Abstract
The recreational use of substituted cathinones continues to grow as a public health concern in the United States. Studies have shown that extended access to intravenous (i.v.) self-administration of stimulants, such as cocaine and methamphetamine, results in escalation of drug intake relative to shorter access; however, little is known about the impact of extended access on self-administration of entactogen class stimulants such as methylone and 4-methylmethcathinone (mephedrone). Male Wistar rats were randomly assigned to short-access (ShA, 2-hr) and long-access (LgA, 6-hr) groups and trained to self-administer methylone or mephedrone (0.5 mg/kg/infusion) using a fixed-ratio 1 response contingency. The methylone-trained groups were evaluated on a progressive-ratio (PR) procedure incorporating dose-substitution of methylone (0.125–2.5 mg/kg/infusion), mephedrone (0.125–2.5 mg/kg/infusion) or methamphetamine (MA; 0.01–0.5 mg/kg/infusion). Mephedrone-trained rats were similarly evaluated on a PR with mephedrone and MA. Rats trained with LgA to methylone and mephedrone earned more infusions during acquisition compared with ShA groups. Mephedrone-trained LgA rats reached significantly higher breakpoints than all other groups in mephedrone and MA PR tests. Methylone-trained LgA rats exhibited a rightward shift of the peak effective dose but no overall efficacy change compared with methylone-trained ShA rats. These findings show that the self-administration of mephedrone escalates under LgA conditions in a manner similar to traditional stimulants whereas escalation of 6 h intakes of methylone is not accompanied by differences in PR performance. Thus mephedrone represents the greater risk for dysregulated drug consumption.
Keywords: Drug addiction, bath salts, Ecstasy, reward, substance abuse
INTRODUCTION
The cathinone derivative drug methylone (3,4-methylenedioxymethcathinone) is more common than 3,4-methylenedioxymethamphetamine (MDMA) in the 2013 and 2014 midyear reports of the U.S. National Forensic Laboratory Information System (DEA, 2014, 2015) and use of the cathinone mephedrone expanded rapidly in the UK from 2009–2010 (Winstock et al., 2010; Winstock et al., 2011) during a reported European shortage of MDMA (Brunt et al., 2012; Brunt et al., 2011). Both methylone and mephedrone have been reported by humans to share some of the empathogenic qualities of MDMA, potentially as a result of their shared propensity to enhance serotonin overflow in the nucleus accumbens more than dopamine overflow (Baumann et al., 2012; Kehr et al., 2011; Wright et al., 2012). In rodent laboratory models however, mephedrone has been shown to result in more robust intravenous self-administration (IVSA) than MDMA, suggesting increased abuse liability, albeit probably lower than that for the restricted transporter inhibitor cathinones MDPV and alpha-PVP (Aarde et al., 2015; Schindler et al., 2015; Watterson et al., 2014). Two early reports found that male adult and adolescent rats obtained more infusions of mephedrone than methamphetamine (MA) at an equivalent infusion dose (Hadlock et al., 2011; Motbey et al., 2013) and another study reported greater mephedrone intake versus MA even when training doses were better matched for potency (Aarde et al., 2013a). Male Sprague-Dawley rats have been reported to readily self-administer methylone (Watterson et al., 2012), but prior reports from this laboratory found the IVSA of methylone to be low, similar to MDMA and dissimilar to mephedrone in female or male Wistar rats (Creehan et al., 2015; Vandewater et al., 2015). Another investigation (Schindler et al., 2015) also reported that IVSA intakes of methylone in male Sprague-Dawley rats were lower than those reported by Watterson and colleagues (2012). As there are relatively few studies of the IVSA of mephedrone or methylone, further investigation is urgently needed to precisely delineate the relative abuse liability of these entactogen cathinone stimulants.
Long (6 h daily sessions) access to cocaine (Ahmed and Koob, 1998; Larson et al., 2007) or methamphetamine (Kitamura et al., 2006; Schwendt et al., 2009) results in both higher daily drug intake and a progressive increase across sessions (termed “escalation”) relative to animals trained only in 1–2 h sessions. In contrast, a prior study found no difference in total session intake of the entactogen MDMA between long (6 h) and short (2 h) access groups over the first 11 sessions (Schenk et al., 2003). We found, however, that male Wistar rat IVSA of MDMA in the dark (active) cycle and at a 0.5 mg/kg/inf dose was higher under 6 h versus 2 h conditions (Vandewater et al., 2015). Furthermore, a modest escalation of 6 h MDMA intake was observed across training sessions (although 2 h intake did not escalate in the long-access group). Significantly greater amounts of mephedrone than MDMA were obtained by male rats self-administering only during 2 h sessions but in that study methylone intakes were intermediate (Vandewater et al., 2015). In female rats, 2 h intakes of methylone were almost identical to MDMA, both of which were significantly lower than for mephedrone (Creehan et al., 2015). Finally Watterson and colleagues also reported effects of ten long-access (6 h) sessions on the self-administration of methylone after animals had been trained under short-access conditions but found no escalation in drug intake (Watterson et al., 2012). A study of long-access conditions comparing mephedrone and methylone was therefore designed to further resolve the differences in reinforcer efficacy and abuse liability between mephedrone and methylone.
