Abstract
2,4-dinitroanisole (DNAN) is an emerging insensitive munitions compound that readily undergoes anaerobic nitro-group reduction to 2-methoxy-5-nitroaniline (MENA) and 2,4-diaminoanisole (DAAN), followed by subsequent formation of unique azo-dimers. Currently there is scarce knowledge on the ecotoxicity of DNAN (bio)transformation products. In this work, mortality, development, and behavioral effects of DNAN (bio)transformation products were assessed using zebrafish (Danio rerio) embryos. We tested individual products, MENA and DAAN, as well as dimer and trimer surrogates. As pure compounds, 3-nitro-4-methoxyaniline and 2,2’-dimethoxy-4,4’-azodianiline caused significant effects with lowest observable effect levels (LOEL) at 6.4 µM on one or two developmental endpoints, respectively. The latter had six-additional significant developmental endpoints with LOELs of 64 µM. Based on light/dark swimming behavioral tests, DAAN (640 µM) caused reduction in swimming, suggestive of neurotoxicity. No significant mortality occurred (≤ 64 µM) for any of the individual compounds. However, metabolite mixtures formed during different stages of MENA (bio)transformation in soil were characterized using high-resolution mass spectrometry in parallel with zebrafish embryo toxicity assays, which demonstrated significant mortality during the onset of azo-dimer formation. Overall the results indicate that several DNAN (bio)transformation products cause different types of toxicity to zebrafish embryos.
Keywords: DNAN, biotransformation, zebrafish, 3-nitro-4-methoxyaniline, azo-dimer
Graphical Abstract
INTRODUCTION
The nitroaromatic compound, 2,4-dinitroanisole (DNAN), is an emerging insensitive munitions compound that has gained the interest of defense industries due to its shock tolerance [1]. As the use of insensitive munitions formulations containing DNAN becomes widespread, discharges to natural environments may threaten diverse organisms vital to ecosystem function. Once released to the environment, nitro groups of DNAN can readily undergo reductive transformation to amino groups due to biological activity of microorganisms [2–4] or abiotic processes [5].
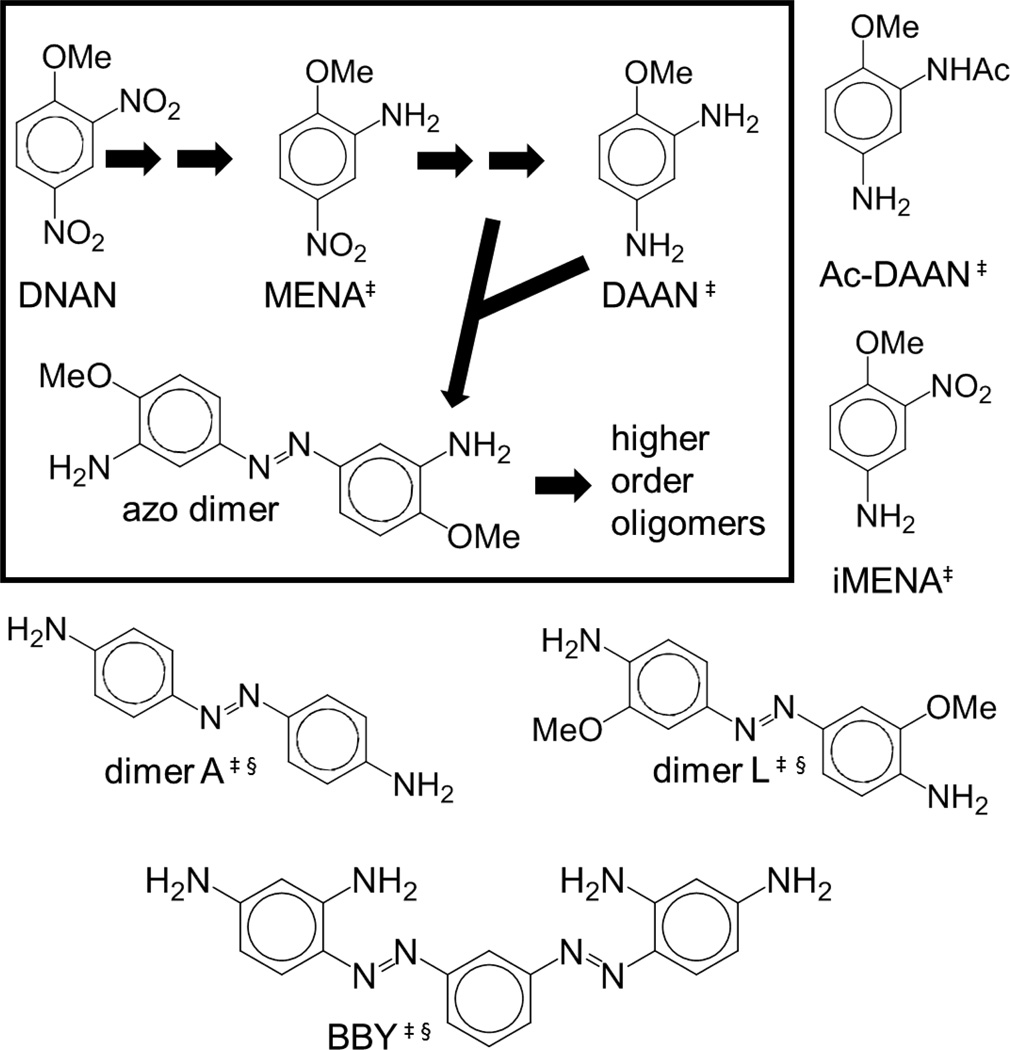
Rapid (bio)transformation of DNAN to 2-methoxy-5-nitroaniline (MENA) and 2,4-diaminoanisole (DAAN) has been reported in anaerobic environments [3, 4]. Reactive species formed during biotransformation can undergo coupling to form azo dimers (Figure 1) [2–4]. MENA and DAAN are more hydrophilic than DNAN [6], thus both metabolites might be more mobile in surface and groundwater impacted by DNAN contamination compared to the parent compound. Since DNAN (bio)transformation occurs readily in anaerobic environments, the environmental impact of DNAN pollution could be largely due to its transformation products.
Figure 1.
