Abstract
Chemotherapeutic agents without cross-resistance to prior therapies may enhance peripheral blood stem cell collection and improve patient outcomes by exacting a more potent direct anti-tumor effect prior to autologous stem cell transplant. Bendamustine has broad clinical activity in transplantable lymphoid malignancies, but concern remains over the potential adverse impact of this combined alkylator-nucleoside analog on stem cell mobilization. We performed a prospective, non-randomized Phase II study including thirty-four patients with multiple myeloma (MM) (n=34; ISS stage-I[35%], II[29%] and III[24%]; not scored[13%]) to evaluate bendamustine’s efficacy and safety as a stem cell mobilizing agent. Patients received bendamustine (120 mg/m2 IV d 1,2), etoposide(200 mg/m2 IV d 1–3) and dexamethasone(40 mg PO d 1–4) (BED) followed by filgrastim (10 mcg/kg/d s.c.; through collection). All patients (100%) successfully collected stem cells (median of 21.60 ×106/kg of body weight; range 9.24–55.5×106/kg), and 88% required a single apheresis. Six non-hematologic SAEs were observed in 6 patients including: neutropenic fever (1, grade 3), bone pain (1, grade 3), and renal insufficiency (1, grade 1). In conclusion, BED safely and effectively mobilizes hematopoietic stem cells.
Introduction
High dose chemotherapy followed by autologous stem cell transplantation (ASCT) is a standard of care for patients with multiple myeloma (MM). PBSC engraftment occurs more rapidly with infusion of ≥5 ×106 CD34+ cells/kg; and >2×106 CD34+ cells/kg is often considered to be the minimum number of cells required to proceed to SCT.1, 2 Stem cell proliferation can be enhanced though the addition of myelosuppressive chemotherapy in concert with hematopoietic cytokine granulocyte colony stimulating factor (GCSF).3 Cyclophosphamide (CY) has frequently been used to augment stem cell collection in patients failing GCSF alone.4 In patients who have not mobilized adequate CD34+ stem cells with CY, etoposide has been used successfully.5 No single chemotherapy regimen has demonstrated clear superiority for mobilization, however, and a wide variety of disease-specific cytoreductive chemotherapy approaches have been incorporated into stem cell collection regimens.
The large majority of patients who receive high dose therapy (HDT) followed by ASCT for hematologic malignancies have prior exposure to multiple cycles of cytotoxic therapy, sometimes involving numerous regimens. In patients with MM, ASCT is often performed as consolidation after initial cytoreductive chemo/immunotherapy, yet MM patients frequently have persistent measurable disease at the time of peripheral blood stem cell (PBSC) collection. Reducing both disease burden and the level of tumor cell contamination in collected PBSCs has been correlated with reduced rates of relapse and improved outcomes. Unfortunately, prior attempts to purge contaminant tumor cells through CD34-selection have led to delayed immune reconstitution following SCT and an increased rate of viral infection. Impaired T-lymphocyte (T-cell) immunity has been proposed as a mechanism for the increased infectious risk.6
Chemotherapeutic agents without cross-resistance to prior therapies may enhance PBSC collection and improve patient outcomes by exacting a more potent direct anti-tumor effect prior to ASCT. Bendamustine (Treanda; Teva Pharmaceuticals, Petah Tikva, Israel) is a unique synthetic chemotherapeutic compound that combines a bifunctional alkylating nitrogen mustard group and a purine–like benzamidazol nucleus and thus shares structural similarities to both purine analog and alkylating agents without significant cross resistance to other compounds in either drug class.7 While alklyating agents (melphalan, chlorambucil and cyclophosphamide) exhibit similar mechanistic features to one another, bendamustine has a unique mechanism of action7 and can overcome resistance to melphalan in MM cell lines.8 Bendamustine has demonstrated activity in a wide range of hematologic malignancies (MM, NHL, and chronic lymphocytic leukemia [CLL]) and is well tolerated.9–12 Bendamustine is approved in the European Community for upfront therapy to treat MM in patients over age 65, with neuropathy, who are not considered candidates for ASCT.13 When combined with high-dose melphalan (200 mg/m2) for myeloablative conditioning in MM, 225 mg/m2 of bendamustine demonstrated no increased toxicity compared to melphalan alone (200 mg/m2); and a maximum tolerated dose of bendamustine was not reached.14
As a single agent in the relapsed/refractory setting, bendamustine has demonstrated response rates in 30 to 55% of MM patients,10, 15 and clinical responses to bendamustine-containing regimens have been rapid, with a median time to initial response of 31 days in relapsed/refractory MM.16 The addition of etoposide to mobilization regimens has been shown to increase the overall rate of successful collection, and previous studies have demonstrated that the combination of bendamustine with etoposide is both safe and tolerable.17 Experience with bendamustine only combined with dexamethasone followed by GCSF (BDG) in 3 patients18 did not result in a predictable pattern for leukocyte nadir and recovery. Although all patients treated with the BDG approach were able to eventually collect PBSC, the variability in time to collection and requirement for frequent monitoring of CD34+ cell levels rendered this approach cumbersome and impractical. Thus, etoposide was combined with bendamustine in the BED mobilization regimen to ensure a predictable pattern of nadir and subsequent enhanced CD34+ cell expansion phase during recovery.