METHODS
Animals
Male Wistar rats (Charles River, New York) were used for these investigations. Animals were housed in a humidity and temperature-controlled (23±1 °C) vivarium on 12:12 hour light:dark cycles. Animals entered the laboratory at 13–14 weeks of age and weighed an average of 383.1 (±SEM: 6.3) grams at the start of the self-administration study. Animals had ad libitum access to food and water in their home cages and were housed in pairs throughout the study. All procedures were conducted in the dark cycle, under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Garber et al., 2011).
Intravenous catheterization
Rats (mephedrone (Long Access, N=8; Short Access, N=8); methylone (Long Access, N = 12; Short Access, N=12) were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and prepared with chronic intravenous catheters as described previously (Aarde et al., 2015; Aarde et al., 2013b; Miller et al., 2012). Briefly, the catheters consisted of an 14-cm length of polyurethane based tubing (Micro-Renathane®, Braintree Scientific, Inc, Braintree MA, USA) fitted to a guide cannula (Plastics One, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3 cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal’s back to the right jugular vein. Catheter tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 1469SB).
A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, an antibiotic (cephazolin) and an analgesic (flunixin) were administered daily. During testing and training, intravenous catheters were flushed with ~0.2–0.3 ml heparinized (166.7 USP/ml) saline before sessions and ~0.2–0.3 ml heparinized saline containing cefazolan (100 mg/mL) after sessions.
Catheter patency was assessed nearly once a week after the last session of the week via administration through the catheter of ~0.2 ml (10 mg/ml) of the ultra-short-acting barbiturate anesthetic Brevital sodium (1% methohexital sodium; Eli Lilly, Indianapolis, IN). Animals with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 sec after infusion. Animals that failed to display these signs were considered to have faulty catheters and were discontinued from the study. Data that was taken prior to failing this test and after the previous passing of this test were excluded from analysis.
Drugs
The racemic 4-methylmethcathinone (mephedrone) HCl used for this study was obtained from Fox Chase Chemical Diversity Center (Doylestown, PA). Racemic 3,4-methylenedioxymethcathinone (methylone) HCl was obtained from Cayman Chemical. Racemic MDMA (3,4-methylenedioxymethamphetamine) HCl was provided by U.S. National Institute on Drug Abuse. All doses are expressed as the salt and were dissolved in physiological saline.
Self-administration procedure
Acquisition
Drug self-administration was conducted in operant boxes (Med Associates) located inside sound-attenuating chambers located in an experimental room (ambient temperature 22 ± 1 °C; illuminated by red light) outside of the housing vivarium. To begin a session, the catheter fittings on the animals’ backs were connected to polyethylene tubing contained inside a protective spring suspended into the operant chamber from a liquid swivel attached to a balance arm. Each operant session started with the extension of two retractable levers into the chamber. Following each completion of the response requirement (response ratio), a white stimulus light (located above the reinforced lever) signaled delivery of the reinforcer and remained on during a 20-sec post-infusion timeout, during which responses were recorded but had no scheduled consequences. Drug infusions were delivered via syringe pump. The training dose for all three drugs (0.5 mg/kg/infusion; ~0.1 ml/infusion) was selected from prior self-administration studies (Creehan et al., 2015; Vandewater et al., 2015). Differences in molecular weight of the drugs are such that mephedrone freebase (mol wt: 177.24) per-infusion dose was 97.6% of the methylone freebase (mol wt: 207.23) dose. Session duration for the normal (Short Access; ShA) acquisition was 2 h, up to 3 h sessions were conducted for Progressive-Ratio dose substitution and the Long Access (LgA) training sessions were 6 h in duration.
Progressive- Ratio (PR) Dose-Response Testing
For the PR task, the sequence of response ratios started with one response then progressed thru ratios determined by the following equation (rounded to the nearest integer): Response Ratio = 5e^(injection number * j) − 5 (Richardson and Roberts, 1996). The value of “j” was 0.2 and was chosen so as to observe a “breakpoint” within ~3 hrs. The last ratio completed before the end of the session (1 h after the last response up to a maximum of 3 h sessions) was operationally defined as the breakpoint. The animals in all four groups were subjected to randomized dose-substitution conditions in which different per-infusion doses were presented on sequential sessions as described previously (Creehan et al., 2015). The methylone-trained groups completed series in which methylone (0.0, 0.125, 0.5, 1.0 and 2.5 mg/kg/inf), mephedrone (0.0, 0.125, 0.5, 1.0 and 2.5 mg/kg/inf), or methamphetamine (0.0, 0.01, 0.05, 0.1 and 0.5 mg/kg/inf) was available. The mephedrone-trained groups completed the mephedrone and methamphetamine series. The dose order was balanced by Latin Square design within each drug series (i.e., the 5 total conditions were randomized) and each series was repeated twice with the average for both determinations used for analysis.