Main anaerobic DNAN (bio)transformation pathway (structures inside box): DNAN undergoes nitroreduction to MENA and DAAN. [4] Reactive intermediates formed during nitro-group reduction enable coupling reactions that form dimers and other oligomers by reacting with aromatic amines. [34, 35] Chemical structures of individual compounds tested based on previously identified DNAN (bio)transformation products (‡) or best available surrogates (§).
There is a lack of information regarding the ecotoxicity of the products formed during the anaerobic (bio)transformation of DNAN. However, (bio)transformation is expected to alter the toxicity effects of DNAN, as confirmed by early evidence that reductive (bio)transformation during DNAN toxicity assays decreased inhibition towards methanogenic archaea [7]. Similarly, chemical reduction of DNAN with zero-valent iron caused a decrease in the zebrafish (Danio rerio) 48-h mortality [8]. DNAN transformation has been reported in ecotoxicity studies [9–12], which may complicate toxicological assessment since the observed toxicity effects might be due to either DNAN and/or its transformation products. A few recent studies have characterized the toxic impacts of DNAN to diverse groups of microorganisms [7, 9], earthworms and plants [9, 10]. Two recent studies have evaluated the in vivo toxicity of DNAN to aquatic vertebrates [8, 12]. However, information on the developmental and chronic toxic effects associated with DNAN biotransformation products is lacking.
In the ecotoxicology field, zebrafish embryos have become widely used to monitor water quality and to aid in environmentally safe product development [13, 14]. The embryonic zebrafish model is amenable for high-throughput studies and allows evaluation of developmental and behavioral endpoints in addition to acute toxicity [15]. Early development is very similar to higher order vertebrates and its transparency allows for non-invasive and specific developmental assessment endpoints [16]. Moreover, a vast amount of transcriptomic information along an expanding library of toxicants tested can provide mechanistic insights of the toxicity effects in zebrafish [14, 17–19].
Using zebrafish as a toxicology model to test the toxicity of DNAN (bio)transformation products, the objective of this work was to evaluate developmental effects of these products (or best commercially-available surrogates). We also tested mixtures of the products formed at different stages of (bio)transformation during anaerobic incubations of soils using as a starting point the primary DNAN transformation product, MENA. Toxicological testing was supported by detailed mass spectrometry studies (using ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry, UHPLC-Q-ToF-MS) to characterize transformation product mixture profiles.
MATERIALS AND METHODS
Zebrafish embryo assays
A previously reported protocol [18] was used with slight modifications. Embryos with intact chorions were manually placed in 96-well plates containing embryo medium (EM) at 6 hours post fertilization (hpf) and exposed to individual toxicants (10 µL + 90 µL EM) or supernatant solutions of different stages of MENA (bio)transformation (50 µL + 50 µL EM). 32 embryos were exposed per concentration and toxicant, and all controls and treatments included 0.64% dimethylsulfoxide (DMSO) to aid toxicant dissolution). The plates were incubated at 28°C in the dark. At 24 and 120 hpf, development abnormality and mortality assessments were performed (endpoints shown in Table 1) [18]. Endpoint scoring and statistical analyses were performed in R software as described previously [18].
Table 1.
Zebrafish embryo toxicity endpoints assessed.
| Time | Endpoints |
|---|---|
| 24 hpf | mortality, developmental delay, spontaneous movement, notochord |
| 120 hpf | mortality, notochord, yolk sac edema, body axis, eye defect, snout, jaw, otic vesicle, pericardial edema, brain somite, pectoral fin, caudal fin, pigment, circulation, truncated body, touch response |
In addition, at 120 hpf a larval locomotor behavior assay was performed with alternating light/dark cycles in a Viewpoint Zebrabox (software version 3.0, Life Sciences, Lyon, France) [20]. Briefly, at 120 hpf, plates were inserted into the Viewpoint Zebrabox where movement was recorded during the following light/dark cycle: initial light acclimation (10 min), light (10 min), dark (5 min). Long swimming distance (> 2.5 mm min−1) was averaged across all surviving replicates at 120 hpf for a single concentration. Malformed and dead zebrafish were not considered in the locomotor assay. Endpoint scoring and statistical analyses were performed according to the protocols described [18, 20].
Individual chemicals tested
The library of compounds tested included DNAN monomer (bio)transformation products [2–4] and best available surrogates for azo dimers and trimers, since no dimer metabolites were commercially available. 2-methoxy-5-nitroaniline (MENA, CAS# 99-59-2, 98%) and 2–4 diaminoanisole (DAAN, CAS# 615-05-4, analytical standard) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 3-nitro-4-methoxyaniline (iMENA, CAS# 577-72-0, purity 97%) was obtained from Accela ChemBio Inc. (San Diego, CA, USA). N-(5-amino-2-methoxyphenyl) acetamide (Ac-DAAN, CAS# 64353-88-4, purity 95%) was acquired from ChemBridge Corporation (San Diego, CA, USA). 4,4’-azodianiline (denoted “dimer A”, CAS# 538-41-0, purity 95%) was obtained from Alfa Aesar (Ward Hill, MA, USA). 2,2’-dimethoxy-4,4’-azodianiline (denoted “dimer L”, CAS# 6364-31-4, purity >90%) was obtained from MolMall Sarl (Lonay, Switzerland). Bismarck Brown Y (m-Bis(2,4-diaminophenylazo)-benzene, denoted “BBY”, CAS# 8005-77-4, 46%) was purchased from Chem-Impex International (Wood Dale, IL, USA). Figure 1 shows chemical structures of these compounds. Solutions were prepared the same day the exposure began and diluted to contain 0.64% DMSO across all wells including controls. This concentration of DMSO is known to not cause effects in zebrafish embryo assays. Zebrafish embryos were exposed to 0–64 µM concentrations of the test compounds, with the exception of MENA and DAAN which had a range 0–640 µM. In all cases the concentrations tested were chosen to span five orders of magnitude. DNAN was not tested in this work due to the commercialization, transportation, and export control restrictions established by the International Traffic in Arms Regulations (ITAR) of the US Department of State [21].