Bendamustine’s potential non-cross resistance and overall tolerability makes it an appealing agent to evaluate for pre-transplant cytoredution. Though bendamustine does not have significant toxicity to stem cells in culture,19, 20 the immediate impact of bendamustine on PSBC mobilization has not been prospectively evaluated. The limited data on stem cell yield immediately following full dose bendamustine together with the potential beneficial anti-tumor effect of this agent provided the scientific rationale for this phase II PBSC mobilization trial in patients with multiple myeloma undergoing ASCT.
Methods
This single-center, open-label prospective trial was open to patients with MM planning to undergo autologous stem cell transplantation (six lymphoma patients were also enrolled and will be reported separately). This trial was approved by the Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Institutional Review Board and written informed consent was obtained from all patients. Eligibility criteria included an ECOG status <2, absolute neutrophils of ≥1.5 × 109/L, platelets ≥100 × 108/L, creatinine clearance greater than 50 ml/minute (Cockcroft-Gault formula), bilirubin <1.5 times the upper limit of normal (ULN) AST and ALT <2.5 times ULN. Patients were excluded if they had prior resistance to bendamustine, ≥4 prior different myelotoxic chemotherapy regimens (e.g. VRD-PACE [bortezomib/lenalidomide/dexamethasone/cis-platin/doxorubicin/cyclophosphamide/etoposide]), symptomatic cardiopulmonary disease, fludarabine therapy in the preceding 24 months, ≥7 cycles of lenalidomide, a prior failed stem cell mobilization attempt, prior autologous or allogeneic stem cell transplant, known HIV, hepatitis B or C, > 3 cycles of multi-agent myelotoxic salvage chemotherapy within 4 months of enrollment, prior pelvic/spinal irradiation, or systemic chemotherapy within 3 weeks of initiating BED.
Study design
Patients were administered 1 cycle of BED [bendamustine (120 mg/m2 IV d 1, 2), etoposide (200 mg/m2 IV d 1–3), dexamethasone (40 mg PO d 1–4), delivered as an outpatient, followed by filgrastim (initially 10 mcg/kg/d s.c.; starting on d 5 through end of collection)]. Apheresis was initiated when peripheral blood CD34+ cell counts were >5/μL. The primary endpoint was successful mobilization in over 80% of patients, defined as collection of ≥2.0 × 106 CD34+ cells/kg. The stem cell mobilization success rate after chemotherapy regimens has typically been 80% or greater.2, 5, 21 This study was not powered to observe a rate that is statistically significantly better than 80%, as such a study would have required a very large number of patients. Rather, potential efficacy was defined by identifying an observed collection success rate of at least 80%. Forty patients were enrolled (including the 6 lymphoma patients to be reported elsewhere), and if the true success rate was 70%, then the probability of seeing 32 or more successes among 40 patients (an observed rate of 80% or more) was 0.11. An observed rate of 80% provides 89% confidence that the true success rate is over 70%. We considered any outcome of >80% successful mobilization to be sufficiently consistent with an acceptable success rate that the regimen could be considered potentially efficacious. Adverse events (AEs) were graded using the CTCAE v4.0. Secondary endpoints included determining the number of apheresis cycles required to collect a minimum of >2 ×106 CD34+ cells/kg and ideally >5 × 106 CD34+ cells/kg, and evaluation of the disease response rate to one cycle of BED. Vital signs and clinical status were closely monitored during bendamustine infusion. Tumor lysis syndrome (TLS) associated with bendamustine treatment has been reported, and volume status, serum chemistries and uric acid levels were monitored. Patients deemed to be at risk for TLS who received prophylactic allopurinol were monitored closely, as some investigators have suggested an increased risk for severe skin toxicity when bendamustine is combined with allopurinol.22 When patients’ absolute neutrophil count dropped below 500 mm3, prophylactic antibiotic therapy (fluoroquinolone) was initiated at the discretion of the treating physician.
Response Criteria
Response in patients with measurable disease was a secondary endpoint and was assessed after a single cycle of BED. Definitions of disease, criteria for evaluation, endpoint definitions, and response criteria were defined by the multiple myeloma response criteria as defined by the International Multiple Myeloma Working Group.23, 24
Results
Patient characteristics
We enrolled 34 MM patients in this trial between May 2011 and October 2013. Patient characteristics are shown in Table 1. Patients were a median age of 61 years (range 46–70). The MM patients received a median of 1 line of prior therapy (range 1–3) with 22 receiving a median of 4 cycles of lenalidomide-based therapy (range 1–6). Twenty-six patients received one line of prior therapy. Six patients received either two (n=4) or three (n=2) lines of therapy prior to stem cell mobilization (Table 2). More than one regimen was administered to deepen disease response prior to ASCT (n=3), in response to intolerance to the prior regimen (n=2) or due to a change in treatment plan (n=1; after once cycle of melphalan containing therapy, the treatment goal were modified to include ASCT). International Staging System (ISS) scores could be calculated for 31/34 MM patients from the time of diagnosis; 12 patients were stage I, 10 were stage II, and 9 were stage III. Eight of the 34 MM patients had high-risk cytogenetic features identified either by conventional cytogenetic analysis or by MM-specific FISH probes. These high risk features included t(4;14)[3 patients]; 17p- [3 patients]; t(14;16) [2 patients]; complex karyotype [1 patient]; 1p- [1 patient] including 2 patients demonstrating 2 high risk features concurrently. Two patients had received prior radiotherapy. Twenty-six patients had measurable disease prior to BED. Thirty-one patients have proceeded to ASCT following collection.