Data Analysis
The analysis was conducted on the number of infusions earned during the acquisition interval and on the breakpoints reached in the PR study. The Long Access acquisition data were analyzed for the first 2 h of the session as well as for the entire 6 h session; one LgA methylone animal was missing data for session 15, thus the Session 14 value was used for analysis. The data were analyzed with repeated-measures Analysis of Variance (rmANOVA) with dose and/or session number as within-subjects factors and access duration and drug identity as between-subjects factors as appropriate. Any significant rmANOVA main effects were followed with post hoc analysis using Tukey (within-subject) or Sidak (between-groups) correction for all possible comparisons. Analyses were conducted using Prism 6 for Windows (v. 6.02; GraphPad Software, Inc, San Diego CA). Graphs were generated with Excel (Microsoft, Redmond WA) and figures created in Canvas (v.12, v.16; ACD Systems of America, Inc, Seattle, WA)
RESULTS
ACQUISITION OF SELF-ADMINISTRATION
The mean number of infusions obtained by the rats that survived and remained patent through the 15 session acquisition phase while being trained on 0.5 mg/kg/inf of Methylone (N=11 LgA; N=12 ShA), mephedrone (N=7 LgA; N=8 ShA) are presented in Figure 1. On the fifteenth session, the methylone LgA animals averaged 41.4 infusions (±4.8 SEM) whereas the mephedrone LgA averaged 75.0 (±15.4) infusions. Short access totals were 15.3 (±2.8) and 19.5 (±5.0) for the methylone and mephedrone trained animals. The ANOVA confirmed significant effects of Session [F (14, 476) = 25.79; P < 0.0001], of Group [F (3, 34) = 10.20; P < 0.0001] and of the interaction of Session with Group [F (42, 476) = 3.40; P < 0.0001] on total infusions obtained. The post hoc test confirmed that, compared with the respective ShA groups, the LgA groups obtained more infusions of methylone (sessions 10, 12–15) or mephedrone (sessions 4–15). Furthermore, the methylone LgA rats obtained more infusions in sessions 9–15 compared with their first three sessions and the mephedrone LgA group obtained more infusions in sessions 10–15 compared with their first five sessions. In contrast, no differences across sessions were confirmed for either of the ShA groups.
Figure 1.
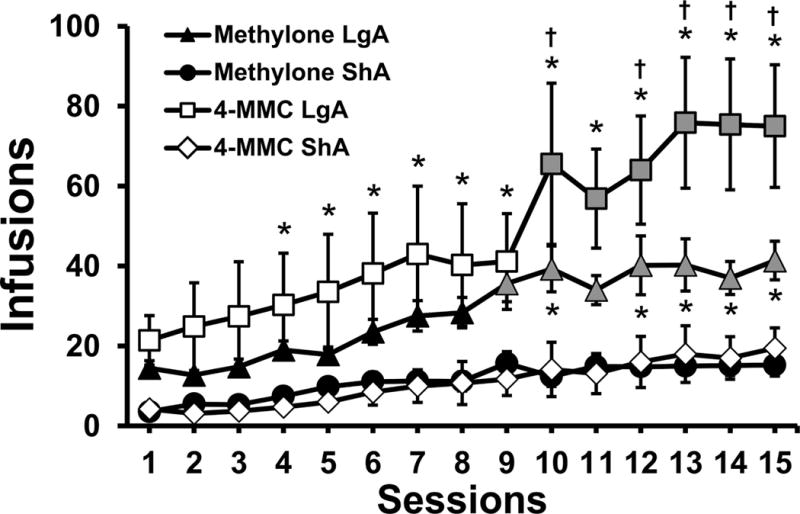
Mean (±SEM) infusions obtained during acquisition for groups of male rats trained to self-administer methylone or mephedrone within Long Access (LgA; 6 h) or Short Access (ShA; 2 h) sessions. Significant differences from the first three sessions within group are indicated by shaded symbols. Significant differences between Access groups within a drug are indicated by * and differences between drugs, within Access condition, by †.