Sampling & metabolite analysis from anaerobic (bio)transformation of MENA
Anaerobic bioassays were performed to obtain mixtures of the metabolites formed during different stages of the (bio)transformation of MENA in soil. Anaerobic tubes (Bellco Glass Inc., Vineland, NJ, USA) were filled with 10 mL of mineral medium (described in [4]) containing 18 mM phosphate buffer (pH= 7.2) and 500 µM MENA. The solution was inoculated with 100 mg of fresh Camp Navajo soil (water content 9.3%), previously characterized [22] and amended with 10 mM pyruvate as an exogenous electron donor. The tubes were flushed with He/CO2 (80/20 %), closed with t-butyl caps and aluminum seals, and subsequently incubated in the dark at 30°C in an orbital shaker at 115 rpm. Samples from the different tubes were all collected on the same day to facilitate simultaneous toxicity testing of all samples and minimize test variability. The tubes were incubated according to a staggered timeline to ensure that samples incubated for 0, 1, 6, 9, 20, and 30 days were available on the sample collection day.
At the end of the incubation period, tubes were opened in an anaerobic hood to avoid autoxidation of unstable products, and the liquid phase was centrifuged (10 min, 9,600×g). Aliquots of the supernatant were collected for immediate analysis by UHPLC-Q-ToF-MS and for toxicity testing. The latter samples were sealed under N2 gas and then frozen (−20°C) for 2 weeks until the zebrafish embryo toxicity assays were performed.
UHPLC-Q-ToF-MS and UV-VIS analyses
Liquid samples from soil (bio)transformation experiments were analyzed on an UltiMate 3000 UHPLC (Dionex, Sunnyvale, CA) coupled to a TripleTOF® 5600 quadrupole Q-ToF-MS (AB Sciex, Framingham, MA). An Acclaim RSLC Explosives E2 column (2.1 × 100 mm, 2.2 µm) (Thermo Fisher Scientific, Waltham, WA, USA) was used for chromatographic separation with an isocratic mobile phase consisting of methanol/H2O (40/60, v/v, 0.25 mL min−1, 15 min) at room temperature. MENA concentration was quantified using UHPLC coupled to a diode-array detector (DAD) at 254 nm (retention time 5 min) before measurement by UHPLC-Q-ToF-MS. The Q-ToF-MS utilized an electrospray ionization (ESI) source operated in positive mode at 450°C with a capillary setting of 5.5 kV, a declustering potential of 80 V, and curtain gas, desolvation gas, and nebulizer gas levels at 30, 35, and 35 psi, respectively, with N2. A parent compound list was created for fragmentation with a collision energy range of 15–45eV, based on previously resolved transformation products from DNAN (bio)transformation [4], as well as other detected compounds that had not been assigned a structure. Analyst TF 1.6 with PeakView 1.2.0.3 and Multiquant version 2.1 software were used to develop the parent list as well as integrate peak areas of each analyte detected.
UV-VIS spectra of the supernatant from MENA samples incubated for 1, 6, 9, 20, and 30 days were recorded (200–600 nm) in quartz cuvettes with a UV-1800 Shimadzu spectrophotometer (Columbia, MD, USA). Samples were diluted with a 50 mM phosphate buffer (pH = 7).
RESULTS AND DISCUSSION
Toxicity of model degradation products
Mortality
Freshwater fish species are reported to be as sensitive or more sensitive to nitroaromatics explosives, such as 2,4,6-trinitrotoluene (TNT), than are invertebrates and amphibians [23–26]; and the lowest 96-h LC50 has been reported for rainbow trout (Oncorhynchus mykiss) at 3.5 µM TNT [23]. DNAN has been reported to be less toxic than TNT, with a 96-h LC50 for DNAN in larval fathead minnows (Pimephales promelas) reported at 187 µM [12], whereas for TNT it was 13.6 µM [27]. The 48-h LC50 for DNAN in adult zebrafish has recently been reported as 177 µM [8].
In our work, no statistically significant mortality at 24 and 120 hpf was detected for any of the pure compounds tested (0–640 µM for MENA and DAAN; 0–64 µM for iMENA, Ac-DAAN, dimer A, dimer L, and BBY). Mortality charts for each compound tested can be found in Supplemental Data (Figures S-1 through S-3). In this study, which used zebrafish embryos with intact chorions, no adverse lethal effect were detected for MENA concentrations up to 640 µM. However, in a previous study with dechorionated zebrafish embryos on compounds from the U.S. EPA ToxCast phase 1 and 2 lists, which included MENA, the 120-hpf mortality lowest observable effect level (LOEL) was reported at 64 µM [18]. Since the chorion is the primary exposure route before hatching [15], this discrepancy suggests that the presence of the chorion could be limiting the diffusion of aromatic amines like MENA. Similar to the monomeric compounds tested in this work, the azo-dimers and trimer (BBY) evaluated did not result in zebrafish mortality at concentrations up to 64 µM. Azo dye dimers and trimers have been tested in aquatic ecotoxicity models, including an extensive dye survey on fathead minnows (P. promelas) [28], and more recently on guppy fish (Poecilia reticulata) [29], and the Western clawed frog (Silurana tropicalis) [30]. In contrast with the lack of lethal effects detected in our study, the 96-h LC50 value reported in guppy fish for Methyl Red (CAS# 493-52-7), an azo-dimer with N-methyl, amino, and carboxylic acid substituents, was 89 µM [29], and for Basic Brown 4 (CAS# 5421-66-9), an azo trimer with amino and methyl substituents, in fathead minnows it was 12.1 µM.
Overall, the low mortality counts obtained with the pure compounds tested suggest that the individual transformation products/surrogates evaluated in this study pose little acute toxicity risk (≤ 64 µM). This argument is strengthened by the observed decrease in toxicity of TNT on fathead minnows, as TNT was reduced to dinitroaniline products in the assay [23]. While mortality effects were not detected in the concentration ranges tested in this work, other toxicity effects may occur at sublethal concentrations, such as with reproductive [23, 25] and developmental [31] endpoints.
