Abstract
Objective
Deep Brain Stimulation (DBS) is an established adjunctive surgical intervention for treating Parkinson’s disease (PD) motor symptoms. Both surgical targets, the globus pallidus interna (GPi) and subthalamic nucleus (STN), appear equally beneficial when treating motor symptoms but effects on nonmotor symptoms are not clear. Lower urinary tract symptoms (LUTS) are a common PD complaint. Given prior data in STN-DBS, we aimed to further explore potential benefits in LUTS in both targets.
Methods
We performed a prospective, non-blinded clinical trial evaluating LUTS in PD patients in both targets pre and post DBS using validated urologic surveys. Participants were already slated for DBS and target selection predetermined before study entry. LUTS was evaluated using: the American Urological Association (AUA-SI), Quality of Life score (QOL), Overactive bladder 8 questionnaire (OAB-q), and sexual health inventory for men (SHIM).
Results
Of 33 participants, 20 underwent STN DBS and 13 had GPi DBS. Patients demonstrated moderate baseline LUTS. The urologic QOL score significantly improved post DBS (3.24±1.77vs 2.52±1.30; p=0.03). Analyzed by target, only the STN showed significant change in QOL (vs. 2.25±1.33; p=0.04). There were no other significant differences in urologic scores post DBS noted in either target.
Conclusion
In PD patients with moderate LUTS, there were notable improvements in QOL for LUTS post DBS in the total sample and STN target. There may be differences in DBS effects on LUTS between targets but this will require further larger, blinded studies.
Keywords: Parkinson’s disease, Deep Brain Stimulation, globus pallidus interna, subthalamic nucleus, bladder dysfunction, pallidal stimulation
Introduction
Parkinson’s disease (PD) is a complicated neurodegenerative disorder with classic diagnostic motor features but also a myriad of burdensome and debilitating nonmotor features that typically have poor treatments and are unresponsive to dopaminergic therapy (1–6). More effective treatments are needed either through non-dopaminergic medicinal therapies or perhaps surgical modalities. Deep brain stimulation (DBS) is a proven surgical treatment for adjunctive symptomatic management of the levodopa responsive motor features of moderate to severe PD patients in cases where medication is becoming less effective or causing complications (e.g. motor fluctuations and dyskinesias). However, it may also have a role in treating nonmotor symptoms (NMS) as well (7–9). While data is limited, DBS has shown some beneficial effects on several NMS including sleep, autonomic symptoms such as urologic dysfunction, and pain among others (9–10).
Lower urinary tract symptoms (LUTS) are a common NMS in PD. Loss of neurons in the basal ganglia and the resulting dopamine depletion central to the pathophysiology of PD results in major disruptions in the connections to the thalamus, motor cortex and limbic system which in turn appears to lead to the urinary storage symptoms typically seen clinically. These structures facilitate the voiding function and overlap in the urine storage facilitating areas. Dopamine and other neurotransmitters such as γ amino butyric acid (GABA) and glycine are inhibitory on the pontine micturition center and potentially reduce detrusor hyperactivity (11, 12). However, dopaminergic therapy used to treat the motor symptoms of PD has been shown to affect LUTS both positively and negatively with data scarce and results inconsistent (12). The main treatment of LUTS relies on identifying the specific voiding complaints and largely mirrors treatment options for those without PD. As urinary storage symptoms predominate, anticholinergics and alpha adrenergic antagonists are the main pharmacological treatment for LUTS in PD. However, most of these drugs have not been specifically evaluated for PD patients. Efficacy is modest and medication compliance is poor as the side effect profile can be burdensome (13).
The downstream effects of nigral degeneration and dopamine deficiency in PD specifically cause excessive subthalamic nucleus (STN) excitation of the globus pallidus interna (GPi) and excessive GPi inhibition of the thalamus. PD DBS targets these structures and modulates their activity. It is theorized DBS could improve LUTS by enhancing afferent bladder information processing through the basal ganglia’s connections to the thalamus, frontal cortex and other structures (14). Herzog et al. has performed two studies using PET scans in PD patients post STN-DBS that would support this theory (14–15).
