Abstract
Objective
To describe pediatric patients with convulsive refractory status epilepticus (RSE) in whom there is intention-to-use an intravenous anesthetic for seizure control.
Design
Two-year prospective observational study evaluating patients (age range one month to 21 years) with RSE not responding to two antiepileptic drug classes and treated with continuous infusion of anesthetic agent.
Setting
Nine pediatric hospitals in the United States.
Patients
In a cohort of 111 patients with RSE (median age 3.7 years, 50% male), 54 (49%) underwent continuous infusion of anesthetic treatment.
Main Results
The median (interquartile range, IQR) intensive care unit length-of-stay was 10 (3–20) days. Up to four ‘cycles’ of serial anesthetic therapy were used and seizure termination was achieved in 94% by the second cycle. Seizure duration in controlled patients was 5.9 (1.9–34) hours for the first cycle, and longer when a second cycle was required (30 [4,−120] hours, p=0.048). Midazolam was the most frequent first-line anesthetic agent (78%); pentobarbital was the most frequently used second-line agent after midazolam failure (82%). An electroencephalographic endpoint was used in over half of the patients; higher midazolam dosing was used with a burst suppression endpoint. In midazolam non-responders, transition to a second agent occurred after a median of one day. Most patients (94%) experienced seizure termination with these two therapies.
Conclusions
Midazolam and pentobarbital remains the mainstay of continuous infusion therapy for RSE in the pediatric patient. The majority of patients experience seizure termination within a median of 30 hours. These data have implications for the design and feasibility of future intervention trials. That is, testing a new anesthetic anticonvulsant after failure of both midazolam and pentobarbital is unlikely to be feasible in a pediatric study, whereas a decision to test an alternative to pentobarbital, after midazolam failure, may be possible in a multicenter multinational study.
Keywords: Anesthetic treatment, Critical Care, All Pediatric, EEG, Status Epilepticus
Introduction
The initial treatment of convulsive status epilepticus (SE) consists of one or two doses of a benzodiazepine followed by fosphenytoin or phenobarbital, which are frequently referred to as first- and second-tier therapy, respectively.[1–3] While there is no consensus definition of refractory status epilepticus (RSE),[4] we have defined it as continued seizure activity despite administration of two antiepileptic drugs (AEDs).[5] RSE accounts for up to 4% of all admissions to the pediatric intensive care unit (PICU) and occurs in 10 – 25% of pediatric patients presenting with acute seizures.[6–10]
After failing to stop seizures with first- and second-tier AEDs, most SE protocols recommend treatment escalation to continuous infusions with either midazolam or pentobarbital.[1–3,5] In a recent systematic review on pediatric RSE (with data largely from retrospective case series) a hierarchy of interventions was noted: midazolam as the primary agent for RSE; progression to barbiturate anesthesia when midazolam fails; and, subsequently, isoflurane, ketamine or hypothermia when other therapies have failed.[11] In this multicenter, prospective observational cohort study we aim to comprehensively describe the intention-to-use an anesthetic strategy in children with RSE, including the medications used, endpoints, and outcomes of these therapies. That is, a characterization of what happens once a clinician has decided to use an anesthetic agent to control an ongoing episode of RSE.
Methods
The Institutional Review Board for Human Subject Study at each center approved the study. Written informed consent was obtained from parents or guardians.
Study design
The pediatric Status Epilepticus Research Group (pSERG) performed this prospective observational study of health care delivery at nine tertiary pediatric hospitals in the United States (US).[5] The purpose of the pSERG network is to describe and subsequently optimize the management of RSE in children. The eligibility criteria for the current study cohort included: 1) hospital admission between 1st June 2011 and 30th June 2013; 2) age from 1 month to 21 years; 3) convulsive seizures at onset; and 4) failure of two or more AEDs of different drug classes to terminate seizures or the use of a continuous infusion of AEDs for seizure termination. The exclusion criteria were: 1) non-convulsive SE identified on electroencephalogram (EEG) without convulsive seizures at onset; and 2) SE with motor manifestations limited to infrequent myoclonic jerks.
If more than one episode of RSE occurred during the study period, only the first episode was included.
Data collection and assessments
Data were collected with a standardized data acquisition tool and entered into an electronic database hosted by Cincinnati Children’s Hospital Medical Center. Additional elements of the consortium and study design have been reported elsewhere.[5]
Variables related to anesthetic agents (i.e., midazolam and pentobarbital) included: bolus dose, initial infusion rate, effective dose at time of seizure termination or change of infusion to a new medication, and duration of infusion. The endpoints for seizure termination and management during the infusion were determined by the local clinicians caring for the child, and included: 1) termination of convulsive seizures, 2) termination of EEG seizures, and 3) achievement of burst suppression.
