increased CD8 T cell cytotoxicity toward oligodendroglial cells in vitro is at least partly dependent on granzyme B, using an inhibitor of V-ATPase (CMA).
In conclusion, elevated IL-15 levels provided by B cells and monocytes in the peripheral blood of MS patients enhance the cytotoxic profile of CD8 T cells. As elevated levels of IL-15 have been detected in several inflammatory diseases (14–16), therapeutic strategies targeting this cytokine are in development; indeed, a humanized anti-human IL-15 Ab was well tolerated in rheumatoid arthritis patients (60), and other clinical trials in inflammatory diseases are ongoing. Thus, therapeutic strategies to reduce IL-15 in the peripheral blood of MS patients could be similarly beneficial through decreasing the CD8 T cell access to the inflamed CNS and the subsequent CD8 T cell-mediated damage observed in these patients.
Acknowledgments
This work was supported by a grant from the Multiple Sclerosis Society of Canada (to N.A.). R.S. received a postdoctoral fellowship from the German Academic Exchange Service (Deutscher Akademischer Austausch Dienst Grant D0743793), the Canadian Institute of Health Research Neuroinflammation Training Program, and the Multiple Sclerosis Society of Canada. A.N.M. received funding from the Canadian Institute of Health Research Neuroinflammation Training Program and holds a studentship from the Multiple Sclerosis Society of Canada. A.P. and N.A. hold Donald Paty Career Development Awards from the Multiple Sclerosis Society of Canada and are Research Scholars from the Fonds de la Recherche en Santé du Québec.
We thank Josée Poirier for drawing blood from MS patients.
Abbreviations used in this article
- BBB
blood–brain barrier
- CMA
concanamycin A
- HBEC
human brain-derived endothelial cell
- MFI
median fluorescence intensity
- MS
multiple sclerosis
- qPCR
quantitative real-time PCR
- TFL4
target fluorescent label 4
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Mars LT, Saikali P, Liblau RS, Arbour N. Contribution of CD8 T lymphocytes to the immuno-pathogenesis of multiple sclerosis and its animal models. Biochim Biophys Acta. 2011;1812:151–161. doi: 10.1016/j.bbadis.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friese MA, Fugger L. Pathogenic CD8+ T cells in multiple sclerosis. Ann Neurol. 2009;66:132–141. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 4.Junker A, Ivanidze J, Malotka J, Eiglmeier I, Lassmann H, Wekerle H, Meinl E, Hohlfeld R, Dornmair K. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789–2799. doi: 10.1093/brain/awm214. [DOI] [PubMed] [Google Scholar]
- 5.Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schröder R, Deckert M, Schmidt S, et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann H, I, Medana M, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- 8.Brisebois M, Zehntner SP, Estrada J, Owens T, Fournier S. A pathogenic role for CD8+ T cells in a spontaneous model of demyelinating disease. J Immunol. 2006;177:2403–2411. doi: 10.4049/jimmunol.177.4.2403. [DOI] [PubMed] [Google Scholar]
- 9.Ip CW, Kroner A, Bendszus M, Leder C, Kobsar I, Fischer S, Wiendl H, Nave KA, Martini R. Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. J Neurosci. 2006;26:8206–8216. doi: 10.1523/JNEUROSCI.1921-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, Waisman A, Lassmann H, Liblau RS, Mars LT. Cutting edge: multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181:1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- 11.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 12.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 14.McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 15.Mention JJ, Ben Ahmed M, Bègue B, Barbe U, Verkarre V, Asnafi V, Colombel JF, Cugnenc PH, Ruemmele FM, McIntyre E, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Kusugami K, Nishimura H, Ando T, Yamaguchi T, Ohsuga M, Ina K, Enomoto A, Kimura Y, Yoshikai Y. Interleukin 15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology. 1998;114:1237–1243. doi: 10.1016/s0016-5085(98)70430-5. [DOI] [PubMed] [Google Scholar]