Several small clinical studies would support DBS potentially helping LUTS as well. Consistently, primary urologic studies in PD patients undergoing DBS demonstrate improvements in urinary symptoms post surgery (16–18). However, all studies are in the STN target. Those specifically examining urinary symptoms after pallidal DBS for PD have not been reported. There is a recent study looking at urinary function after pallidal DBS for dystonia which demonstrated DBS may have benefit here as well by potentially creating a relaxing effect on detrusor function (19).
Given the available data, the aim of this study was to expand the understanding of the benefits of DBS on LUTS by assessing urologic function via validated questionnaires in patients pre and post DBS at our center. We studied patients undergoing DBS in both targets (STN and GPi) given the lack of information on the GPi target in PD. Ultimately, our hope is that better understanding of the effects of DBS on NMS like LUTS from this study and others like it will provide valuable information in tailoring DBS targets to individual patients.
Materials and Methods
This study was an institutional board review approved prospective, non-blinded clinical trial designed to further evaluate changes in urologic function in PD patients pre and post DBS in the STN and GPi target. Study participants were selected from PD patients at our center already slated for DBS with targets predetermined before study entry. During the study period from November 2013-December 2014, every DBS candidate was approached for entry into the study until the final 2 months whereupon GPi targets were selectively recruited given this center tended to perform a much higher number of STN surgeries. Every eligible candidate who met criteria for entry into the study provided written informed consent if they wished to proceed. PD patients were only excluded if they personally refused entry into the trial.
Our center performs a high volume of DBS and is well experienced in determining good surgery candidates. Candidacy evaluation and target selection for impending DBS cases occurs at a monthly interdisciplinary conference attended by neurologists, neurosurgeons, physical therapists and neuropsychiatrists. There are no set criteria for selecting the GPi target versus the STN target but our center places particular emphasis on depression, postural instability, lower levels of medication, and impaired verbal fluency as reasons to proceed with the GPi target (20–22). Urinary function was not discussed at the conference during target selection throughout the study period and is not common practice. DBS implantation consisted of lead placement into either the STN or GPi target during a multi-staged surgery using the DBS miniframe-the STarFix platform (FHC, Inc., Bowdoin, ME). Details of the surgery process have been published prior (23–24). Urologic assessments were done preoperatively after target selection and then 6 months post-operatively whereupon Parkinson’s drugs had been titrated as clinically able and DBS settings optimized. All participants completed the validated American Urological Association symptom index (AUA-SI), Overactive bladder 8 questionnaire (OAB-q), the sexual health inventory for men (SHIM), and, for follow up, the patient global impression of improvement score (PGI-I) for urinary symptoms. The AUA-SI consists of seven questions on LUTS. Symptoms are scored on a frequency scale from 0 (not at all) to 5 (almost always). The symptom index categories are mild (1–7), moderate (8–19), and severe (20–35). An additional question included in the AUA-SI but not tabulated in the score on general satisfaction of urinary symptoms is termed the quality of life score (QOL). This score determines overall satisfaction with urinary function with ranges from 0 (delighted) to 6 (terrible) (25). The OAB-q is an 8-item questionnaire that can discriminate between normal and clinically diagnosed continent and incontinent patients with overactive bladder. Symptoms are scored on a bother scale from 0 (not at all) to 5 (a very great deal) (26). The sexual health inventory for men (SHIM) is a patient reported diagnostic tool for erectile dysfunction severity (27). The classifications are severe (1–7), moderate (8–11), mild to moderate (12–16), and mild (17–21). The PGI-I ranges from 1 (very much improved) to 7 (very much worse). Consequently, with the exception of the SHIM, which was the inverse, higher scores on all surveys corresponded to worse clinical outcome.