Analysis of the first 2 h of infusions obtained by the LgA groups (Figure 2) confirmed a significant effect of Session [F (14, 224) = 11.93; P < 0.0001] and a significant interaction of Session with Group [F (14, 224) = 1.77; P < 0.05]. The post-hoc test confirmed that methylone LgA animals obtained more infusions in the first 2 h of Session 13 (mean 19.5; ± 3.6 SEM compared with the first seven sessions (means 5.5–8.7 infusions) and in the first 2 h of Sessions 9–10 compared with Sessions 2–3. Likewise, the mephedrone LgA animals obtained more infusions in the first 2 h of Sessions 12–15 (means 26.3–31.6) compared with the first 2 h of the first five sessions (means 8.4–13.7). The post-hoc test also confirmed that mephedrone LgA group obtained more infusions on Session 14 (30.3; ± 8.0) than did the methylone LgA group (14.1; ± 2.5). A follow up ANOVA contrasting the 2 h intakes with the ShA groups’ intakes did not confirm any group differences.
Figure 2.
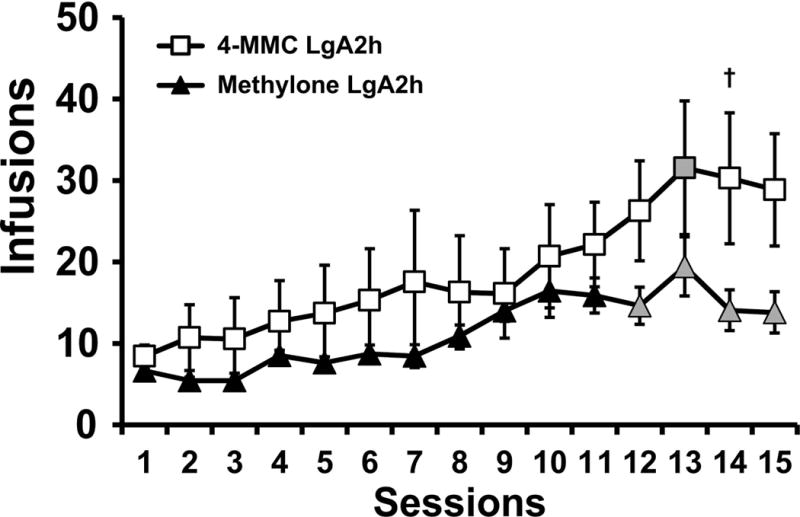
Comparison of mean (±SEM) infusions obtained during the first 2 h of the sessions during acquisition for the LgA groups trained on mephedrone (4-MMC) and methylone. Significant differences from the first five sessions within group are indicated by shaded symbols and significant differences between groups by †.
PROGRESSIVE RATIO DOSE SUBSTITUTION
Methylone-trained
The rats originally trained on methylone under LgA (N=11) and ShA (N=12) conditions exhibited group differences during the initial substitution of methylone doses. The ANOVA confirmed significant effects of Dose [F (4, 84) = 8.49; P < 0.0001] and the interaction of Dose with Access Group [F (4, 84) = 4.11; P < 0.005] on breakpoint. The post hoc test within group confirmed that breakpoints were higher in the LgA group in the 1.0 mg/kg condition (mean 36.7; ± 7.8 SEM) compared with the vehicle (13.9; ±2.0), 0.125 (16.5; ±2.2), 0.5 (20.1; ±3.3) and 2.5 (24.1; ±5.2) mg/kg doses. In the ShA animals, the post hoc test confirmed that significantly higher breakpoints were reached in the 0.5 mg/kg condition (28.2; ±5.4) compared with the 2.5 mg/kg dose (16.1; ±3.2). No between-groups differences at specific doses were confirmed by the post-hoc test.
Substitution of doses of mephedrone or methamphetamine in the methylone-trained LgA (N=11) and ShA (N=10 completed this series) rats did not result in confirmation of any group differences. Specifically, the ANOVA confirmed significant effects of mephedrone Dose [F (4, 76) = 7.03; P < 0.0001] but not of Access Group or of the interaction of Dose with Access Group. Correspondingly, the post hoc test confirmed that significantly higher breakpoints were reached by the LgA rats in the 0.5 (34.9; ±9.2) and 1.0 (38.2; ±10.7) mg/kg mephedrone conditions compared with the saline substitution (15.2; ±1.8) and similarly by the ShA group in 0.5 (47.7; ±9.6) and 1.0 (46.5; ±10.5) mg/kg mephedrone conditions versus vehicle (26.7; ±3.9). The breakpoints in the 0.5 and 1.0 mg/kg mephedrone conditions were also higher than the 2.5 mg/kg (23.8; ±6.9) dose in the ShA group. Likewise, the ANOVA for the methamphetamine dose substitution confirmed significant effects of Dose [F (4, 76) = 5.82; P < 0.001] but not of Access Group or of the interaction of Dose with Access Group. The post hoc test confirmed that significantly higher breakpoints were reached in the 0.1 mg/kg methamphetamine condition (91.6; ±37.6) compared with either vehicle (16.5; ±2.3) or the 0.5 mg/kg MA condition (14.7; ±2.0) in the LgA group and in the 0.05 mg/kg MA condition (105.3; ±47.5) compared with vehicle (25.9; ±4.0) for the ShA group.