Table 1.
| Gender | 71% male |
| Median Age | 61 (46–70) |
| Multiple Myeloma | 34 patients |
| Median prior regimens | 1 (range1–3) |
| Median cycles of prior chemotherapy | 5 (range 1–12) |
| Prior lenalidomide (MM pts) | 22 patients |
| Median prior cycles | 4 |
| 1–4 cycles | 13 patients |
| 5–6 cycles | 9 patients |
| Prior radiotherapy | 3 patients |
| ISS Stage I (MM) | 12 (40%) |
| ISS Stage II (MM) | 10 (33%) |
| ISS Stage III (MM) | 8 (27%) |
| High Risk Cytogenetics (MM) | 9 patients |
| Median Monoclonal Protein (n=32) | 0.3 g/dL (range 0–1.6) |
| Median Bone Marrow % Involvement by Morphology (MM) | 3% (range 0–35%) |
Table 2.
| Prior Therapy | Number of Patients |
|---|---|
| Bortezomib, Lenalidomide, Dexamethasone (BLD) | 15 |
| Bortezomib, Cyclophosphamide, Dexamethasone (BCD) | 9 |
| Bortezomib, Cyclophosphamide, Liposomal-doxarubicin, Dexamethasone (BCLD) | 1 |
| Lenolidomide, Dexamethasone (LD) | 1 |
| Bortezomib, Dexamethasone (BD) | 1 |
| BCD; BLD | 1 |
| Bortezomib, Melphalan;BCD | 1 |
| Bortezomib, Thalidomide, Dexamethasone (BTD); LD | 1 |
| BD; BLD | 1 |
| BCLD; BTD | 1 |
| LD; BLD; BCD | 1 |
| BCLD; BTD; BLD-Cisplatin, Adriamycin, Cyclophosphamide, Etoposide | 1 |
Stem cell mobilization and collection
All MM patients (34/34) were successfully mobilized. No patient required dose reduction of the chemotherapy agents. The median number of CD34+ cells collected was 21.60 × 106/kg (range 9.24 to 55.51 × 106, Figure 1A). A sufficient number of CD34+ cells for two future ASCTs were collected from all subjects including 33 of 34 (97%) collecting ≥10 × 106 CD34+ cells/kg and all collecting ≥9 × 106 CD34+ cells/kg. Twenty-two MM patients received lenalidomide therapy prior to mobilization, and there was no difference in the absolute number of CD34+ stem cells collected after BED among those who had received <4 cycles of lenalidomide (21.64 × 106 CD34+ cells/kg, n=13) and those treated with >4 but <7 cycles of lenalidomide (20.8 × 106 CD34+ cells/kg, n=9) (p=0.98). The median time from the start of BED mobilization therapy to the first day of CD34+ stem cell collection was 11 days (range 9 to 13 days, Figure 1B). The median number of apheresis days was 1 (range 1 to 4, Figure 1C). A predictable pattern of leukocyte nadir and recovery was demonstrated (all patients started apheresis between days 9–13). One patient (3%) was given plerixafor (administered at the discretion of the transplant service attending physician of record) on day 12 after BED. This patient was defined as a “collection failure” based on the pre-specified goal of achieving adequate collection without requiring plerixafor support; however, the patient was successfully mobilized and collected 13.45 × 106 CD34+ cells/kg over three days. Time to collection, days of apheresis, and number of stem cells collected for this patient was not included in the study collection results because the plerixafor represents a variable that was not equally applied to all study participants and the decision to use plerixafor was based on the clinical judgment of the transplant attending of record however, the subject was not excluded from the response assessment. One patient received plerixafor, this patient had previously received 6 cycles of bortezomib/cyclophosphamide/dexamethasone and had previously required bortezomib dose reductions due to cytopenias. In 1 MM patient (3%) the GCSF dose was increased to 16 mcg/kg twice daily, in response to prolonged neutrophil recovery. This patient had previously received 3 cycles of cyclophosphamide/liposomal doxorubicin/bortezomib/dexamethasone therapy; 4 cycles of bortezomib/thalidomide/dexamethasone and one cycle of VRD-PACE. Among the 21 evaluable patients ≥60 years of age, the mean number of CD34+ cells/kg was 23.01 × 106 (SD 11.80), and for the 12 patients <60, the mean number was 27.21 × 106 (SD 15.47, p=0.38) [the plerixafor-treated patient was 61 years old, collected 13.45 × 106 and was not included in the analysis].
Figure 1.