Statistical analysis was performed with commercially available software SPSS® 22.0. Due to the small sample size, only nonparametric statistical tests were performed. Parameters including questionnaire scores and medication doses were compared within the same individual separately by target and in the overall sample using the Wilcoxon signed rank test to identify differences as a result of treatment effect. Baseline patient demographics and clinical parameters were compared between the DBS-STN and DBS-GPi with the Wilcoxon rank sum test or chi square analysis. The differences in change in questionnaire scores and medication equivalent doses pre and post surgery between the two targets were also compared using the Wilcoxon rank sum test.
Results
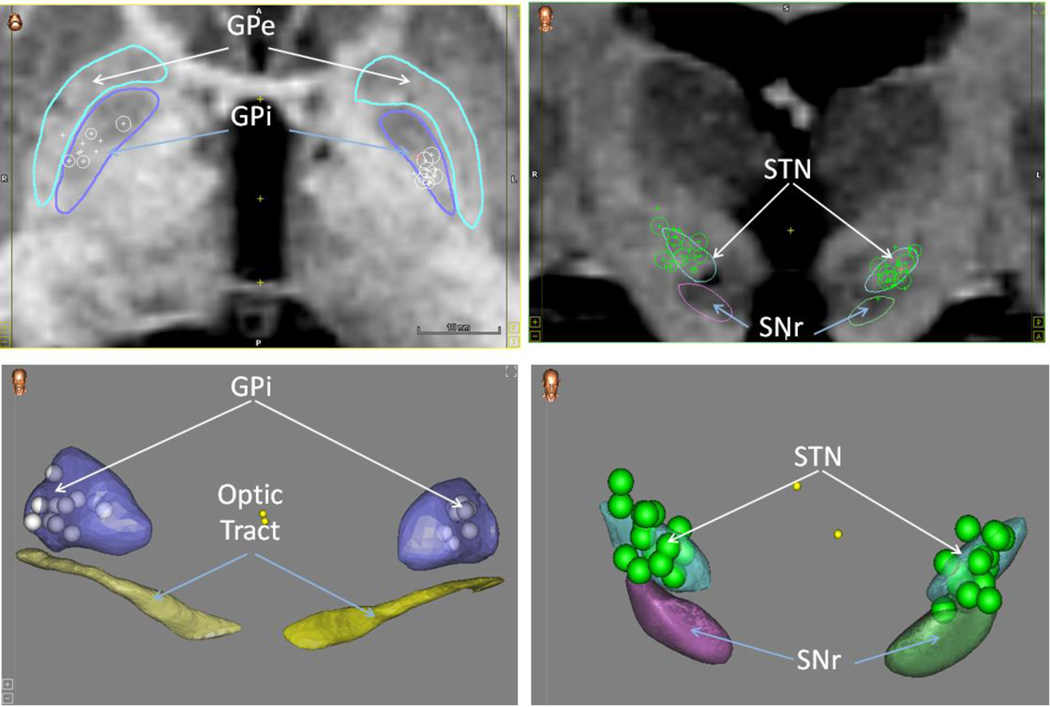
Between November 2013 and December 2014, a total of 45 patients enrolled in the study but only 33 were included in the final analysis. Two patients sustained falls and were excluded since they did not undergo DBS implantation. One patient required explantation after developing cranial wound dehiscence and hardware exposure. One patient died of aspiration pneumonia within 1 month of implantation. Four patients did not complete follow up questionnaire forms. Finally, four additional patients completed the study protocol but were excluded after review over concerns they had confounding variables during their surgery course (3 patients had unilateral implants and 1 patient received an anticholinergic during the post-operative study period). Statistical outcomes with and without these last four patients were not significantly different with the exception that the change in total levodopa equivalent daily dose (LEDD) post-operatively was no longer significant after exclusion of these four patients. Out of the analyzed sample, there were 24 men and 9 women. DBS targets included the STN in 20 and GPi in 13 patients. Targeting accuracy was confirmed for all patients using a functional atlas in a multipatient normalized space (Figure 1). Nonlinear normalization has been proven to provide a truer estimate of the postoperative anatomical location of DBS leads as opposed to more conventional approaches that rely on an anterior-posterior commissure coordinate system (28). We have previously demonstrated the accuracy of this method based on predictions of anatomical landmarks as well as by correlating statistical maps of electrophysiological data with underlying anatomy (28–29).