Mephedrone-trained
The rats originally trained on mephedrone under LgA (N=7) and ShA (N=8) conditions exhibited group differences in the initial substitution of mephedrone dose (Figure 4). The ANOVA confirmed significant effects of Access Group [F (1, 13) = 6.49; P < 0.05] and Dose [F (4, 52) = 2.74; P < 0.05], but not the interaction, on breakpoint. The post hoc test confirmed that breakpoints were higher in the LgA group in the 1.0 mg/kg mephedrone condition (mean 101.1; ± 29.7 SEM) compared with either vehicle (30.3; ±4.3) or the 0.125 dose (37.9; ±7.6) and higher in the 2.5 mg/kg mephedrone condition (98.1; ±40.1) compared with vehicle. There were no dose-related differences confirmed within the ShA group and breakpoints were lower in the ShA group when 1.0 mg/kg (20.3; ±4.0) or 2.5 mg/kg (31.1; ±15.1) mephedrone was available and LgA groups.
Figure 4.
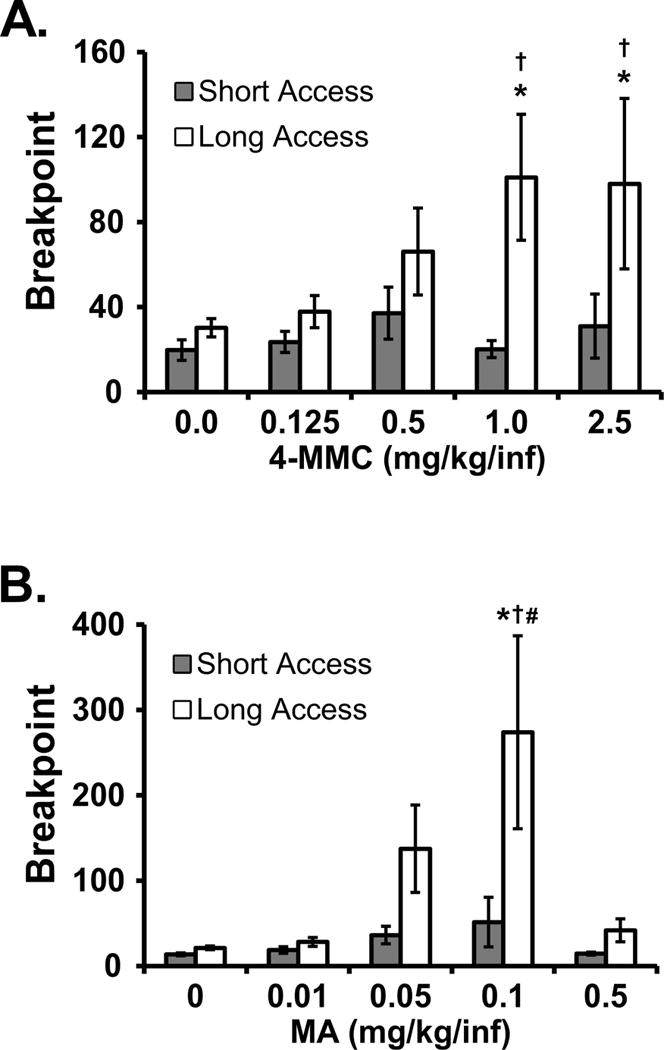
Mean (±SEM) breakpoints reached by mephedrone-trained rats under the progressive ratio procedure following training under Long Access (N=7) and Short Access (N=8) conditions. The top panel includes breakpoints during mephedrone (4-MMC) dose substitution. The bottom panel includes data from methamphetamine (MA) substitution. Significant differences from vehicle control are indicated by *, from the highest dose by # and differences between LgA and ShA groups are indicated by †.
The substitution of doses of methamphetamine in the mephedrone-trained LgA (N=7) and ShA (N=8) rats illustrated higher breakpoints for the LgA group. The ANOVA confirmed significant effects of Access Group [F (1, 13) = 5.08; P < 0.05], Dose [F (4, 52) = 6.61; P < 0.0005] and of the interaction of Dose with Access Group [F (4, 52) = 3.60; P < 0.05] on breakpoint. Correspondingly, the post hoc test confirmed that the LgA animals reached significantly higher breakpoints in the 0.1 mg/kg MA condition (273.9; ±113) compared with when vehicle (21.4; ±2.1), 0.01 (28.3; ±5.3) or 0.5 (41.9; ±13.4) mg/kg MA was available. There were no dose-related differences within the ShA group, however the LgA rats achieved higher breakpoints in the 0.1 mg/kg MA condition compared with the ShA animals at the same dose (51.6; ±29.1).