Each center followed its own policy and procedure for RSE management, as there were no common treatment pathways across centers. For the purpose of this study we identified patients within our whole cohort with RSE in whom clinicians had made a decision to start treatment with a continuous infusion of an anesthetic agent, i.e., intention-to-use. Such “intention-to-use” does not imply that anesthesia had to be induced. Rather, it is entirely possible that some clinicians may choose to administer the minimum bolus dose and continuous infusion rate necessary to produce the endpoint that they are concerned about, e.g., seizure control. Other clinicians may choose to administer the minimum dose necessary to avoid hypotension. In this study, a “cycle” of intention-to-use anesthetic treatment was defined as use of a single agent (i.e., cycle one describes the initial agent used, cycle two the next agent used, and so on). There was no pre-specified pathway for when a clinician should persist or abandon a particular agent and proceed to the next treatment cycle.
Statistical analyses
Demographic and clinical characteristics were summarized with descriptive statistics. Since these data were non-parametric, by inspection, the information was summarized using median and interquartile range (IQR). Differences between groups were performed using non-parametric analyses (Kruskal-Wallis test and Wilcoxon rank-sum test) for continuous variables. Proportions are described as percentages; the exact 95% confidence interval (95% CI) of percentages for N = 2–100 is used when describing success or failure rates. Differences in two independent proportions were assessed using the chi-squared test and the Hypothesis Test for Proportions. All analyses were performed using JMP™ 9.02 Statistical Discovery software (SAS Institute Inc., Cary, NC).
Results
Out of a cohort of 111 patients with RSE (median age 3.7 years, 50% male), 54 (49%) patients progressed to receiving an anesthetic agent by continuous infusion for seizure termination, and 57 did not receive such treatment. Table 1 shows the demographic features of these subgroups. There was no statistically significant difference in any of the variables between patients who received and patients who did not receive continuous infusion. In the symptomatic causes of seizure, there was a trend in difference between the subgroups: febrile or fever-associated SE occurred in 5 of 30 patients going on to treatment with continuous infusion, versus 13 of 38 patients with the same etiology but not going on to receive continuous infusion (trend to significance with 1.6 standard deviations difference, hypothesis test for proportions).
Table 1.
Demographic characteristics of the RSE cohort.
| Intention-to-use Continuous infusion (CI) |
No use of CI | |
|---|---|---|
| n = 54 | n = 57 | |
|
| ||
| Age, Median (IQR) | 4.5 (1.8 – 10.2) years | 2.4 (1.1 – 7.1) yrs |
|
| ||
| Male sex, n (%) | 28 (52%) | 28 (50%) |
|
| ||
| Race, n (%) | ||
| • White | 37 (69%) | 33 (58%) |
| • Black / African American | 12 (22%) | 11 (19%) |
| • Asian | 1 (2%) | 5 (9%) |
| • Unknown | 4 (7%) | 8 (14%) |
|
| ||
| Ethnicity, n (%) | ||
| • Not Hispanic / Latino | 38 (70%) | 42 (74%) |
| • Hispanic / Latino | 13 (24%) | 10 (17%) |
| • Unknown | 3 (6%) | 5 (9%) |
|
| ||
| Baseline condition, n* | ||
| • None | 21 | 19 |
| • History of FC | 6 | 6 |
| • Previous SE | 11 | 7 |
| • Cerebral palsy | 8 | 6 |
| • Developmental delay | 25 | 26 |
| • Epilepsy | 21 | 25 |
|
| ||
| Proximate cause, n (%) | ||
| • Symptomatic | 30 (56%) | 38 (67%) |
| • Genetic / Metabolic | 10 (19%) | 5 (9%) |
| • Unknown / Other | 14 (25%) | 14 (24%) |
Key: FC, febrile convulsion; IQR, interquartile range; n*, the same patient may present with more than one condition; RSE, refractory status epilepticus; SE, status epilepticus
In the whole cohort of 111 cases the length-of-stay in the PICU was 3 (IQR 2, 10) days. The stay was longer in those undergoing continuous infusion of anesthetic agent for seizure control: 10 (IQR 3, 20) days vs 2 (IQR 1.8, 4) days, P <0.0001. Eighty of 111 cases (72%) underwent endotracheal (ETT) intubation with supportive mechanical ventilation; there was a greater proportion in the continuous infusion subgroup, 83% vs 61%, P <0.01. Furthermore, in those who had ETT intubated, the duration of intubation was longer in those receiving continuous infusion: 6 (IQR 2.3, 13) days vs 2 (IQR 0.5, 2.8) days. Vasoactive drugs were used in 20 of 111 (18%) patients, and those on continuous infusion therapy were more often treated with vasopressors: 15/54 (28%) vs 5/57 (9%), P <0.01). Last, the mortality rate was 3.6% (4/111), with no significant difference between the subgroups (3/54 vs 1/57 in the continuous infusion and non-continuous infusion subgroups, respectively).