Figure 1.
Legend-The upper two panels are coronal MRI Scans. The bottom two panels are a 3D representation of pertinent structures. Upper panels: Left- Arrows point to the outline of the globus pallidus interna (GPi) in dark blue and the globus pallidus externa (GPe) which is in light blue. Right- Arrows point to the outline of the substantia nigra (SNr) and subthalamic nucleus (STN). The 2D circles are the actual points representing the midpoint of the lead on the 2D MRI whereas the pluses are projections of the points lying in other slices. Bottom panels: Left-Arrows point to a 3D representation of the globus pallidus interna (GPi) in purple and the optic tract in yellow. Right- Arrows point to a 3D representation of the subthalamic nucleus (STN) and substantia nigra (SNr). 3D circles represent the center of the leads in both panels.
In the cohort, there were no significant differences between GPI and STN groups. Mean time from PD diagnosis to DBS implantation was 7.79 years and the majority (64%) had a Hoehn and Yahr score of 3 in the OFF state at the time of surgery (mean 3.18). The vast majority scored above mild cognitive impairment (MCI) on cognitive screening but there was a higher number of MCI patients in the GPi group (p=0.21). 23 patients were simultaneously implanted whereas 11 were staged one week apart. Complete patient demographics and clinical parameters are shown in Table 1. 26 of 33 patients (79%) were taking dopamine agonist medication at baseline with no difference by target (STN-75%, GPi- 85%; p=0.51). Mean dopamine agonist LEDD was lower in the STN target but total LEDD was essentially the same (mean dopamine agonist LEDD: 159.00 ± 158.34 vs. 281.92± 252.34, mean total LEDD 1222.30 ± 625.46 vs. 1249.62 ± 508.20). Neither difference was significant (p=0.23, p=0.91). One patient was on anticholinergic medication for bladder storage symptoms prior to DBS and remained on it at unchanged doses post-operatively.
Table 1.
Patient Demographics
| Overall | STN | GPI | P value | |
|---|---|---|---|---|
| N | 33 | 20 | 13 | |
| Age at Diagnosis (years) | 54.49 (8.69) | 23.20 (9.51) | 56.51 (7.16) | 0.51 |
| Age at DBS (years) | 62.29 (9.00) | 60.88 (9.82) | 64.45 (7.40) | 0.42 |
| PD Diagnosis to implantation (years) |
7.79 (2.55) | 7.69 (2.61) | 7.95 (2.56) | 0.67 |
| Gender | ||||
| M | 24(73%) | 15(75%) | 9(61%) | 1.0 |
| F | 9(27%) | 5 (25%) | 4(39%) | |
| Cognitive Status Above MCI |
25 (76%) | 17(85%) | 8 (62%) | 0.21 |
| MCI | 8(24%) | 3 (15%) | 5 (38%) | |
| UPDRS | ||||
| "off" | 40.64 (11.10) | 43.20 (10.74) | 36.69 (10.88) | 0.12 |
| "on" | 20.39 (8.09) | 20.50 (8.11) | 20.23 (8.39) | 0.84 |
| Hoehn and Yahr score | 3.18(0.58) | 3.25(0.55) | 3.08(0.64) | |
| 2 | 3 (9%) | 1 (5%) | 2 (15%) | 0.58 |
| 3 | 21 (64%) | 13 (65%) | 8 (62%) | |
| 4 | 9 (27%) | 6 (30%) | 3 (23%) | |
| Total LEDD | 1233.10 (573.86) | 1222.30 (625.46) | 1249.62 (508.20) | 0.91 |
| Dopamine agonist LEDD | 207.42 (206.12) | 159.00 (158.34) | 281.92 (252.34) | 0.23 |
Legend: Patient demographics. Numerical values correspond to mean with standard deviation or percentages in parentheses. STN=Subthalamic nucleus, GPI=globus pallidus, DBS=Deep Brain Stimulation, PD=Parkinson’s disease, M=male, F=female, MCI=Mild Cognitive Impairment, LEDD=Levodopa equivalent daily dose. P values were calculated using the Wilcoxon rank sum test with the exception of values marked with a *. These were calculated using chi square analysis.