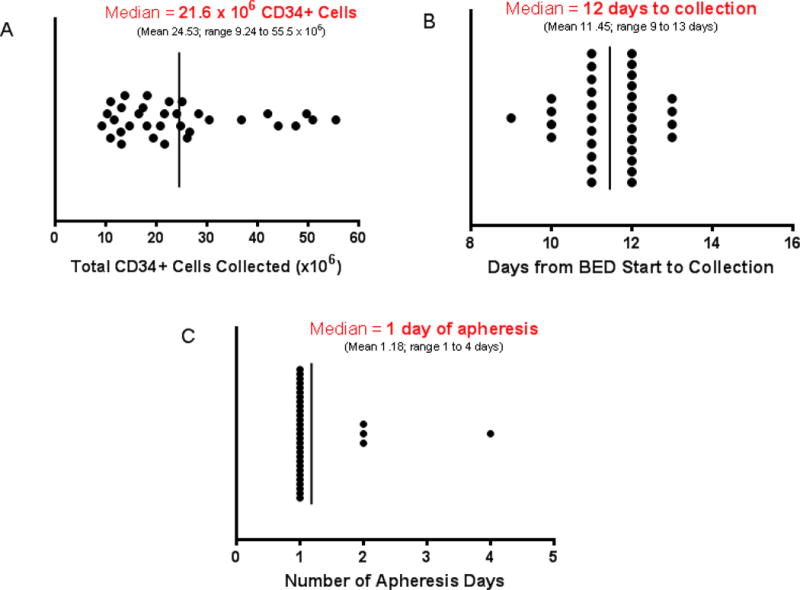
(A) Median number of CD34+ cells collected/kg body weight, (B) median days to collection (C) median number of apheresis days after bendamustine, etoposide and dexamethasone mobilization (n=38, 2 patients were given plerixafor and are not included).
Among the 30 MM patients with ISS scores available from diagnosis, there was no correlation between ISS and median number of stem cells/kg mobilized (25.96 × 106 for stage I [n=12], 19.82 × 106 for stage II [n=10] and 20.79 × 106 for stage III [n=8]), and no relationship between stem cell yield and the presence of high risk cytogenetic features.
Toxicity and engraftment
Expected grade 3 or 4 thrombocytopenia, leukopenia, and lymphopenia were seen in most patients (Table 3). Six SAEs were observed in 6 patients. SAEs included: neutropenic fever (1, grade 3), bone pain (1, grade 3), renal insufficiency (1, grade 1), atrial fibrillation (1, grade 2), hypotension (1, grade 3), and stroke (1, grade 2). Among the 34 patients mobilized and collected, 31 have thus far undergone ASCT, and 100% (31/31) achieved an unsupported neutrophil count ≥500/μL at a median of 15 days (range 7–19, Figure 2A) after PBSC infusion and a platelet count ≥20K/μL at a median of 11 days (range 8–15, Figure 2B).
Table 3.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | SAE |
|---|---|---|---|---|---|---|
| Hematologic | ||||||
| Lymphopenia | 0 | 0 | 0 | 34 | 0 | No |
| Thrombocytopenia | 0 | 0 | 30 | 1 | 0 | No |
| Neutropenia | 0 | 0 | 11 | 21 | 0 | No |
| Leukopenia | 0 | 0 | 0 | 26 | 0 | No |
| Non-Hematologic | ||||||
| Hypophosphatemia | 0 | 0 | 5 | 1 | 0 | No |
| Hyperglycemia | 0 | 0 | 2 | 0 | 0 | No |
| Muscle Weakness | 0 | 0 | 1 | 0 | 0 | No |
| Bone Pain | 0 | 0 | 1 | 0 | 0 | No |
| Rash Maculo-papular | 0 | 0 | 1 | 0 | 0 | No |
| Hypotension | 0 | 0 | 2 | 0 | 0 | Yes |
| Muscle Weakness | 0 | 0 | 1 | 0 | 0 | No |
| Stroke | 0 | 1 | 0 | 0 | 0 | Yes |
| Renal Insufficiency | 1 | 0 | 0 | 0 | 0 | Yes |
| Atrial Fibrillation | 0 | 1 | 0 | 0 | 0 | Yes |
| Neutropenic Fever | 0 | 0 | 1 | 0 | 0 | Yes |
| Sepsis | 0 | 0 | 1 | 0 | 0 | Yes |
Figure 2.
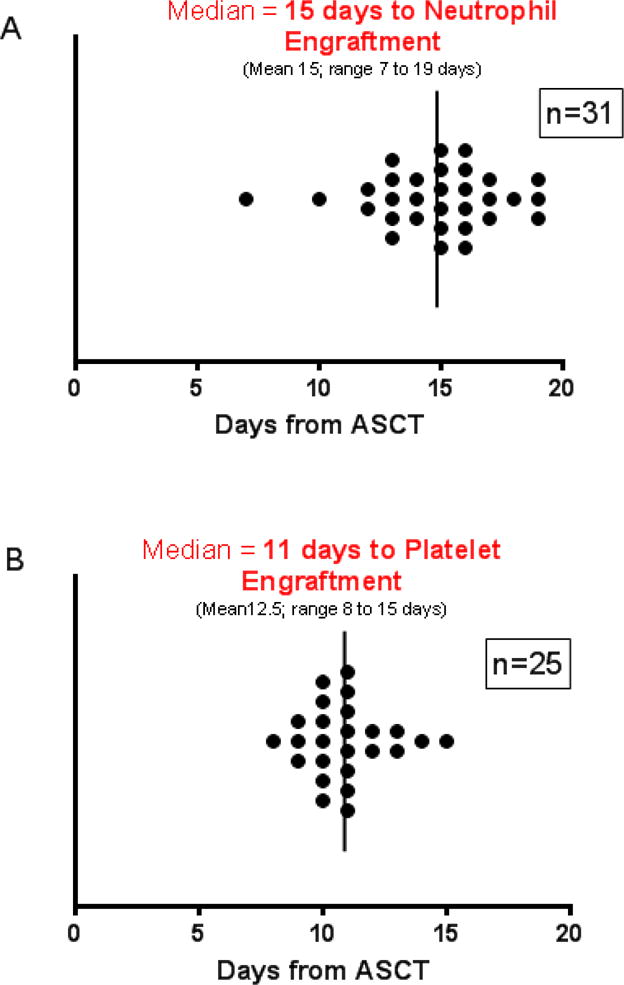
Median time to (A) neutrophil [N=31] and (B) platelet [N=25] engraftment among patients who have proceeded to ASCT following BED mobilization. Neutrophil engraftment is based on absolute neutrophil count ≥500 for two consecutive days. Platelet engraftment is based on an unsupported platelet count of ≥20,000. Post-transplant platelet counts were evaluable in 25 patients, patients who required ongoing platelet support (i.e. those receiving active anticoagulation) were not included.