Use of Anesthetic Agents
Anesthetic agents initially used to stop RSE included midazolam (42/54), propofol (4/54), pentobarbital (2/54) and other agents (ketamine, valproate or isoflurane) in 6/54. By the time of initiating such drug administration it should be noted that a median of 5 (IQR 4, 7) doses of AEDs had been given before the infusion started. This number of AED doses was similar to that used in the other 57 patients with RSE (i.e., those not receiving continuous infusions) who received 5 (IQR 4, 6) doses of AEDs. This finding implies, in general, that in the subgroup receiving a continuous infusion for RSE, therapeutic trial of bolus AEDs had been tested to the same extent as those not receiving continuous infusion; the difference being, lack of seizure control in those undergoing infusion.
Figure 1 illustrates the initial endpoint for seizure termination used in the 54 patients. The drug administration endpoints were clinical seizure termination in 26 patients, of which 21 (81% [95% CI: 61% – 93%]) achieved control by 4.2 (IQR 2.2, 52.3) hours. Among the other 28 patients, an EEG-based endpoint was used, including: EEG seizure termination in 18 patients, of which 14 (78% [95% CI: 52% – 94%]) achieved control by 22 (IQR 3.2, 39) hours; and, burst suppression in 10 patients, of which 4 (40% [95% CI: 12% – 74%]) achieved this target by 81.5 (IQR 16.5, 408) hours. The proportions where the target had been achieved (i.e., 21/26, 14/18, and 4/10 for clinical seizure, EEG-seizure, and burst suppression endpoints, respectively) depict the relative ease of clinical seizure termination compared to achieving EEG-based endpoint targets (chi-squared for all three proportions = 6.4, 2 degrees of freedom, p = 0.041). The durations from SE onset to seizure termination were significantly shorter for the clinical endpoint compared to the burst suppression endpoint (p = 0.008). There was a trend in significance for timing to EEG seizure termination compared to burst suppression (p = 0.053). There was no difference in duration for the comparison between clinical seizure termination and EEG seizure termination.
Figure 1. Cycles of anesthetic therapy for termination of RSE.
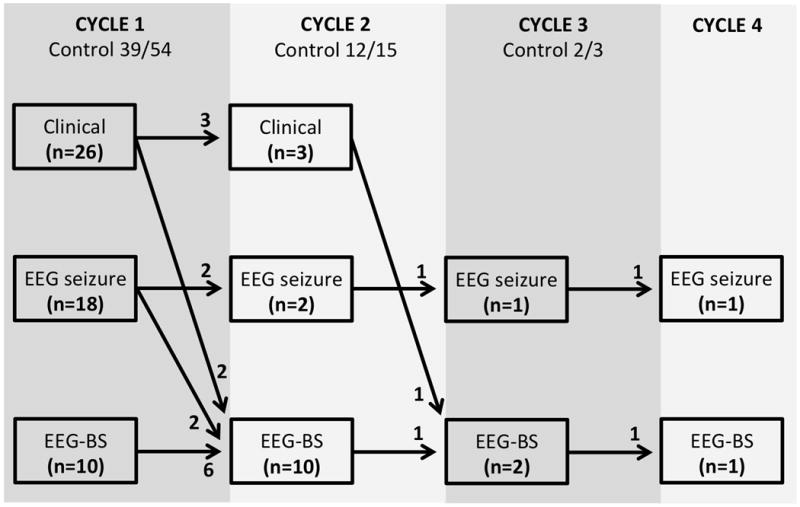
Key: Clinician goals were clinical seizure termination (Clinical), EEG seizure termination (EEG seizure) or achievement of burst suppression (EEG-BS).
Patients underwent one cycle (n = 54), two cycles (n = 15), three cycles (n = 3), or four cycles (n = 2) of anesthetic treatment. In the first cycle of anesthetic treatment management success, as defined by not needing another anesthetic agent, occurred in 39/54 (72% [95% CI: 58% – 84%]). Comparing these 39 controlled patients, with the 15 not achieving seizure control, there was no difference in the number of AED doses before the initial infusion started: 5 (IQR 3, 7) vs 5 (IQR 4, 7) in those controlled on the initial infusion versus those not, respectively. Furthermore, assessment of use of benzodiazepine-type (BDZ) and non-BDZ AEDs before use of continuous infusion, showed that there was no difference between those coming under control with the first continuous infusion, and those not: in controlled patients 2.5 (IQR 2, 4) BDZ AED doses and 2 (IQR 1, 3) non-BDZ AED doses vs non-controlled patients 2 (IQR 2, 3) BDZ AED doses and 3 (IQR 2, 5) non-BDZ AED doses. Based on these observations, dosing of AEDs (number and type) did not appear to be associated with responsiveness to infusion therapy.
In the second cycle, seizure termination was achieved in 12/15 (80% [95% CI: 52% – 96%]) patients who had persisting seizures during the first cycle of treatment. Ultimately, 51/54 (94% [95% CI: 85% – 99%]) patients had seizure termination with two cycles of anesthetic treatment. In regard to seizure duration, in patients in whom seizures were terminated, timing from onset was 5.9 (IQR 1.9, 34) hours in the first cycle, which represents a significantly shorter (p = 0.048) duration when compared to the 30 (IQR 4, 120) hours noted in the second cycle.