From a urologic standpoint based on questionnaire scores, baseline scores indicated moderate urinary symptoms with a mean AUA-SI of 10.67±6.29, QOL of 3.24±1.77, and OABq of 12.06±6.55. Scores were similar by gender and target. Two patients had severe symptoms by AUA-SI (>20) pre implant. Six months post operatively there was a significant improvement in urologic QOL scores (3.24±1.77 vs. 2.52±1.30; p=0.03). Other surveys demonstrated marginal post-operative change (Table 2). With these survey findings, patients reported on average on the PGI-I that they were between “minimally improved” to having “no change.” Analyzed by individual targets, the STN patients had a significant improvement in QOL (3.20±1.61 vs. 2.25±1.33; p=0.04) while mean AUA-SI and OAB-q scores demonstrated no significant change postoperatively. The scales did improve by 0.35 and 1.1 points respectively (p=0.93, p=0.59). GPi patients had milder, nonsignificant improvement in QOL (3.31±2.06 vs. 2.92±1.19; p=0.37). Here, AUA-SI and OAB-q scores conversely had a statistically nonsignificant worsening of 0.91 and 2.46 points respectively (p=0.39, p=0.21). PGI-I score was in the “minimally improved” to “no change” range in the STN target while fully in the “no change” range in the GPi target. SHIM scores had no significant changes post surgery in either target but data trended differently in the two targets (Table 3). Comparing between targets, the differences in score change pre and post operatively did not reveal a significant difference in AUA-SI, QOL, SHIM, OAB-q or PGI-I scores (Table 3).
Table 2.
Pre and post DBS outcomes.
| Baseline | 6 month post implant |
p-value | |
|---|---|---|---|
| AUA-SI | 10.67±6.29 | 10.82±6.04 | 0.64 |
| QOL | 3.24±1.77 | 2.52±1.30 | 0.03 |
| OAB-q | 12.06±6.55 | 12.36±8.25 | 0.79 |
| SHIM | 8.70±8.26 | 8.63±7.66 | 0.70 |
| Total LEDD | 1233.10±573.8666 | 1044.38±645.76 | 0.07 |
| Dopamine agonist LEDD | 207.42±206.12 | 136.67±159.17 | 0.02 |
| PGI-I | 3.76±1.28 |
Legend: Numerical values correspond to mean ±standard deviation. AUA-SI=American Urological Association symptom index, QOL= Quality of Life score, OAB-q=Overactive Bladder 8 Questionnaire, SHIM=The Sexual Health Inventory for Men, PGI-I=The Patient Global Impression of Improvement Score, LEDD=Levodopa equivalent daily dose.
Table 3.
Pre and post DBS outcomes by target.