Developmental toxicity
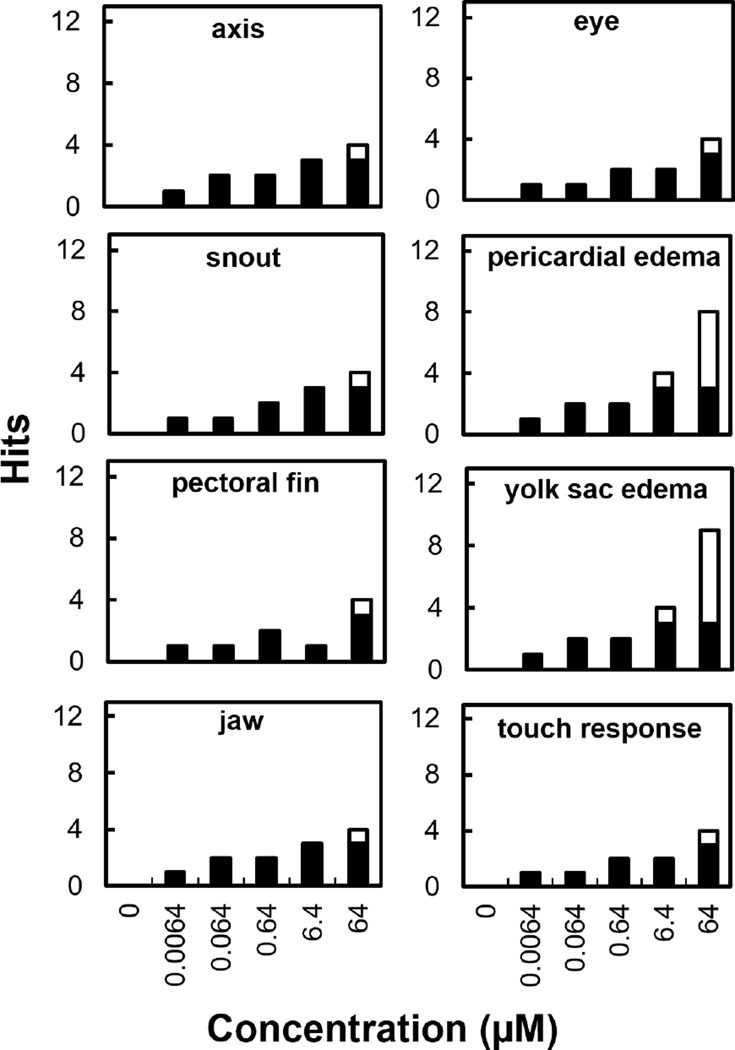
Detailed developmental endpoint scoring for all the chemicals tested in this work is shown in the Supplemental Data, in Figures S-4 through S-10. While most of the compounds tested did not cause developmental abnormalities, dimer L and iMENA caused significant malformations in zebrafish embryos (Table 2). iMENA had a developmental LOEL of 6.4 µM based on yolk sac edema formation (Figure S-5), but no other abnormalities were detected. The rest of monomer compounds did not show developmental activity. Developmental toxicity studies with the monomeric compounds tested here have not been previously reported. However, some aromatic amines, such as aniline have been shown to cause developmental toxicity in African clawed frog (Xenopus laevis) embryos, albeit at much higher concentrations, 3.9 mM [32]. Of the dimers and trimer tested, only dimer L caused significant developmental abnormalities in the embryos (Figure S-9). Dimer L caused a significant occurrence of yolk sac and pericardial edemas (LOEL = 6.4 µM), and several malformations in six other endpoints at 64 µM (Figure 2). A photograph illustrating some of these abnormalities is shown in Figure 3. While BBY and dimer A did not show any developmental toxicity in the tested range, in other aquatic species, BBY has been reported to cause malformations in Western clawed frogs at 2.4 mM. [30].
Table 2.
Developmental Lowest Observable Effect Levels (LOELs) and endpoints with significant morbidity.
| Chemical compound (identifier) | Surrogate for | Developmental LOEL (µM) |
Active Endpoints (µM) |
|---|---|---|---|
| 2-methoxy-5-nitroaniline (MENA) | N/A | >640 | N.D. |
| 3-nitro-4methoxyaniline (iMENA) | N/A | 6.4 | yolk sac edema (6.4) |
| 2,4-diaminoanisole (DAAN) | N/A | >640 | N.D. |
| N-(5-amino-2-methoxyphenyl) acetamide (Ac-DAAN) | N/A | >64 | N.D. |
| 4,4'-azodianiline (dimer A) | azo dimer | >64 | N.D. |
| 2,2’-dimethoxy-4,4’-azodianiline (dimer L) | azo dimer | 6.4 | yolk sac and pericardial edemas (6.4); axis, eye, snout, jaw, pectoral fin, touch response (64) |
| Bismarck Brown Y (BBY) | azo trimer | >64 | N.D. |
N/A = Not applicable; N.D. = None detected
Figure 2.
Developmental endpoints that caused significant malformations in the zebrafish embryo assay for dimer L. White bars indicate hits above the statistically significant threshold (p ≤ 0.05). The rest of the developmental endpoints did not show significant activity.
Figure 3.
Representative visual comparison between 120 hpf zebrafish embryos: Control (A), 64 µM dimer L (B). The embryo exposed to dimer L showed developmental abnormalities and visible dimer L uptake (coloration).