Midazolam strategy, dosing and duration
Forty-two of 111 patients in the whole cohort received midazolam as their first continuous infusion AED (38% [95% CI: 29% – 47%]). Seizures had been ongoing in the hospital for 2.4 (IQR 1.6, 9.5) hours at the time of infusion initiation. The number of AEDs administered before treatment with midazolam was four (IQR 4, 6) drugs, of which two (IQR 1, 3) were benzodiazepines and two (IQR 2, 3) were non-benzodiazepine drugs.
Figure 2 illustrates the initial endpoint goals used in the 42 patients receiving midazolam; 30/42 (71%) patients were controlled. Only 12/42 (29%) midazolam patients required treatment with a second anesthetic agent, most commonly pentobarbital (see below). Four of 12 patients requiring a second anesthetic went on to receive a third anesthetic. Overall, 38/42 (90% [95% CI: 77% – 97%) patients achieved seizure termination with two cycles of anesthetic treatment when midazolam was the first administered anesthetic.
Figure 2. Termination of RSE when midazolam administered as the first-line anesthetic agent.
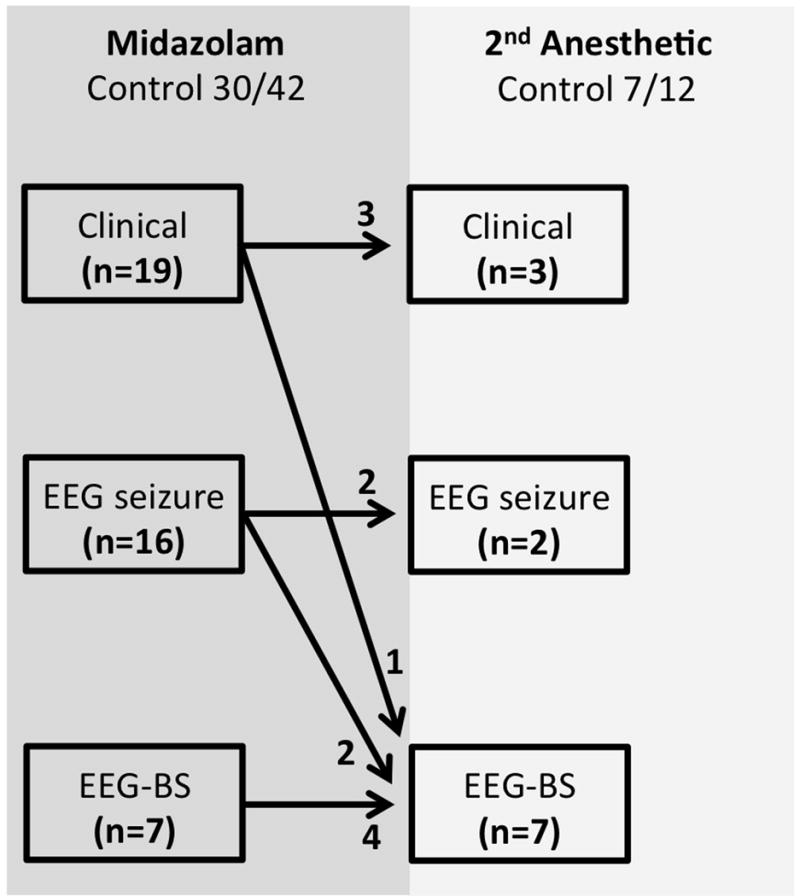
Key: Clinician goals were clinical seizure termination (Clinical), EEG seizure termination (EEG seizure) or achievement of burst suppression (EEG-BS).
Table 2 summarizes the bolus dose, initial and highest infusion rates of midazolam. There was no significant difference between the midazolam infusion rates required for clinical seizure termination (0.30 [IQR 0.13, 0.88] mg/kg/hr or 5.0 [IQR 2.2, 14.7] mcg/kg/min) or EEG seizure termination (0.23 [IQR 0.17, 0.35) mg/kg/hr or 3.8 [IQR 2.8, 5.8] mcg/kg/min). However, significantly higher dosing rates were used when burst suppression was the chosen endpoint (i.e., 1.00 [IQR 0.55, 1.50] mg/kg/hr, or 16.7 [IQR 9.2, 25.0] mcg/kg/min) when compared with either of the other endpoints (p <0.05). The duration of treatment in responders was 47.5 (IQR 14.5, 72) hours, which was significantly longer than the duration of use in non-responders (p = 0.012). In the 12 non-responders requiring a second anesthetic agent, midazolam was used for 24 (IQR 12.5, 38.5) hours before transitioning to the next agent. We could not identify any specific characteristics of these 12 non-responders that would have differentiated them from the other 30 responders, a priori. (They could not be differentiated based on sex, age, presence of known epilepsy, the endpoint used for seizure control, hospital or out-of-hospital onset of seizure, and number of AEDs administered before the continuous infusion).