- 17.Rentzos M, Cambouri C, Rombos A, Nikolaou C, Anagnostouli M, Tsoutsou A, Dimitrakopoulos A, Triantafyllou N, Vassilopoulos D. IL-15 is elevated in serum and cerebrospinal fluid of patients with multiple sclerosis. J Neurol Sci. 2006;241:25–29. doi: 10.1016/j.jns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Blanco-Jerez C, Plaza JF, Masjuan J, Orensanz LM, Alvarez-Cermeño JC. Increased levels of IL-15 mRNA in relapsing—remitting multiple sclerosis attacks. J Neuroimmunol. 2002;128:90–94. doi: 10.1016/s0165-5728(02)00146-7. [DOI] [PubMed] [Google Scholar]
- 19.Kivisäkk P, Matusevicius D, He B, Söderström M, Fredrikson S, Link H. IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS) Clin Exp Immunol. 1998;111:193–197. doi: 10.1046/j.1365-2249.1998.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaknin-Dembinsky A, Brass SD, Gandhi R, Weiner HL. Membrane bound IL-15 is increased on CD14 monocytes in early stages of MS. J Neuroimmunol. 2008;195:135–139. doi: 10.1016/j.jneuroim.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pashenkov M, Mustafa M, Kivisäkk P, Link H. Levels of interleukin-15–expressing blood mononuclear cells are elevated in multiple sclerosis. Scand J Immunol. 1999;50:302–308. doi: 10.1046/j.1365-3083.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- 22.Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185:5693–5703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
- 23.Arbour N, Holz A, Sipe JC, Naniche D, Romine JS, Zyroff J, Oldstone MB. A new approach for evaluating antigen-specific T cell responses to myelin antigens during the course of multiple sclerosis. J Neuroimmunol. 2003;137:197–209. doi: 10.1016/s0165-5728(03)00080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbour N, Lapointe R, Saikali P, McCrea E, Regen T, Antel JP. A new clinically relevant approach to expand myelin specific T cells. J Immunol Methods. 2006;310:53–61. doi: 10.1016/j.jim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naïve CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 26.Prat A, Biernacki K, Pouly S, Nalbantoglu J, Couture R, Antel JP. Kinin B1 receptor expression and function on human brain endothelial cells. J Neuropathol Exp Neurol. 2000;59:896–906. doi: 10.1093/jnen/59.10.896. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 28.Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teleshova N, Bao W, Kivisäkk P, Ozenci V, Mustafa M, Link H. Elevated CD40 ligand expressing blood T-cell levels in multiple sclerosis are reversed by interferon-β treatment. Scand J Immunol. 2000;51:312–320. doi: 10.1046/j.1365-3083.2000.00688.x. [DOI] [PubMed] [Google Scholar]
- 31.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, Malavasi F, Ponzi AN, Paus R, Bulfone-Paus S. Human monocytes constitutively express membrane-bound, biologically active, and interferon-γ–upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- 32.Ifergan I, Wosik K, Cayrol R, Kébir H, Auger C, Bernard M, Bouthillier A, Moumdjian R, Duquette P, Prat A. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol. 2006;60:45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- 33.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-γ–expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 34.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Allie R, Conant K, Haughey N, Turchan-Chelowo J, Hahn K, Rosen A, Steiner J, Keswani S, Jones M, et al. Granzyme B mediates neurotoxicity through a G-protein-coupled receptor. FASEB J. 2006;20:1209–1211. doi: 10.1096/fj.05-5022fje. [DOI] [PubMed] [Google Scholar]
- 36.Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171:368–379. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- 37.McLaurin J, Trudel GC, Shaw IT, Antel JP, Cashman NR. A human glial hybrid cell line differentially expressing genes subserving oligodendrocyte and astrocyte phenotype. J Neurobiol. 1995;26:283–293. doi: 10.1002/neu.480260212. [DOI] [PubMed] [Google Scholar]