Mephedrone and Methamphetamine Substitution Across Training Groups
The PR substitutions for mephedrone and methamphetamine were conducted in all four groups, thus it is possible to compare across training group for these challenges (Figure 5). For the mephedrone PR, the ANOVA confirmed main effects of Dose [F (4, 128) = 6.07; P < 0.0005] and Training Group [F (3, 32) = 3.95; P < 0.05] and of the interaction [F (12, 128) = 2.27; P < 0.05]. The post-hoc test confirmed that a higher breakpoint was reached by the mephedrone LgA animals when 1.0 (mean 101.1; ± 29.7 SEM) or 2.5 (98.1;± 40.1) mg/kg/inf were available compared with methylone LgA rats who reached breakpoints of 38.2 (±10.7) and 32.0 (±9.7), respectively, and each of the ShA groups. For the methamphetamine PR, the ANOVA confirmed main effects of Dose [F (4, 128) = 13.10; P < 0.0001] and Training Group [F (3, 32) = 3.28; P < 0.05] and of the interaction [F (12, 128) = 2.43; P < 0.01]. The post-hoc test confirmed that a higher breakpoint was reached by the mephedrone LgA animals when 0.1 mg/kg/inf was available (273.9; ±113) compared with methylone LgA (91.6; ±37.6), and each of the ShA, groups.
Figure 5.
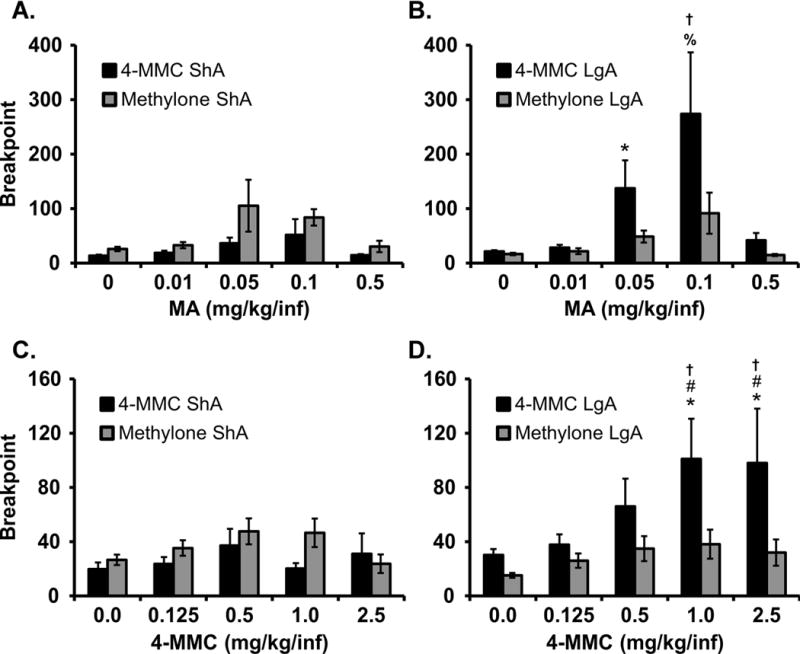
Mean (±SEM) breakpoints reached in the progressive ratio procedure were analyzed by challenge drug. The top panels contrast breakpoints during methamphetamine (MA) substitution in A) ShA and B) LgA groups. The bottom panels contrast breakpoints reached during mephedrone (4-MMC) dose substitution in C) ShA and D) LgA groups. Significant differences from vehicle control within-group are indicated by *, from the 0.125 dose by # and from all other dose conditions by %. Significant differences from all other groups, within a dose condition, are indicated by †.
DISCUSSION
This investigation demonstrates for the first time that male Wistar rats will obtain more infusions of the entactogen-class substituted cathinone drugs mephedrone and methylone (0.5 mg/kg/inf) during intravenous self-administration (IVSA) when trained in Long Access (LgA; 6 h) sessions compared with the intake of a 2 h session trained Short Access (ShA) group. Furthermore it was found that daily (6 h total) or initial 2 h intake in LgA animals increased over the first 15 sessions of training. This outcome is consistent with prior reports of escalated intake of the better-studied stimulants, cocaine (Ahmed and Koob, 1999; Ahmed et al., 2002) or methamphetamine (Anker et al., 2012; Jang et al., 2013; Kitamura et al., 2006; Schwendt et al., 2009), but inconsistent with one prior report for the entactogen MDMA (Schenk et al., 2003). Escalation of either mephedrone or methylone intake in the LgA rats was greater than that found for rats trained to self-administer MDMA (0.5 mg/kg/inf) in 6 h sessions in a prior study (Vandewater et al., 2015), suggesting an enhanced abuse liability of these entactogen cathinones relative to the most closely-related amphetamine. Finally, the self-administration of mephedrone increased to a greater extent than did that of methylone under the LgA but not the ShA training conditions, which demonstrates a further ncreased abuse liability of mephedrone.