| STN | GPI | STN vs GPI | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 month post-implant |
p-value | Baseline | 6 month post-implant |
p-value | Diff. in STN vs GPi delta |
p-value | |
| AUA-SI | 10.15±6.22 | 9.80±5.67 | 0.93 | 11.47±6.58 | 12.38±6.48 | 0.39 | −1.26 | 0.48 |
| QOL | 3.20±1.61 | 2.25±1.33 | 0.04 | 3.31±2.06 | 2.92±1.19 | 0.37 | −0.56 | 0.36 |
| OAB-q | 10.95±6.73 | 9.85±7.58 | 0.59 | 13.77±6.13 | 16.23±7.99 | 0.21 | −3.56 | 0.18 |
| SHIM | 9.40±8.24 | 8.67±7.58 | 0.47 | 7.38±8.70 | 8.56±8.25 | 0.18 | −1.91 | 0.10 |
| LEDD Total |
1222.30±625.46 | 1040.65±678.35 | 0.25 | 1249.62±508.20 | 1050.12±619.17 | 0.17 | 18.15 | 0.77 |
| LEDD DA |
159.0±158.34 | 100.0±146.11 | 0.11 | 281.92±252.34 | 193.08±167.55 | 0.04 | 29.4 | 0.34 |
| PGI-I | 3.60±1.31 | N/A | 4.00±1.22 | N/A | −0.40 | 0.44 | ||
Legend: DBS outcomes by target. Numerical values correspond to mean ± standard deviation. AUA-SI=American Urological Association symptom index, QOL= Quality of Life score, OAB-q=Overactive Bladder 8 Questionnaire, SHIM=The Sexual Health Inventory for Men, PGI-I=The Patient Global Impression of Improvement Score, LEDD=Levodopa equivalent daily dose, DA=dopamine agonist, STN=subthalmic nucleus, GPi=globus pallidus interna, Diff.=Difference.
Patients were consistently able to decrease medication after surgery (Table 2, Table 3). There was a significant decrease in mean dopamine agonist LEDD of 70.75 units in the overall group post implantation (p=0.02). Total LEDD decreased by 188.72 units (p=0.07). When analyzed by individual target, only the GPi site demonstrated a significant medication decrease, solely in reduction of dopamine agonist LEDD (0.04). There was no difference in medication reduction when comparing between targets. Of the 26 patients taking dopamine agonists prior to DBS, 14 patients (54%) were able to decrease their dose and 5 (19%) were able to stop taking dopamine agonist entirely. There was no difference between targets in decreasing or stopping dopamine agonists.
Discussion
We performed a prospective nonrandomized clinical trial looking at the change in urologic function in a PD cohort after undergoing DBS in both approved targets-the GPi and STN. The overall cohort had moderate baseline LUTS. After DBS, there was significant improvement in the mean QOL score in the overall cohort while other survey scores showed marginal change. Direct comparison of the changes in urologic scores post surgery in both targets demonstrated no significant differences either. Analyzing the targets individually, the STN group had a statistically significant improvement in urologic QOL score while other scores demonstrated no statistically significant difference. There was some mild improvement noted in the AUA-SI and OAB-q scores in this target while the SHIM score in turn appeared to worsen. . Conversely, in the GPi target, there was a worsening also nonsignificant trend in the AUA-SI and OAB-q scores while the SHIM scores appeared to improve. Here, QOL score only mildly improved. Larger sample sizes and more in depth testing will be needed to see if these trends in data continue and could move towards statistical significance. The QOL score, which did achieve statistical significance in some cases, was derived from a single question at the end of the AUA-SI and stated “How would you feel if you had to live with your current condition the rest of your life?” Despite being statistically nonsignificant, we felt the differing quantifiable changes in the other survey data in each target important to note. These differences suggest that there could be a primary urologic contribution behind the significant improvements in QOL in the overall cohort and STN group as opposed to improvements purely in motor features or other nonmeasured variables.