- 38.Kataoka T, Takaku K, Magae J, Shinohara N, Takayama H, Kondo S, Nagai K. Acidification is essential for maintaining the structure and function of lytic granules of CTL: effect of concanamycin A, an inhibitor of vacuolar type H+-ATPase, on CTL-mediated cytotoxicity. J Immunol. 1994;153:3938–3947. [PubMed] [Google Scholar]
- 39.Hart G, Avin-Wittenberg T, Shachar I. IL-15 regulates immature B-cell homing in an Ly49D-, IL-12–, and IL-18–dependent manner. Blood. 2008;111:50–59. doi: 10.1182/blood-2007-07-099598. [DOI] [PubMed] [Google Scholar]
- 40.Bo H, Wei XQ, Dong H, Zhang Y, Lv P, Liu W, Koutoulaki A, Gao XM. Elevated expression of transmembrane IL-15 in immune cells correlates with the development of murine lupus: a potential target for immunotherapy against SLE. Scand J Immunol. 2009;69:119–129. doi: 10.1111/j.1365-3083.2008.02197.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsukamoto K, Huang YC, Dorsey WC, Carns B, Sharma V. Juxtacrine function of interleukin-15/interleukin-15 receptor system in tumour derived human B-cell lines. Clin Exp Immunol. 2006;146:559–566. doi: 10.1111/j.1365-2249.2006.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 44.Mita Y, Dobashi K, Endou K, Kawata T, Shimizu Y, Nakazawa T, Mori M. Toll-like receptor 4 surface expression on human monocytes and B cells is modulated by IL-2 and IL-4. Immunol Lett. 2002;81:71–75. doi: 10.1016/s0165-2478(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 45.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 46.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. doi: 10.1016/j.jneuroim.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 48.Ota N, Takase M, Uchiyama H, Olsen SK, Kanagawa O. No requirement of trans presentations of IL-15 for human CD8 T cell proliferation. J Immunol. 2010;185:6041–6048. doi: 10.4049/jimmunol.0901834. [DOI] [PubMed] [Google Scholar]
- 49.Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-αβ and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J Immunol. 2010;185:1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perera LP, Goldman CK, Waldmann TA. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol. 1999;162:2606–2612. [PubMed] [Google Scholar]
- 51.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells In vitro and in the SCID mouse-human rheumatoid arthritis model In vivo. J Clin Invest. 1998;101:1261–1272. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nieto M, del Pozo MA, Sánchez-Madrid F. Interleukin-15 induces adhesion receptor redistribution in T lymphocytes. Eur J Immunol. 1996;26:1302–1307. doi: 10.1002/eji.1830260619. [DOI] [PubMed] [Google Scholar]
- 53.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 55.Göbel K, Melzer N, Herrmann AM, Schuhmann MK, Bittner S, Ip CW, Hünig T, Meuth SG, Wiendl H. Collateral neuronal apoptosis in CNS gray matter during an oligodendrocyte-directed CD8+ T cell attack. Glia. 2010;58:469–480. doi: 10.1002/glia.20938. [DOI] [PubMed] [Google Scholar]
- 56.Niland B, Miklossy G, Banki K, Biddison WE, Casciola-Rosen L, Rosen A, Martinvalet D, Lieberman J, Perl A. Cleavage of transaldolase by granzyme B causes the loss of enzymatic activity with retention of antigenicity for multiple sclerosis patients. J Immunol. 2010;184:4025–4032. doi: 10.4049/jimmunol.0804174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rensing-Ehl A, Malipiero U, Irmler M, Tschopp J, Constam D, Fontana A. Neurons induced to express major histocompatibility complex class I antigen are killed via the perforin and not the Fas (APO-1/CD95) pathway. Eur J Immunol. 1996;26:2271–2274. doi: 10.1002/eji.1830260945. [DOI] [PubMed] [Google Scholar]
- 58.Wang T, Lee MH, Johnson T, Allie R, Hu L, Calabresi PA, Nath A. Activated T-cells inhibit neurogenesis by releasing granzyme B: rescue by Kv1.3 blockers. J Neurosci. 2010;30:5020–5027. doi: 10.1523/JNEUROSCI.0311-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, Petersen LJ, Beurskens FJ, Schuurman J, van de Winkel JG, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]