Table 2.
Dosing (median, IQR) of midazolam and pentobarbital for RSE
| Dosing | Midazolam | Pentobarbital |
|---|---|---|
| Bolus dose | 0.11 (0.09 – 0.22) mg/kg | 3.7 (0.6 – 5.2) mg/kg |
| Initial infusion rate | 0.10 (0.06 – 0.23) mg/kg/hr (or 1.7 [1.0 – 3.8] mcg/kg/min) |
1.0 (1.0 – 2.0) mg/kg/hr |
| Highest subsequent infusion rate in responders | 0.10 (0.06 – 0.50) mg/kg/hr (or 1.7 [1.0 – 8.3] mcg/kg/min) |
3.0 (1.5 – 4.0) mg/kg/hr |
| Highest subsequent infusion rate in non-responders | 0.20 (0.20 – 1.5) mg/kg/hr (or 3.3 [3.3 – 25.0] mcg/kg/min) |
Treatment with a vasoactive drug infusion occurred in 12/42 (29%) of patients initially managed with midazolam. Of these 12 patients, five were patients that responded to midazolam and did not need another continuous infusion. The other seven required pentobarbital after midazolam infusion (see below).
Pentobarbital strategy, dosing and duration
Eleven of the 12 patients that failed to respond to first-cycle therapy with midazolam ultimately received pentobarbital: nine received it as the second anesthetic agent and two received it as the third anesthetic agent. The bolus dosing, initial and highest infusion rates are given in Table 2. The endpoint used for titrating drug administration was clinical seizure termination in one patient and EEG seizure termination in 10 patients. In the patients undergoing EEG for titration of pentobarbital, the endpoint was EEG seizure termination in two patients, and induction of burst suppression in eight patients. Out of the 11 patients receiving pentobarbital at some time after initial use of midazolam, two failed to respond and were subsequently treated with another anesthetic agent: propofol in one patient and ketamine in the other. In the nine patients that responded to pentobarbital, the duration of pentobarbital treatment was three (IQR 2, 12.3) days. The combined duration of using midazolam and pentobarbital infusion was four (IQR 3, 14) days. The total duration of intensive care length-of-stay in all 11 patients was 15.5 (IQR 7.3, 42.3) days.
Last, in regard to vasoactive drug administration during pentobarbital infusion, seven of 11 (64%) cases received such therapy. There was higher likelihood of requiring a vasoactive drug during pentobarbital infusion compared with midazolam infusion (see above 5/30 vs 7/11, p <0.05).
Discussion
In this prospective multicenter observational cohort study of RSE from nine tertiary pediatric hospitals in the US, we have found that supervising clinicians embark on treatment with anesthetic agents in almost half of the patients and that this is an “intention-to-use” or induce anesthesia, but anesthetic dosing is infrequently required. All of these patients were admitted to a PICU, as the majority of patients undergo mechanical ventilation. The length-of-stay in the PICU is a median of 10 days. Seizure termination in those receiving continuous infusions was achieved in 94% (95% CI: 85% – 99%) of patients by the second cycle of therapy, although a minority of patients required up to four cycles of serial anesthetic therapies. The timing from SE onset to anesthetic initiation was a median of six and 30 hours for the first- and second-line anesthetic agents used, respectively. Midazolam was the most frequently administered first-line anesthetic agent and pentobarbital was the most frequently administered second-line agent. These data are unique in that they provide information on contemporary practice and potential areas of quality improvement, with description of actual drug dosing and control rates.
To date, there have been 11 retrospective pediatric RSE studies that provide details of the dosing of midazolam when used as the first AED by continuous infusion.[12–23] The current prospective pSERG cohort from 2011 to 2013 adds contemporary data to this literature. The proportion of RSE patients presenting to emergency services needing midazolam infusion is substantial, occurring in 29% to 47% of patients (95% CI), a finding that may have implications for hospital emergency services. The overall success rate with midazolam used as the initial AED by continuous infusion in our cohort (95% CI: 55% – 84%) is similar to the 76% rate of control previously described in a meta-analysis of the historical data.[11] However, in contrast to the previous reports, the pSERG cohort provides data regarding the prolonged duration of seizure activity before initiation of midazolam infusion (median of 145 minutes). This period appears long and warrants further investigation into potential causes for delay. For example, previously we knew of a delay in time to administer the initial drugs and escalate between AED types,[24] now, we know of delay in escalation to anesthetic therapies. Whether this delay is due to timing of endotracheal intubation and availability of central intravenous access is unknown. Quality improvements in these matters may have significant impact on seizure control and response rate to the initial continuous infusion.