Locomotor behavior assay
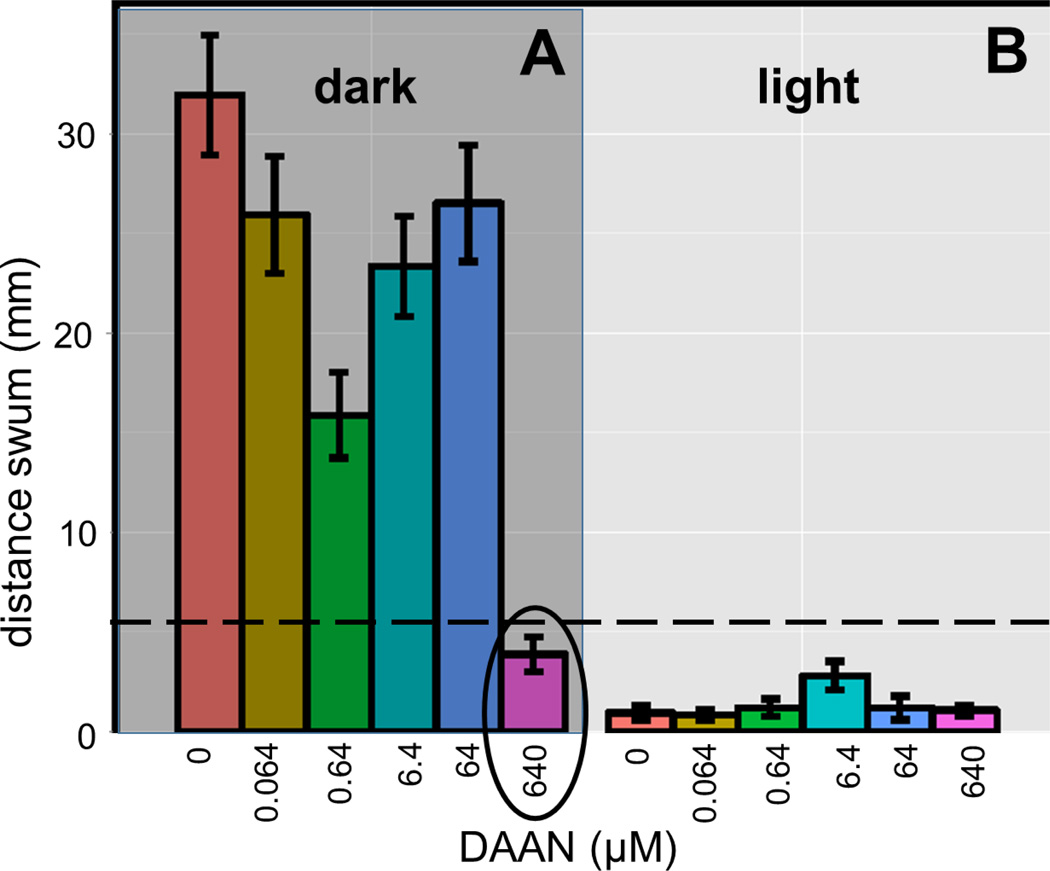
The impact of the various model compounds on zebrafish swimming locomotor behavior was also tested at 120 hpf. Zebrafish have been reported to be more static in presence of light compared to dark periods, and comparing the difference in light/dark cycles can indicate changes in behavior due to exposure to toxicants during development [20]. In this study, long distance swimming motion ranged from 18 to 30 mm in the dark, while it was below 5 mm in light (Figure 4). There were no detectable effects in locomotion for any of the chemicals tested, except for DAAN. While there were some minor variations in the distance swum in the dark periods when considering most compounds, the average movement in zebrafish that had been exposed to 640 µM DAAN (4 mm), was eight-fold less than the toxicant–free control (32 mm). In essence 640 µM DAAN caused the motion in the dark period to be reduced to the level normally observed by the embryos in the light periods. This suggests that 640 µM DAAN impaired response to light/dark cycles. Since there were no developmental active endpoints for DAAN (Figure S-6), the lack of response to light/dark periods is not associated with swimming impairment due to malformations. Therefore, this suggests that exposure to 640 µM DAAN could have neurotoxicity effects. To the best of our knowledge, this is the first work to study potential locomotor effects caused by a nitroaromatic, an aromatic amine, and an azo-dimer exposure in zebrafish during the developmental stage.
Figure 4.
Average long distance swum recorded in larval locomotor response Viewpoint assay for 120 hpf zebrafish larvae exposed to DAAN (0–640 µM) in dark (Panel A) and light (Panel B) stages.
Toxicity evaluation of MENA (bio)transformation products in anaerobic soil microcosms
Product characterization and semi-quantitation
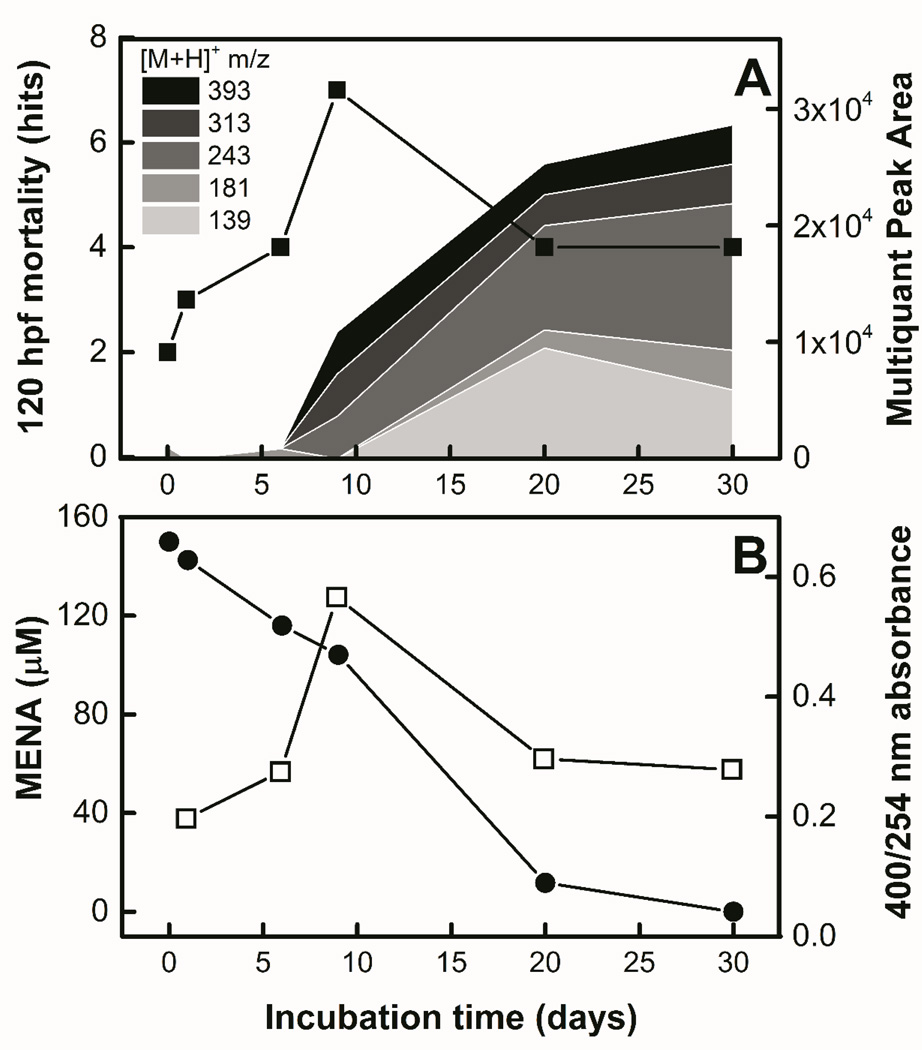
Based on UHPLC-Q-ToF-MS analyses, six parent ions were selected for semi-quantitation according to their chromatogram peak area abundance (> 1000) (Table 3). From these compounds, three had been reported previously as monomer products formed during DNAN (bio)transformation in anaerobic conditions: DAAN and Ac-DAAN [4]. In this work, we report a new transformation product, 3-amino-3’-nitro-azobenzene (m/z 243, Figure S-11), as well as two parent ions that have not been assigned chemical structures [M+H]+ m/z 313.1343 (putative molecular formula C16H16N4O3, Figure S-12) and 393.0836 (putative molecular formula C19H12N4O6, Figure S-13). Based on these formulae, these ions could represent a dimer and a trimer, respectively. Transformation products were quantified using parent ions (m/z [M+H]+), or daughter ion (m/z [M+H-R]+) transitions when the daughter yielded a stronger ionization signal than the precursor (Table 3). While standards were available for DAAN and Ac-DAAN, the rest of the analytes were not commercially available, difficult to synthesize, and potentially not stable in air. For consistency, all analytes are reported in peak area units based on UHPLC-Q-ToF-MS, which cannot be unequivocally translated to concentration because of potential variation in ionization efficiencies (Figure 5A). MENA was not added to the selected ion list since it ionized several orders of magnitude more strongly than the rest of the analytes. Therefore, its concentration during the (bio)transformation is reported in µM as quantified using UHPLC-DAD (Figure 5B).