Response Rates
Responses among all 34 patients were evaluated and include: CR = 4, VGPR = 0 (myeloma) PR=6, SD=22, and PD=2. The ORR to this single cycle of therapy was 29%. Response was measured in MM patients at a median of 26.5 days (range 10–69) after initiating BED. Two of the 9 MM patients (22%) with high-risk cytogenetic were in a CR after one cycle of BED. Eleven of the 34 MM patients had no evidence of bone marrow involvement at enrollment and 8 had no measurable monoclonal protein. Among the 4 patients in CR, 3 had a CR and 1 a PR to their most recent regimen prior to BED mobilization. Among the 6 patients with PR, 4 had a PR, 1 a CR and 1 was not evaluable for response to their most recent regimen. Among the 22 patients with SD after BED, 1 was in VGPR, 1 in CR, 1 with SD, 18 with a PR and 1 was not evaluable for response to their most recent treatment regimen. Among the 2 patients with PD, 1 had SD and 1 a PR to their most recent pre-BED regimen.
Discussion
Important considerations in selecting an effective stem cell chemotherapy based mobilization regimen for multiple myeloma include: (1) non-cross resistance with prior therapies, (2) the potency from a single cycle of treatment, (3) capacity for predictable robust CD34+ cell mobilization (facilitating short apheresis duration and cost containment), and (4) safety. Recent consensus guidelines addressing stem cell collection approaches advocate for the identification of novel mobilization strategies designed to improve yields, efficiency, and cost.25, 26 This study demonstrates that BED mobilization offers advantages in each of these categories.
Cyclophosphamide (CY) is the most frequently used chemomobilization agent in MM. CY can effectively increase CD34+ cell yields;27–29 however CY mobilization does not improve rates of CR, time to progression, EFS, or OS in MM, while the risk of developing bacteremia is increased when compared with GCSF mobilization alone.30 This absence of a documented anti-tumor effect, in conjunction with increased cost and toxicity, has led some groups to recommend limiting chemotherapy-based mobilization to MM patients presenting with circulating plasma cells or disease in frank relapse.31 Identifying an alternative mobilization regimen capable of safely improving disease control in MM patients may be important, however, because disease control pre-transplant has been associated with improvements in long-term patient outcomes;32 achieving a CR or stringent CR (sCR) after HDT-ASCT has been shown to significantly improve EFS, PFS, and OS.33–35
The time to response is an important consideration when selecting non-cross resistant mobilization regimens designed to reduce disease burden, because the time window between stem cell mobilization and subsequent myeloablative conditioning therapy is frequently short. When bendamustine was compared to oral melphalan (both in combination with prednisone) in newly diagnosed MM patients, bendamustine demonstrated a significantly faster time for maximum response and had a longer time to treatment failure (14 vs 10 months, p <0.02).11 Measurable responses to a single cycle of bendamustine-based therapy have been reported at a median 31 days after treatment among patients with relapsed or refractory MM (≥PR, for bendamustine combined with bortezomib and dexamethasone among MM patients [63% with prior bortezomib treatment]), and an overall response rate of 61% was reported in this heavily pretreated patient population.16 Rapid responses have also been reported when bendamustine is combined with lenalidomide and dexamethasone in patients with relapsed or refractory MM.36, 37 In our study, thirty-one patients proceeded to ASCT within 3 months of study enrollment. By necessity, restaging was performed a median of only 26.5 days (range 10–69) after BED. The short time interval between treatment and disease response assessment may contribute to an underestimation of MM response rates in this study, because established MM response criteria are reliant on serologic markers that lack short term sensitivity23, 24 (the clearance half-life for IgG monoclonal protein is more than 3 weeks).38
Following ASCT, improved outcome has been associated with time to engraftment of platelets, neutrophils, and lymphocytes, and the time to recovery has correlated with stem cell dose.39, 40 Patients receiving BED mobilization collected a median of 21.60 × 106 CD34+ stem cells (mean 24.53; range 9.24 to 55.5 × 106). The absolute number of CD34+ cells collected after CY alone or in combination with etoposide mobilization is variable (see Table 4) and reflects differences in patient populations on clinical trials. While different study populations make comparisons of CD34+ cell yield between trials less informative, some studies have suggested that CY based regimens impair stem cell engraftment,27 while with BED, engraftment is rapid (Figure 2).