Bolus dosing and initial infusion rates for midazolam were similar to those reported in previous studies,[11–23] with a median dose of 0.11 mg/kg (average in the literature 0.15 – 0.50 mg/kg) and 1.7 mcg/kg/min (average in the literature 1 – 2 mcg/kg/min) respectively. However, previous studies identified an apparent discrepancy: treatment dosing from studies using EEG monitoring to guide therapy[13,19] used considerably higher doses of midazolam when compared with studies using a clinical endpoint to guide treatment (10.7 mcg/kg/min versus 2.8 mcg/kg/min).[12,14–18,20,21] The current pSERG cohort illustrates that an EEG endpoint is currently used in over half of patients and also confirms the utilization of higher midazolam dosing when EEG serves as the endpoint measure (i.e., cessation of EEG seizures 3.8 [2.8 – 5.8] mcg/kg/min or burst suppression 16.7 [9.2 – 25.0] mcg/kg/min). Our study is novel in demonstrating the median duration of midazolam therapy in those whose seizures become controlled (two days), while also illustrating the median time until therapy escalation occurs in subjects not responding to first-cycle infusion therapy (1 day). Again, this latter observation of care is of concern, since it tells us that there may be a delay in recognizing those patients not responding to initial infusion therapy.
There have been six previous pediatric studies describing the dosing of pentobarbital anesthesia for RSE, with the majority of these patients failing to respond to first cycle midazolam therapy.[21,22,25–28] In these reports, when the data are considered together, pentobarbital infusion was successful at achieving burst suppression and seizure control in 65% of the patients.[11] In two of these studies, the median interval between seizure onset and initiation of barbiturate infusion was 24-hours[25] and 35-hours.[28] In the pSERG cohort we have detailed the progression from presentation with RSE to midazolam failure, and the subsequent use of pentobarbital. Out of 111 patients with RSE, 42/111 (38%) of the series received midazolam as first-line continuous infusion; 12/42 (29%) treated with midazolam continued to have uncontrolled seizures and, of these, 11/12 (92%) subsequently received pentobarbital (10% of the whole series).
Three previous studies reported on the use of vasoactive support during pentobarbital for RSE: 28/30 patients reported by Baberio et al,[28] 20/20 patients reported by Gestel et al,[26] and 10/11 patients reported by Patten et al.[22] Our data indicates that 7/11 patients required such support. When these data are combined with the prior data, 65/72 patients (90.3% [95% CI: 81% – 96%]) required vasoactive support in the setting of pentobarbital for RSE.
This analysis of midazolam and pentobarbital infusions for RSE has two main limitations. Firstly, the treatment cohort sizes are relatively small, but represent the cumulative data over a two-year period from nine US academic, tertiary care centers. Secondly, specific clinical data (e.g., subsequent seizure frequency, seizure recurrence during therapy, long-term survival and cognitive outcomes in relation to treatment) are not available for further analysis. Recent class III evidence in adults with RSE questions whether use of intravenous anesthestic agents causes harm, due to conflicting data from a number of large case series.[29–33] Our study was not designed to compare outcomes for different AED strategies. Despite these limitations, this report does provide a contemporary description of how midazolam and “intention-to-use” an anesthetic infusion strategy are administered in current clinical pediatric practice and how successful this approach is at controlling RSE.
Taken together with the above discussion, our study identifies midazolam and pentobarbital as the preferred continuous infusions for RSE in children. Midazolam is typically used first, and is followed by seizure termination in 71% of patients. In patients who required pentobarbital as a second cycle of treatment, after midazolam, seizure termination occurred in 90% of patients. These data should help inform the design and recruitment of randomized clinical trials for new strategies and AEDs for critical RSE. For example, based on our unique data it would appear that there are two natural decision points for randomization: determination of failure of midazolam with a view to escalating to the next anesthetic agent, or continued up-titration of this treatment at 24 hours; or, failure of to successfully wean the second anesthetic agent after 72 hours. By our assessment of current practice in our nine pediatric tertiary centers, for every 100 patients presenting with RSE, 49 would be treated by an infusion of AED/anesthetic agent. Out of these 49, ~14 would fail such therapy and need a second anesthetic agent. Out of these 14, ~3 would fail such therapy and need to progress to another course of treatment. From these estimates it is apparent that testing a new anesthetic AED agent after failure of a second agent is unlikely to be feasible without being tested in a multicenter international, adult and pediatric study. Selecting an earlier decision point in which to undertake a randomized controlled trial, such as the original decision to use an intravenous agent (most commonly midazolam), might also prove problematic since it is not clear what prompts this decision as only half of the patients with RSE underwent such treatment in our centers. We therefore recommend further comparative effectiveness assessment of these earlier decisions in order to better understand the clinical decision-making.
Conclusions
Midazolam and then pentobarbital remains the mainstay of continuous infusion anesthetic management for RSE in the pediatric patient. The timing from SE onset to initiation of an infusion is a median of six and 30 hours for these first- and second-line anesthetic agents. The majority (94%) of patients experience seizure termination with these two therapies and the report provides contemporary dosing. Testing a new anesthetic AED after failure of midazolam and pentobarbital is not feasible in a pediatric study.
Acknowledgments
Study funding
This study and the pSERG consortium was funded by the Epilepsy Foundation of America (EF-213583, Targeted Initiative for Health Outcomes) and by the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award.