Table 3.
Selected parent/daughter ion list for semi-quantitative LC-MS determination of soluble products formed during anaerobic MENA soil (bio)transformation. Daughter ions shown indicate they were used to determine the abundance of the products.
| Calculated | Measured | ||||
|---|---|---|---|---|---|
| Chemical compound or isdentifier; Molecular formula | CAS # | Parent [M+H]+ |
Parent [M+H]+ |
Daughter [M+H-R]+ |
Retention time (min) |
| 2,4-diaminoanisole (DAAN); C7H10N2O | 615-05-4 | 139.0866 | 139.0866 | N/A | 2.86 |
| N-(5-amino-2-methoxyphenyl) acetamide (Ac-DAAN); C9H12N2O2 | 64353-88-4 | 181.0972 | 181.0972 | N/A | 2.36 |
| 3-amino-3’-nitro-azobenzene (m/z 243)§; C12H10N4O2 | 61390-99-6 | 243.0877 | 243.0870 | N/A | 2.3 |
| (m/z 313)*§; C16H16N4O3 | N/A | 313.1295 | 313.1312 | 250.8794 | 1.60 |
| (m/z 393)*§; C19H12N4O6 | N/A | 393.0830 | 393.0836 | 276.0833 | 1.59 |
N/A = Not applicable.
These ions detected have not been assigned a chemical structure.
These compounds are reported for the first time as (bio)transformation products.
Figure 5.
Characterization of products formed during MENA (bio)transformation coupled to zebrafish toxicity. Panel A: Temporal semi-quantitation of transformation products ([M+H]+ m/z = 139, 181, 243, 313, 393) shown with stacked areas and increasing m/z shown with darker shades and zebrafish mortality assessed at 120 hpf (■). Mortality statistical significance (p < 0.05) is above 4 hits (n = 32). Panel B: MENA concentration (●) and 400 nm/ 254 nm absorbance index (□) during MENA (bio)transformation.
From initial time to six days of (bio)transformation, trace amounts of all of the analytes were detected, but 23% of MENA had already been removed, indicating more transformation products not shown in Table 3 were possibly formed. From day 6 to 9 the formation of oligomers (mainly m/z 243, 313, 393) was visible, followed by accumulation of DAAN and Ac-DAAN after day 10. The concentration of all of the analytes increased until day 30, with exception of DAAN, which decreased by 40% from days 20–30.
In order to consider transformation products that might be difficult to separate chromatographically or ionize in the Q-ToF-MS, UV-VIS spectra were also recorded along the course of the (bio)transformation of MENA (Figure S-14). Since oligomers were detected in Q-ToF-MS, the spectral data are summarized as an oligomer index, based on the ratio of absorbance at 400 to 254 nm (Figure 5B), the former wavelength being chosen to quantify polymers [33] that have visible absorbance (e.g. azo dyes), and the latter a common wavelength for aromaticity. The 400/254 nm ratio increased from days 1–6, reached a maximum at 9 days of incubation, and then decreased until day 30. The initial increase might be due to the onset of oligomer formation as evidenced by Q-ToF-MS data, whereas the subsequent decrease following day 9 indicates precipitation of larger insoluble oligomers out of solution, even if soluble dimers such as m/z 243 continue being formed.
Zebrafish mortality and absence of developmental abnormalities
Zebrafish embryo mortality assessed at 120 hpf was statistically significant only for the sample taken at 9 days of (bio)transformation (Figure 5A), coinciding with the maxima recorded for the 400/254 nm absorbance oligomer index (Figure 5B) and slightly after the onset of oligomer formation. This suggests that the early formation of azo dimers is responsible for an increase in zebrafish embryo mortality. Oligomerization occurs when reactive nitroso-intermediates react with amines to cause coupling reactions [34, 35]. Thus, mortality is potentially associated with the reactive intermediates.