The escalation of self-administration of methamphetamine (MA) or cocaine has been inferred in some cases from a pattern of increased intake in LgA groups across training sessions in the absence of an increase in the intake of parallel ShA groups (Anker et al., 2012; Du et al., 2016; Zlebnik et al., 2012). Other studies have additionally found increased drug intake in the initial 1–2 h of a 6 h LgA session (Ahmed and Koob, 1998; Jang et al., 2013) relative to the same interval used for ShA control groups. It is clear, however, that significant increases in MA infusions obtained in the LgA IVSA procedure depends on the training dose. That is, behavioral patterns inconsistent with “escalation” with a high training dose of methamphetamine (0.2 mg/kg/inf) in combination with canonical escalation with lower training doses (0.05–0.1 mg/kg/inf) have been reported when identical cumulative session drug intakes were obtained in both groups (Kitamura et al., 2006). Additional studies have shown that progressive increases in self-administered MA indexed by either total session or initial 1–2 h intake may (Wee et al., 2007; Whitfield et al., 2015) or may not (Crawford et al., 2013) be accompanied by changes in responding under a Progressive Ratio (PR) schedule. The present study therefore took the additional step of contrasting IVSA under a PR schedule in the four training groups to provide further insight into the drug seeking behavior.
The ShA and LgA methylone-trained animals exhibited clear but subtle differences on the PR dose-substitution testing after acquisition. In general the three drugs evaluated in these groups were less potent in the LgA animals, with a rightward shift of the breakpoint curve observed for MA, mephedrone and most robustly for methylone (Figure 3). There were not, however, any indications of an upward shift indicative of altered efficacy such as was observed for MDMA-trained LgA animals when subjected to a dose-substitution of methylone under a Fixed-Ratio contingency in our prior study (Vandewater et al., 2015). This is probably most parsimoniously interpreted as evidence of drug tolerance in the absence of the changes interpreted in prior studies of LgA to cocaine or methamphetamine as compulsive and dysregulated drug-seeking. In contrast, the mephedrone-trained animals exhibited clear efficacy differences attributable to the Lg/Sh access condition since the LgA reached higher peak breakpoints compared with the ShA animals in the mephedrone and methamphetamine dose-substitution tests (Figure 4). The inclusion of two of the same drug substitutions (mephedrone and methamphetamine) in all groups facilitated a further comparison across training drugs, within the Access durations. This analysis found that while no significant differences were observed between ShA groups, the LgA mephedrone-trained animals reached significantly higher breakpoints than all other groups in both mephedrone and methamphetamine substitutions (Figure 5). This result is congruent with the differences in LgA intakes during acquisition and should alleviate any concerns about the 2.4% difference in the freebase dose of the training drugs. Together, the evidence leads to the inference that mephedrone has the greatest potential to produce the dysregulated drug seeking that characterizes drugs of the highest abuse potential in humans.
Figure 3.
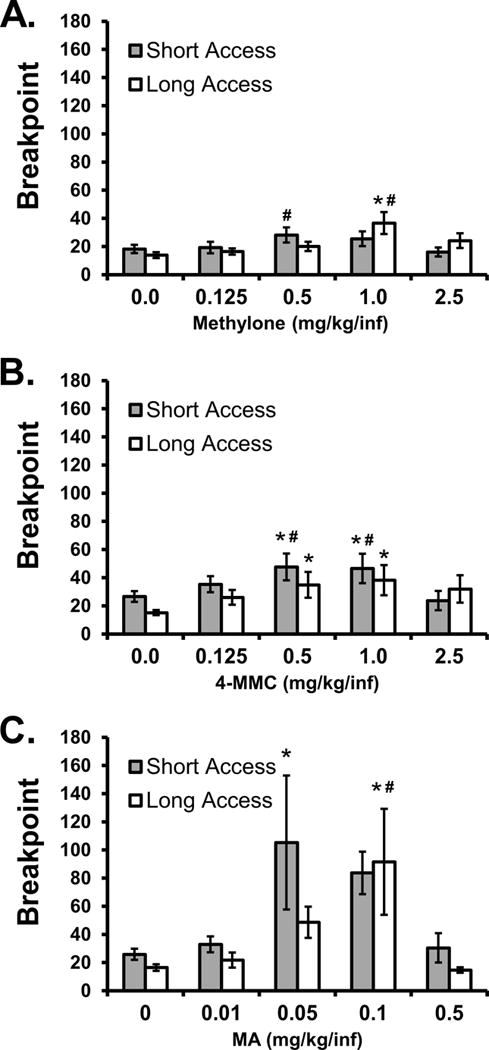
Mean (N=10–11; ±SEM) breakpoints reached by methylone-trained rats under the progressive ratio procedure following training under Long Access and Short Access conditions. The breakpoints reached during A) methylone, B) mephedrone (4-MMC) and C) methamphetamine (MA) substitution are presented. Significant differences from vehicle control are indicated by * and from the highest dose by #.