Footnotes
Financial Disclosures:
All authors confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
In regard to “disclosure of Conflicts of Interest” the authors declarations are as follows: R. Tasker and H. Goodkin report no disclosures relevant to the manuscript. I. Sánchez Fernández is funded by a grant for the study of epileptic encephalopathies from “Fundación Alfonso Martín Escudero” and by the HHV6 Foundation. K. Chapman reports no disclosures relevant to the manuscript. N. Abend reports that he is an Epilepsia editorial board member, 2013–present, and Seizure editorial board member, 2012–present. He receives publishing royalties for Pediatric Neurocritical Care, Demos, 2013. He has grant funding from NIH (National Institute of Neurological Disorders and Stroke), K23NS076550, PI, 2011–present, and Epilepsy Foundation of America. R. Arya, J. Brenton and J. Carpenter report no disclosures relevant to the manuscript. W. Gaillard’s income derives from clinical revenue generated for CNMC clinical care. Federal support provided by National Institute of Neurological Disorders and Stroke 1P30HD40677-01, 2K12NS052159-06A1 NIMH RO1 MH084961, 1R21MH092615, NSF 095998, CDC 1UO1DP003255, DOD/USAMRAA W81XWH-11-2-0198, and PCORI 527. Foundation support from Epilepsy Foundation of America, American Epilepsy Society, Pediatric Epilepsy Research Foundation, and CURE. The department conducts industry-supported trials from which no salary support is derived including Ovation Pharmaceuticals, King Pharmaceuticals, and PRA International/Eisai. Stock (held with spouse): Johnson & Johnson, Lily, GlaxoSmithKline, Pfizer, Siemens, and General Electric. Editorial board of Epilepsia and Epilepsy Research. T. Glauser, J. Goldstein, A. Helseth, and M. Jackson report no disclosures relevant to the manuscript. K. Kapur has received support for statistical work on the grants from the NIH, National Institute of Neurological Disorders and Stroke, Guthy-Jackson Charitable Foundation, International Rett Syndrome Foundation, Simons Foundation, Nancy Lurie Marks Foundation, and Lundbeck and Danny Did Foundation. M. Mikati and K. Peariso report no disclosures relevant to the manuscript. M. Wainwright serves as a scientific consultant to Sage Therapeutics and as a Co-Investigator on a study of treatment of super-refractory status epilepticus sponsored by Sage Therapeutics. A. Wilfong and K. Williams report no disclosures relevant to the manuscript. T. Loddenkemper serves on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an Associate Editor for Seizure, as Contributing Editor for Epilepsy Currents, and as an Associate Editor for Wyllie’s Treatment of Epilepsy, 6th edition. He is part of pending patent applications to detect seizures and to diagnose epilepsy. He receives research support from the American Epilepsy Society, the Epilepsy Foundation of America, the Epilepsy Therapy Project, PCORI, the Pediatric Epilepsy Research Foundation, Cure, Danny-Did Foundation, HHV-6 Foundation, Lundbeck, Eisai, and Upsher-Smith.
Other pSERG members, not included in the authorship:
Rajit Basu, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH (Investigator). John Condie, Phoenix Children’s Hospital, Phoenix, AZ (Investigator). Nathan Dean, Children’s National Medical Center, Washington DC (Investigator). Abeer Hani, Duke University Medical Center, Durham, NC (Investigator). Tewodros Kebede, Children’s National Medical Center, Washington DC (Investigator). Jacquelyn Klehm, Boston Children’s Hospital, Boston MA (Clinical Research Coordinator). Melissa Sacco, The University of Virginia Health System, Charlottesville, VA (Investigator). Kristi Schmidt, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, (Investigator). Alexis Topjian, The Children’s Hospital of Philadelphia, Philadelphia, PA (Investigator). David Turner, Duke University Medical Center, Durham, NC (Investigator)
References
- 1.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–22. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 2.Wilkes R, Tasker RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin. 2013;29:239–257. doi: 10.1016/j.ccc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Abend NS, Loddenkemper T. Pediatric status epilepticus management. Curr Opin Pediatr. 2014;26:668–674. doi: 10.1097/MOP.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus – Report of the the ILAE Task Force on classification of status epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez Fernández I, Abend NS, Agadi S, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG) Seizure. 2014;23:87–97. doi: 10.1016/j.seizure.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacroix J, Deal C, Gauthier M, et al. Admissions to a pediatric intensive care unit for status epilepticus: a 10-year experience. Crit Care Med. 1994;22:827–832. doi: 10.1097/00003246-199405000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Chin RF, Verhulst L, Neville BG, et al. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75:1584–1588. doi: 10.1136/jnnp.2003.032797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson K, Metsäranta P, Huhtala H, et al. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65:1316–1318. doi: 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]