There were no active developmental endpoints in any of the samples collected during MENA biotransformation. The absence of developmental abnormalities in the assay could be due to several factors, which may contribute synergistically. Firstly, the compound mixtures were produced at very low concentrations and below any LOEL. Particularly for dimers and trimer products, for which the maximum molar concentration would be initial parent molarity (MENA in this case) divided by the number of monomer units (aromatic rings). The most abundant non-monomer product, according to ionization signal detected in the mixtures, was an azo-dimer metabolite observed at m/z 243. However, since no developmental abnormalities in the embryos were observed, either the compound was present below the LOEL for the surrogate dimer tested, dimer L, or it had an important difference in biological activity compared to dimer L that made m/z 243 less toxic towards developmental endpoints in the MENA (bio)transformation product mixtures. Secondly, fewer oligomers may be bioavailable to the embryos if extensive polymerization occurs that result in oligomers precipitating from solution or oligomer incorporation into the soil humus [36]. These mechanisms are supported by the overall decrease of aromaticity of the aqueous phase as evidenced by the decline in 254 nm absorbance during the course of the (bio)transformation (Figure S-14). Aromatic amines and azo polymers from similar nitroaromatic compounds, such as DNAN and TNT, are known to bind irreversibly to soil humic substances [6, 36]. All of these factors could have contributed to an overall lower amount of bioavailable oligomers, and in consequence, no developmental abnormalities in the embryos.
Toxicity implications of DNAN biotransformation
The results of this study demonstrate that intermediates of DNAN biotransformation can cause detectable developmental and behavioral toxicity endpoints in zebrafish embryos. Most concerning was the high level of developmental toxicity caused by a surrogate azo-dimer intermediate (dimer L) and iMENA (both at 6.4 µM) as well as evidence of locomotor toxicity caused by DAAN at higher concentrations. Our work approach coupled transformation product identification with toxicological testing to determine the environmental impact of organic pollutants as they undergo transformation in natural systems. The findings from our study will help to advance an understanding of the potential adverse impacts of DNAN biotransformation products in natural systems to aquatic species and vertebrates in general.
Supplementary Material
Acknowledgments
This study was partly supported by the Strategic Environmental Research and Development Program (SERDP) project ER-2221. CIO was supported in part by fellowships from the Mexican National Council for Science and Technology (CONACyT), the University of Arizona Superfund Program Training Core (National Institute of Environment and Health Sciences grant P42 ES04940), and the Water Sustainability Program. His research stay at the Tanguay lab was supported by a NIEHS KC Donnelly Externship 2014 award. Analyses in the Arizona Laboratory for Emerging Contaminants (ALEC) were supported by NSF CBET 0722579, AB Sciex, and additional funding from the University of Arizona. We would like to thank the Sinnhuber Research Laboratory/ Tanguay lab members and screening team (Greg Gonnerman).
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Davies PJ, Provatas A, Defence S Technology Organisation. Weapons Systems D. Characterisation of 2,4-dinitroanisole an ingredient for use in low sensitivity melt cast formulations. Edinburgh, Australia: Science Defence Technology Organization; 2006. [Google Scholar]
- 2.Perreault NN, Manno D, Halasz A, Thiboutot S, Ampleman G, Hawari J. Aerobic biotransformation of 2, 4-dinitroanisole in soil and soil Bacillus sp. Biodegradation. 2012;23:287–295. doi: 10.1007/s10532-011-9508-7. [DOI] [PubMed] [Google Scholar]
- 3.Platten WE, Bailey D, Suidan MT, Maloney SW. Biological transformation pathways of 2,4-dinitroanisole and N-methylparanitroaniline in anaerobic fluidized-bed bioreactors. Chemosphere. 2010;81:1131–1136. doi: 10.1016/j.chemosphere.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Olivares C, Liang J, Abrell L, Sierra-Alvarez R, Field JA. Pathways of reductive 2,4-dinitroanisole (DNAN) biotransformation in sludge. Biotechnol Bioeng. 2013;110:1595–1604. doi: 10.1002/bit.24820. [DOI] [PubMed] [Google Scholar]
- 5.Niedzwiecka JB, Finneran KT, Arnett CM. Biological and chemical mechanisms for 2,4-dinitroanisole (DNAN) degradation; Graduate Research and Discovery Symposium (GRADS) Paper 64; 2013. http://tigerprintsclemsonedu/grads_symposium/64. [Google Scholar]
- 6.Hawari J, Monteil-Rivera F, Perreault NN, Halasz A, Paquet L, Radovic-Hrapovic Z, Deschamps S, Thiboutot S, Ampleman G. Environmental fate of 2,4-dinitroanisole (DNAN) and its reduced products. Chemosphere. 2015;119:16–23. doi: 10.1016/j.chemosphere.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Olivares C, Field JA, Sierra-Alvarez R. Microbial toxicity of the insensitive munitions compound, 2,4-dinitroanisole (DNAN), and its aromatic amine metabolites. J Hazard Mater. 2013;262:281–287. doi: 10.1016/j.jhazmat.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Ou C, Zhang S, Liu J, Shen J, Liu Y, Sun X, Li J, Wang L. Removal of multi-substituted nitroaromatic pollutants by zero valent iron: a comparison of performance, kinetics, toxicity and mechanisms. Phys Chem. 2015;17:22072–22078. doi: 10.1039/c5cp02518d. [DOI] [PubMed] [Google Scholar]
- 9.Dodard SG, Sarrazin M, Hawari J, Paquet L, Ampleman G, Thiboutot S, Sunahara GI. Ecotoxicological assessment of a high energetic and insensitive munitions compound: 2,4-dinitroanisole (DNAN) J Hazard Mater. 2013;262:143–150. doi: 10.1016/j.jhazmat.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Dumitras-Hutanu CA, Pui A, Jurcoane S, Rusu E, Drochioiu G. Biological effect and the toxicity mechanisms of some dinitrophenyl ethers. Rom Biotech Lett. 2009;14:4893–4899. [Google Scholar]