Table 4.
| Regimen | Diagnosis | CD34 + cell yield (× 106) | Collection Failure (%) | N |
|---|---|---|---|---|
| GCSF + BED (current study) | MM | 21.60 | 0 | 34 |
| GCSF + CY27 | MM | 10.3 | NR | 370 |
| GCSF + CY43 | MM | 14.2 | 0 | 19 |
| GCSF + VP-1644 | MM | 12 | 0 | 152 |
| GCSF + VP-1645 | Lymphoma | 6.2 | 6 | 159 |
| GCSF + CY46 | Lymphoma | 7.2 | 4.2 | 24 |
| GCSF + CY47 | MM | 33.4 | 18 | 22 |
| GCSF + CY48 | NHL | 6.41 | NR | 12 |
| GCSF + CY49 | MM | 4.65 | 0 | 37 |
| GCSF + CY50 | NHL | 7.1 | 10.5 | 34 |
| GCSF + CY43 | MM | 16 | NR | 34 |
| GCSF + CY + VP-1651 | MM | 22.5 | 4 | 49 |
| GCSF + CY52 | MM | 5.9 | 4 | 51 |
| GSCF + CY53 | MM | 8.6 | 0 | 15 |
Advanced age has been associated with inferior CD34+ cell collection yields with other mobilization regimens39, 40, but among patients receiving BED there was no significant difference in stem cell collection yield between patients ≥60 (mean = 23.01 × 106 CD34/kg [SD 11.80])) and those <60 (27.21 × 106 CD34/kg [SD 15.47, p=0.38]). While the findings reported here for BED mobilization are encouraging, results from this trial should be further evaluated in a phase III randomized mobilization study comparing BED to a Cytoxan containing regimen. Extrapolation of the current results to the general ASCT patient population is limited by the study’s enrollment criteria. To assess safety and efficacy associated with a bendamustine-containing regimen, the trial design necessarily excluded patients who had received prior radiation to bone marrow, seven or more cycles of lenalidomide, and significant exposure to myelotoxic regimens. While data in these populations would be clinically useful, inclusion of such patients would have limited our capacity to evaluate the impact of the BED regimen on stem cell mobilization. As a result, the population described herein does not fully represent the spectrum of patients who may benefit from BED. The finding that stem cell yield after BED was not impaired among patients who had previously received up to 6 prior cycles of lenalidomide was encouraging, as some groups have reported decreased CD34+ stem cell yields after more than 4 prior cycles of lenalidomide containing therapy.41
While etoposide likely contributed to the efficacy of the BED regimen, this study validates the safety of a bendamustine and etoposide combination for mobilization. Moreover, the single agent activity of etoposide in MM is limited, and bendamustine represents a non-cross resistant agent capable of improving disease response rates. Recent studies have demonstrated synergy between the proteasome inhibitor bortezomib and bendamustine, suggesting the possibility that a four-agent bendamustine-containing mobilization regimen could further improve response rates.42
In conclusion, bendamustine does not appear to be an acute stem cell toxin and PBSC mobilization with BED is safe and effective. Large numbers of stem cells were rapidly mobilized and resulted in short durations of apheresis. No patient collected fewer than 9 × 106 CD34+ cells/kg (sufficient for 2 ASCTs). In patients who were transplanted, the time to neutrophil and platelet engraftment was comparable to other chemotherapy-based mobilization regimens. The BED regimen was well tolerated and these findings suggest a role for BED in PBSC mobilization.
Acknowledgments
Teva Pharmaceutical Industries, NCI K08 CA151682(D.J.G.), NCI P01CA44991, NCI R01CA076287, NCI R01 CA138720, 1K24CA184039, Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant P30 CA015704 and philanthropic gifts from Frank and Betty Vandermeer. AKG is a Scholar in Clinical Research for the Leukemia and Lymphoma Society.
Research funding was provided by Teva Pharmaceuticals for this investigator initiated research study. The funding was used to support the salaries of research study staff. Bendamustine was provided to patients by Teva Pharmaceuticals at no cost. Teva Pharmaceuticals played no role in the study design, the collection and analysis of the data, the decision to publish this work or the writing of this manuscript. Drs. Green, Gopal and Budde have received research support from Teva Pharmaceuticals. Dr. John Pagel has received compensation as a consultant to Teva Pharmaceuticals.
Footnotes
Trial Registration: ClinicalTrials.gov, NCT01110135, https://clinicaltrials.gov/ct2/show/NCT01110135
Conflict of interest statement:
The authors have no personal financial interests in Teva Pharmaceuticals and no other conflicts of interest to disclose.