- 9.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 10.Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368:222–229. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 11.Wilkes R, Tasker RC. Intensive care treatment of uncontrolled status epilepticus in children: systematic literature search of midazolam and anesthetic therapies. Pediatr Crit Care Med. 2014;15:632–639. doi: 10.1097/PCC.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 12.Rivera R, Segnini M, Baltodano A, et al. Midazolam in the treatment of status epilepticus in children. Crit Care Med. 1993;21:991–994. doi: 10.1097/00003246-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Igartua J, Silver P, Maytal J, et al. Midazolam coma for refractory status epilepticus in children. Crit Care Med. 1999;27:1982–1985. doi: 10.1097/00003246-199909000-00043. [DOI] [PubMed] [Google Scholar]
- 14.Singhi S, Murthy A, Singhi P, et al. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17:106–110. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 15.Koul RL, Raj Aithala G, Chacko A, et al. Continuous midazolam infusion as treatment of status epilepticus. Arch Dis Child. 1997;76:445–448. doi: 10.1136/adc.76.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koul R, Chacko A, Javed H, et al. Eight-year study of childhood status epilepticus: midazolam infusion in management and outcome. J Child Neurol. 2002;17:908–910. [PubMed] [Google Scholar]
- 17.Brevoord JC, Josten KF, Arls WF, et al. Status epilepticus: clinical analysis of a treatment protocol based on midazolam and phenytoin. J Child Neurol. 2005;20:476–481. doi: 10.1177/08830738050200060201. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir D, Gulez P, Uran N, et al. Efficacy of continuous midazolam infusion and mortality in childhood refractory generalized convulsive status epilepticus. Seizure. 2005;14:129–132. doi: 10.1016/j.seizure.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Morrison G, Gibbons E, Whitehouse WP. High-dose midazolam therapy for refractory status epilepticus in children. Intensive Care Med. 2006;32:2070–2076. doi: 10.1007/s00134-006-0362-8. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Osawa M, Aihara M, et al. Efficacy of intravenous midazolam for status epilepticus in childhood. Pediatr Neurol. 2007;36:366–372. doi: 10.1016/j.pediatrneurol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Saz EU, Karapinar B, Ozcetin M, et al. Convulsive status epilepticus in children: etiology, treatment protocol, and outcome. Seizure. 2011;20:115–118. doi: 10.1016/j.seizure.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Patten W, Naqvi SZ, Raszynski A, Totapally BT. Complications during the management of pediatric refractory status epilepticus with benzodiazepine and pentobarbital infusions. Ind J Crit Care Med. 2015;19:275–277. doi: 10.4103/0972-5229.156476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kravljanac R, Djuric M, Jankovic B, Pekmezovic T. Etiology, clinical course and response to the treatment of status epilepticus in children: a 16 year single-center experience based on 602 episodes of status epilepticus. Eur J Paediatr Neurol. 2015;19:584–590. doi: 10.1016/j.ejpn.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez Fernández I, Abend NS, Agadi S, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015;84:2304–2311. doi: 10.1212/WNL.0000000000001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Lee DY, Kim JS. Neurologic outcomes of pediatric epileptic patients with pentobarbital coma. Pediatr Neurol. 2001;25:217–220. doi: 10.1016/s0887-8994(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel JP, Blusse van Oud-Albias HJ, Malingre M, et al. Propofol and thiopental for refractory status epilepticus in children. Neurology. 2005;65:591–592. doi: 10.1212/01.wnl.0000173066.89001.f9. [DOI] [PubMed] [Google Scholar]
- 27.Sakuma H, Awaya Y, Shiomi M, et al. Acute encephalitis with refractory, repetitive partial seizures (AERRPS): a peculiar form of childhood encephalitis. Acta Neurol Scand. 2010;121:251–256. doi: 10.1111/j.1600-0404.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 28.Barberio M, Reiter PD, Kaufman J, et al. Continuous infusion pentobarbital for refractory status epilepticus in children. J Child Neurol. 2012;27:721–726. doi: 10.1177/0883073811424941. [DOI] [PubMed] [Google Scholar]
- 29.Sutter R, Marsch S, Fuhr P, Kaplan PW, Ruegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology. 2014;82:656–664. doi: 10.1212/WNL.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugin D, Foreman B, De Marchis GM, et al. Is pentobarbital safe and efficacious in the treatment of super-refractory status epilepticus: a cohort study. Crit Care. 2014;18:R103. doi: 10.1186/cc13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez A, Lantigua H, Lesch C, et al. High-dose midazolam infusion for refractory status epilepticus. Neurology. 2014;82:359–365. doi: 10.1212/WNL.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang BS, Jung KH, Shin JW, et al. Induction of burst suppression or coma using intravenous anesthetics in refractory status epilepticus. J Clin Neurosci. 2015;22:854–858. doi: 10.1016/j.jocn.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Marshi NA, Novy J, Faouzi M, et al. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med. 2015;43:1003–1009. doi: 10.1097/CCM.0000000000000881. [DOI] [PubMed] [Google Scholar]