- 11.Lent EM, Crouse LCB, Hanna T, Wallace SM. U.S. Army Public Health Command. Toxicology Portfolio (MCHB-IP-TEP) MD: Aberdeen Proving Ground; 2012. The subchronic oral toxicity of 2,4-dinitroanisole (DNAN) in rats. [Google Scholar]
- 12.Kennedy AJ, Laird JG, Lounds C, Gong P, Barker ND, Brasfield SM, Russell AL, Johnson MS. Inter- and intraspecies chemical sensitivity: A case study using 2,4-dinitroanisole. Environ Toxicol Chem. 2015;34:402–411. doi: 10.1002/etc.2819. [DOI] [PubMed] [Google Scholar]
- 13.Lin HC, Hwang PP. Acute and chronic effects of gallium chloride (GaCl3) on tilapia (Oreochromis mossambicus) larvae. B Environ Contam Tox. 1998;60:931–935. doi: 10.1007/s001289900717. [DOI] [PubMed] [Google Scholar]
- 14.Kim K-T, Tanguay RL. Integrating zebrafish toxicology and nanoscience for safer product development. Green Chem. 2013;15:872–880. doi: 10.1039/C3GC36806H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholz S, Fischer S, Gündel U, Küster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment—applications beyond acute toxicity testing. Environ Sci Pollut R. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- 16.Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. In: Gautier J-C, editor. Drug Safety Evaluation. Vol 691-Methods in Molecular Biology. Humana Press; 2011. pp. 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai Y-J, Jia Y-F, Chen N, Bian W-P, Li Q-K, Ma Y-B, Chen Y-L, Pei D-S. Zebrafish as a model system to study toxicology. Environ Toxicol Chem. 2014;33:11–17. doi: 10.1002/etc.2406. [DOI] [PubMed] [Google Scholar]
- 18.Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci. 2014;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL, Begum S, Lloyd C, Lanz C, Raddatz G, Schuster SC. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong L, Saili KS, Miller JM, Hutchison JE, Tanguay RL. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp Biochem Phys C. 2012;155:269–274. doi: 10.1016/j.cbpc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amendment to the International Traffic in Arms Regulations: Third Rule Implementing Export Control Reform, 79 Fed. Reg. 34 CFR pts. 121, 123, 124, 125. Vol 79 Fed. Reg. 34. 2014:34–47. [Google Scholar]
- 22.Krzmarzick MJ, Khatiwada R, Olivares CI, Abrell L, Sierra-Alvarez R, Chorover J, Field JA. Biotransformation and degradation of the insensitive munitions compound, 3-nitro-1,2,4-triazol-5-one, by soil bacterial communities. Environ Sci Technol. 2015;49:5681–5688. doi: 10.1021/acs.est.5b00511. [DOI] [PubMed] [Google Scholar]
- 23.Lotufo GR, Rosen G, Wild W, Carton G. US Army Corps of Engineers. Engineer Research and Development Center. Vicksburg, MS: Environmental Laboratory; 2013. Summary review of the aquatic toxicology of munitions constituents. [Google Scholar]
- 24.Talmage SS, Opresko DM, Maxwell CJ, Welsh CJE, Cretella FM, Reno PH, Daniel FB. Nitroaromatic munition compounds: Environmental effects and screening values. Rev Environ Contam Toxicol. 1999:1–156. doi: 10.1007/978-1-4757-6427-7_1. [DOI] [PubMed] [Google Scholar]
- 25.Nipper M, Carr RS, Lotufo GR. Aquatic toxicology of explosives. In: Sunhara GI, Lotufo GR, Kuperman RG, J H, editors. Ecotoxicology of explosives. Boca Raton, FL: CRC Press; 2009. pp. 77–115. [Google Scholar]
- 26.Robidoux PY, Hawari J, Thiboutot S, Sunahara GI. Ecotoxicological risk assessment of an explosives-contaminated site. In: Sunhara GI, Renoux AY, Thellen C, Gaudet CL, Pilon A, editors. Environmental analysis of contaminated sites. West Sussex, UK: John Wiley & Sons; 2002. pp. 335–359. [Google Scholar]
- 27.Pearson JG, Glennon JP, Barkley JJ, Highfill JW. An approach to the toxicological evaluation of a complex industrial wastewater. Aquat Toxicol ASTM STP 667. 1979:284. [Google Scholar]
- 28.Little LW, Lamb JCI. UNC Wastewater Research Center, Department of Environmental Sciences and Engineering. School of Publich Health. Chapel Hill, NC: University of North Carolina; 1972. Acute toxicity of 46 selected dyes to the fathead minnow, Pimpehales promelas. Final Report to the American Dye Manufacturers Institute, Inc. [Google Scholar]
- 29.Sharma S, Upreti N, Sharma KP. Monitoring toxicity of an azo dye methyl red and a heavy metal Cu, using plant and animal bioassays. Toxicol Environ Chem. 2009;91:109–120. [Google Scholar]
- 30.Soriano J, Mathieu-Denoncourt J, Norman G, de Solla S, Langlois V. Toxicity of the azo dyes Acid Red 97 and Bismarck Brown Y to Western clawed frog (Silurana tropicalis) Environ Sci Pollut Res. 2014;21:3582–3591. doi: 10.1007/s11356-013-2323-4. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu-Denoncourt J, Martyniuk CJ, de Solla SR, Balakrishnan VK, Langlois VS. Sediment contaminated with the azo dye Disperse Yellow 7 alters cellular stress and androgen-related transcription in Silurana tropicalis larvae. Environ Sci Technol. 2014;48:2952–2961. doi: 10.1021/es500263x. [DOI] [PubMed] [Google Scholar]
- 32.Davis KR, Schultz TW, Dumont JN. Toxic and teratogenic effects of selected aromatic amines on embryos of the amphibian Xenopus laevis. Arch Environ Contam Toxicol Archives of Environmental Contamination and Toxicology. 1981;10:371–391. doi: 10.1007/BF01055639. [DOI] [PubMed] [Google Scholar]
- 33.Koch M, Yediler A, Lienert D, Insel G, Kettrup A. Ozonation of hydrolyzed azo dye reactive yellow 84 (CI) Chemosphere. 2002;46:109–113. doi: 10.1016/s0045-6535(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 34.Moglie Y, Vitale C, Radivoy G. Synthesis of azo compounds by nanosized iron-promoted reductive coupling of aromatic nitro compounds. Tetrahedron Lett. 2008;49:1828–1831. [Google Scholar]
- 35.Zhao R, Tan C, Xie Y, Gao C, Liu H, Jiang Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011;52:3805–3809. [Google Scholar]
- 36.Drzyzga O, Bruns-Nagel D, Gorontzy T, Blotevogel KH, von Low E. Anaerobic incorporation of the radiolabeled explosive TNT and metabolites into the organic soil matrix of contaminated soil after different treatment procedures. Chemosphere. 1999;38:2081–2095. doi: 10.1016/s0045-6535(98)00426-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.