Our prior two studies found that fewer infusions were obtained during the acquisition of methylone (0.5 mg/kg/inf) vs mephedrone (0.5 mg/kg/inf) IVSA under ShA conditions in female and male rats, albeit not consistently so in the latter sex (Creehan et al., 2015; Vandewater et al., 2015). The present results reinforce a likely similarity of acquisition of these two entactogens in male rats under ShA sessions and therefore the likely difference with the results for MDMA (0.5 mg/kg/inf) reported by Creehan et al. (2015). Methylone IVSA escalated under LgA conditions in a manner distinct from the previous results for LgA MDMA intake (Vandewater et al., 2015), since average session intake after 15 sessions in the methylone LgA group in this study (41.4 infusions; 4.84 SEM) was higher than MDMA LgA group intake (17.8 infusions; 3.12 SEM) in that prior study. (A statistical analysis including all three groups confirmed significantly lower 6 h and 2 h intakes of MDMA relative to the present methylone group.) This shows that despite the tremendous structural (MDMA, methylone) and indeed pharmacological and neuropharmacological (MDMA, methylone and mephedrone) similarity of these drugs, the abuse liability profile differs significantly. Importantly, this study made a clearer distinction between methylone and MDMA, which differ only in the beta ketone motif which distinguishes cathinones from amphetamines. Although it is still unclear why results for male rats self-administering methylone in 2 h sessions in this and prior (Schindler et al., 2015; Vandewater et al., 2015) studies should differ from the findings of Watterson and colleagues (Watterson et al., 2012), this study bridges the gap to the extent it shows that methylone IVSA is higher than MDMA IVSA under the right conditions. These results also provide a possible explanation for why methylone has surpassed mentions of MDMA in the 2013 and 2014 midyear reports of the National Forensic Laboratory Information System (DEA, 2014, 2015). That is, methylone may be a drug with the entactogen-like subjective effects which appeal to the traditional Ecstasy user population, but it has a greater risk for the development of compulsive use and dependence than does MDMA. In comparison, mephedrone exhibits the greatest risk amongst the entactogen psychostimulants based on multiple measures of escalated drug seeking behavior.
Acknowledgments
This work was funded by support from the United States Public Health Service National Institutes of Health (R01 DA024105) which had no direct input on the design, conduct, analysis or publication of the findings. This is manuscript #29256 from The Scripps Research Institute.
Footnotes
Authors Contribution: JDN, YG, KC, and SAV conducted the experiments. JDN and MAT searched the literature, designed the experiments, analyzed the data, created figures and wrote the final version of the manuscript. All authors approved the manuscript.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addiction biology. 2013a;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology. 2015;232:3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013b;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Koob G. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug and alcohol dependence. 2012;124:149–153. doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt TM, Niesink RJ, van den Brink W. Impact of a transient instability of the ecstasy market on health concerns and drug use patterns in The Netherlands. The International journal on drug policy. 2012;23:134–140. doi: 10.1016/j.drugpo.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Crawford JT, Roberts DC, Beveridge TJ. The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug and alcohol dependence. 2013;132:414–419. doi: 10.1016/j.drugalcdep.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEA. National Forensic Laboratory Information System: Midyear Report 2013. U.S. Drug Enforcement Administration; Springfield, VA: 2014. [Google Scholar]
- DEA. National Forensic Laboratory Information System: Midyear Report 2014. U.S. Drug Enforcement Administration; Springfield, VA: 2015. [Google Scholar]
- Du HY, Cao DN, Chen Y, Wang L, Wu N, Li J. Alterations of prefrontal cortical microRNAs in methamphetamine self-administering rats: From controlled drug intake to escalated drug intake. Neurosci Lett. 2016;611:21–27. doi: 10.1016/j.neulet.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Wurbel H. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press; Washington D.C: 2011. [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology. 2013;225:753–763. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. British journal of pharmacology. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Experimental and clinical psychopharmacology. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug and alcohol dependence. 2012;121:90–96. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013;27:823–836. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology. 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the "bath salts" constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology. 2015 doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. The Journal of pharmacology and experimental therapeutics. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology. 2015;99:538–545. doi: 10.1016/j.neuropharm.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The Reinforcing and Rewarding Effects of Methylone, a Synthetic Cathinone Commonly Found in “Bath Salts”. J Addict Res Ther. 2012;S9(002):1–8. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Schlosburg JE, Wee S, Gould A, George O, Grant Y, Zamora-Martinez ER, Edwards S, Crawford E, Vendruscolo LF, Koob GF. kappa Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J Neurosci. 2015;35:4296–4305. doi: 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Marsden J. Mephedrone: still available and twice the price. Lancet. 2010;376:1537–1537. doi: 10.1016/S0140-6736(10)62021-1. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction (Abingdon, England) 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PloS one. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology. 2012;224:387–400. doi: 10.1007/s00213-012-2760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