References
- 1.Blystad AK, Delabie J, Kvaloy S, Holte H, Valerhaugen H, Ikonomou I, et al. Infused CD34 cell dose, but not tumour cell content of peripheral blood progenitor cell grafts, predicts clinical outcome in patients with diffuse large B-cell lymphoma and follicular lymphoma grade 3 treated with high-dose therapy. British journal of haematology. 2004;125(5):605–12. doi: 10.1111/j.1365-2141.2004.04951.x. [DOI] [PubMed] [Google Scholar]
- 2.Gazitt Y, Freytes CO, Callander N, Tsai TW, Alsina M, Anderson J, et al. Successful PBSC mobilization with high-dose G-CSF for patients failing a first round of mobilization. Journal of hematotherapy. 1999;8(2):173–83. doi: 10.1089/106161299320442. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(8):1191–203. doi: 10.1016/j.bbmt.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Gazitt Y, Callander N, Freytes CO, Shaughnessy P, Liu Q, Tsai TW, et al. Peripheral blood stem cell mobilization with cyclophosphamide in combination with G-CSF, GM-CSF, or sequential GM-CSF/G-CSF in non-Hodgkin’s lymphoma patients: a randomized prospective study. Journal of hematotherapy & stem cell research. 2000;9(5):737–48. doi: 10.1089/15258160050196786. [DOI] [PubMed] [Google Scholar]
- 5.Reiser M, Josting A, Draube A, Mapara MY, Scheid C, Chemnitz J, et al. Successful peripheral blood stem cell mobilization with etoposide (VP-16) in patients with relapsed or resistant lymphoma who failed cyclophosphamide mobilization. Bone marrow transplantation. 1999;23(12):1223–8. doi: 10.1038/sj.bmt.1701791. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg LA, Boeckh M, Hooper H, Leisenring W, Rowley S, Heimfeld S, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999;94(12):4029–35. [PubMed] [Google Scholar]
- 7.Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(1):309–17. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 8.Cives M, Ciavarella S, Rizzo FM, De Matteo M, Dammacco F, Silvestris F. Bendamustine overcomes resistance to melphalan in myeloma cell lines by inducing cell death through mitotic catastrophe. Cellular signalling. 2013;25(5):1108–17. doi: 10.1016/j.cellsig.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(15):3383–9. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 10.Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H. The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica. 2005;90(9):1287–8. [PubMed] [Google Scholar]
- 11.Ponisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone–a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO) Journal of cancer research and clinical oncology. 2006;132(4):205–12. doi: 10.1007/s00432-005-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rummel MJ. Bendamustine in chronic lymphocytic leukemia and refractory lymphoma. Seminars in hematology. 2008;45(3 Suppl 2):S7–10. doi: 10.1053/j.seminhematol.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo A, Offidani M, Patriarca F, Petrucci MT, Cavo M. Bendamustine for the treatment of multiple myeloma in first-line and relapsed-refractory settings: a review of clinical trial data. Leukemia & lymphoma. 2014:1–9. doi: 10.3109/10428194.2014.915545. [DOI] [PubMed] [Google Scholar]
- 14.Mark TM, Reid W, Niesvizky R, Gergis U, Pearse R, Mayer S, et al. A phase 1 study of bendamustine and melphalan conditioning for autologous stem cell transplantation in multiple myeloma. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19(5):831–7. doi: 10.1016/j.bbmt.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damaj G, Malard F, Hulin C, Caillot D, Garidi R, Royer B, et al. Efficacy of bendamustine in relapsed/refractory myeloma patients: results from the French compassionate use program. Leukemia & lymphoma. 2012;53(4):632–4. doi: 10.3109/10428194.2011.622422. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig H, Kasparu H, Leitgeb C, Rauch E, Linkesch W, Zojer N, et al. Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood. 2014;123(7):985–91. doi: 10.1182/blood-2013-08-521468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118(12):3419–25. doi: 10.1182/blood-2011-04-351924. [DOI] [PubMed] [Google Scholar]
- 18.Green DJ, Bensinger WI, Holmberg L, Gooley TA, Till BG, Budde LE, et al. Bendamustine (Treanda®), Etoposide and Dexamethasone (BED) Followed by GCSF Effectively Mobilizes Autologous Peripheral Blood Hematopoietic Stem Cells. Blood. 2012;120(21):4126–4126. [Google Scholar]
- 19.El-Mabhouh AA, Ayres ML, Shpall EJ, Baladandayuthapani V, Keating MJ, Wierda WG, et al. Evaluation of bendamustine in combination with fludarabine in primary chronic lymphocytic leukemia cells. Blood. 2014;123(24):3780–9. doi: 10.1182/blood-2013-12-541433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt-Hieber M, Busse A, Reufi B, Knauf W, Thiel E, Blau IW. Bendamustine, but not fludarabine, exhibits a low stem cell toxicity in vitro. Journal of cancer research and clinical oncology. 2009;135(2):227–34. doi: 10.1007/s00432-008-0453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lie AKW, Hui CH, Rawling T, Dyson PG, Thorp D, Benic J, et al. Granulocyte colony-stimulating factor (G-CSF) dose-dependent efficacy in peripheral blood stem cell mobilization in patients who had failed initial mobilization with chemotherapy and G-CSF. Bone Marrow Transplantation. 1998;22(9):853. doi: 10.1038/sj.bmt.1701463. [DOI] [PubMed] [Google Scholar]
- 22.Alamdari HS, Pinter-Brown L, Cassarino DS, Chiu MW. Severe cutaneous interface drug eruption associated with bendamustine. Dermatology Online Journal. 2010;16(7) [PubMed] [Google Scholar]
- 23.Alexanian R, Anderson K, Attal M, Barlogie B, Beksac M, Belch A, et al. International uniform response criteria for multiple myeloma. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20:1467. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Harousseau J-L, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. 2011;117 doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2014;20(3):295–308. doi: 10.1016/j.bbmt.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(10):1904–1912. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 27.Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone marrow transplantation. 2009;43(8):619–25. doi: 10.1038/bmt.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moog R. Management strategies for poor peripheral blood stem cell mobilization. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2008;38(3):229–36. doi: 10.1016/j.transci.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Costa LJ, Nista EJ, Buadi FK, Lacy MQ, Dispenzieri A, Kramer CP, et al. Prediction of poor mobilization of autologous CD34+ cells with growth factor in multiple myeloma patients: implications for risk-stratification. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2014;20(2):222–8. doi: 10.1016/j.bbmt.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clinical lymphoma & myeloma. 2006;6(5):384–8. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 31.Gertz MA, Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood. 2014;124(6):882–90. doi: 10.1182/blood-2014-03-544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(15):2612–24. doi: 10.1200/JCO.2009.25.4250. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Delasalle K, Feng L, Thomas S, Giralt S, Qazilbash M, et al. CR represents an early index of potential long survival in multiple myeloma. Bone marrow transplantation. 2010;45(3):498–504. doi: 10.1038/bmt.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. The New England journal of medicine. 2006;354(10):1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(36):4529–35. doi: 10.1200/JCO.2013.49.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponisch W, Bourgeois M, Moll B, Heyn S, Jakel N, Wagner I, et al. Combined bendamustine, prednisone and bortezomib (BPV) in patients with relapsed or refractory multiple myeloma. Journal of cancer research and clinical oncology. 2013;139(3):499–508. doi: 10.1007/s00432-012-1339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119(20):4608–13. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(11):5512–6. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oran B, Malek K, Sanchorawala V, Wright DG, Quillen K, Finn KT, et al. Predictive factors for hematopoietic engraftment after autologous peripheral blood stem cell transplantation for AL amyloidosis. Bone marrow transplantation. 2005;35(6):567–75. doi: 10.1038/sj.bmt.1704826. [DOI] [PubMed] [Google Scholar]
- 40.Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1995;13(10):2547–55. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 41.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;22(6):1280–1281. doi: 10.1038/sj.leu.2405035. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Wang X, Chen L, Liang J, Suvannasankha A, Abonour R, et al. Synergistic Activity of Bendamustine in Combination with Doxorubicin and Bortezomib in Multiple Myeloma Cells. ASH Annual Meeting Abstracts. 2008;112(11):5171. [Google Scholar]
- 43.Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2004;10(6):395–404. doi: 10.1016/j.bbmt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Wood WA, Whitley J, Moore D, Sharf A, Irons R, Rao K, et al. Chemomobilization with Etoposide is Highly Effective in Patients with Multiple Myeloma and Overcomes the Effects of Age and Prior Therapy. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2011;17(1):141–6. doi: 10.1016/j.bbmt.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biological research for nursing. 2006;8(2):157–69. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- 46.Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98(7):2059–64. doi: 10.1182/blood.v98.7.2059. [DOI] [PubMed] [Google Scholar]
- 47.Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16(4):1547–53. doi: 10.1200/JCO.1998.16.4.1547. [DOI] [PubMed] [Google Scholar]
- 48.Dazzi C, Cariello A, Rosti G, Argnani M, Sebastiani L, Ferrari E, et al. Is there any difference in PBPC mobilization between cyclophosphamide plus G-CSF and G-CSF alone in patients with non-Hodgkin’s Lymphoma? Leukemia & lymphoma. 2000;39(3–4):301–10. doi: 10.3109/10428190009065829. [DOI] [PubMed] [Google Scholar]
- 49.Schiller G, Vescio R, Freytes C, Spitzer G, Sahebi F, Lee M, et al. Transplantation of CD34+ peripheral blood progenitor cells after high-dose chemotherapy for patients with advanced multiple myeloma. Blood. 1995;86(1):390–7. [PubMed] [Google Scholar]
- 50.Pavone V, Gaudio F, Guarini A, Perrone T, Zonno A, Curci P, et al. Mobilization of peripheral blood stem cells with high-dose cyclophosphamide or the DHAP regimen plus G-CSF in non-Hodgkin’s lymphoma. Bone marrow transplantation. 2002;29(4):285–90. doi: 10.1038/sj.bmt.1703364. [DOI] [PubMed] [Google Scholar]
- 51.Gojo I, Guo C, Sarkodee-Adoo C, Meisenberg B, Fassas A, Rapoport AP, et al. High-dose cyclophosphamide with or without etoposide for mobilization of peripheral blood progenitor cells in patients with multiple myeloma: efficacy and toxicity. Bone marrow transplantation. 2004;34(1):69–76. doi: 10.1038/sj.bmt.1704529. [DOI] [PubMed] [Google Scholar]
- 52.Lefrere F, Zohar S, Ghez D, Delarue R, Audat F, Suarez F, et al. The VAD chemotherapy regimen plus a G-CSF dose of 10 microg/kg is as effective and less toxic than high-dose cyclophosphamide plus a G-CSF dose of 5 microg/kg for progenitor cell mobilization: results from a monocentric study of 82 patients. Bone marrow transplantation. 2006;37(8):725–9. doi: 10.1038/sj.bmt.1705308. [DOI] [PubMed] [Google Scholar]
- 53.Bruns I, Steidl U, Kronenwett R, Fenk R, Graef T, Rohr UP, et al. A single dose of 6 or 12 mg of pegfilgrastim for peripheral blood progenitor cell mobilization results in similar yields of CD34+ progenitors in patients with multiple myeloma. Transfusion. 2006;46(2):180–5. doi: 10.1111/j.1537-2995.2006.00699.x. [DOI] [PubMed] [Google Scholar]