Previous published studies address the potential positive effect of DBS on LUTS. Navarro et al. evaluated the impact of bilateral STN-DBS on the prevalence of numerous PD NMS one year after implantation and found a mean decrease in the administered Non-Motor Symptom questionnaire from 12.1 ±4.8 to 7.3 ±4.4 post implantation (p<0.001). Autonomic symptoms demonstrated the greatest reductions (nocturia decreased from 62.5% to 45.8% and urinary urgency decreased from 62.5% to 37.5%). The reduction in NMS was significantly correlated with total QOL scores (9). Winge et al. studied urologic function prospectively in 16 advanced PD patients undergoing STN-DBS regardless of baseline urinary function. They evaluated LUTS with the International prostate symptom score (IPSS; also known as the AUA-SI), the Danish Prostate symptom score (DanPSS) and urodynamics pre and 6 months post implantation. Composite DanPSS and IPSS (baseline median score 18 and 11.5 respectively) and urodynamics parameters (only 2 patients had detrusor overactivity (DO)) did not change after implantation. However, on sub-analysis, the authors separated out the components of the questionnaires and found that the storage question portion of the IPSS (3/7 questions) and DanPSS (4/12 questions) were significantly different. The authors concluded that STN-DBS might improve symptoms of overactive bladder but not LUTS in general (18). Finally, Seif et al. performed urodynamics in 16 randomly selected patients regardless of LUTS after bilateral STN-DBS. Urodynamics were performed with DBS in the “off” and “on” state (interval 20 minutes). In the “off” state, there were 5/16 patients with DO that were not present in the “on” stimulation phase. Overall, there was a significant difference between the “off” vs. “on” stimulation state in regards to initial desire to void and maximum bladder capacity (p<0.005) (17). While the findings in the STN target in our study were not significant with the exception of the QOL score, they did trend positively, mirroring some of these published results.
In the PD population, the potential changes in urinary function after DBS has not been explored in primary urologic studies within the GPi target. The only known study evaluating the GPi target’s effect on bladder function has been in patients with dystonia. Mordasini et al. reported on 11 patients with severe dystonia and evaluated bladder function by questionnaire scores and urodynamics at baseline and after 3 months. Baseline LUTS was mild and urodynamics revealed DO in only 4 patients. Follow up urodynamics after implantation “on” stimulation revealed resolution of DO. Furthermore, there was a decrease in maximum flow rate (p=0.019) and increase in post void residual (0.018) when the authors compared the parameters with the DBS “on” vs. “off” (19). This relaxing effect on detrusor function was not captured in our study as no urodynamics parameters were assessed. The AUA-SI, a tool developed primarily at evaluating men with obstructive voiding symptoms, did not reveal a significant benefit and even trended in a negative direction in our study. This may reflect differences in the underlying pathophysiology of urinary dysfunction in dystonia and PD.
Non motor features are an important part of PD as they can cause significant disability and quality of life impairment. For many patients, NMS may be more bothersome than their motor symptoms (9). As DBS of both the STN and GPi has been established as equally beneficial for PD motor symptoms (8,20), NMS such as LUTS could be important symptoms to consider when making decisions about target selection. Already, published studies are showing some subtle differences. In large studies comparing STN and GPi DBS, STN-DBS showed a detrimental effect on phonological and semantic verbal fluency tasks, which was not seen as prominently in the GPi target (20,30). Similar trends are seen regarding mood disorders such as depression and impulsivity (20,31–32). In a 4-year follow up of adverse events of STN and GPi DBS patients, Hariz et al reported a greater frequency of balance and gait dysfunction in the STN group (26.6%) vs. the GPi group (0%) (33). A variety of other studies looking at NMS symptoms such as sleep, pain, and autonomic symptoms have been performed as well but these have only been done in the STN target (9–10). Currently, at our institution, very few nonmotor features are discussed outside of neuropsychiatric and cognitive measures as we make a target selection. This is common practice in major DBS centers as data is still lacking on the nonmotor effects of DBS with the biggest paucity of data in the GPi target.
There are several limitations to this study. The study was designed to evaluate LUTS in PD patients slated for DBS implantation but without regard to their urologic function. Only 2 patients had severe baseline symptoms by questionnaire and it is unknown whether results would differ for those presenting with more consistently severe symptoms. Further, while there were no statistically significant differences seen between several important variables in the target groups, target selection was not blinded or randomized. It was intentially influenced by some characterizations of the patient’s disease due to our target selection process making truly objective comparisons of urologic changes post surgery between the STN and GPi group difficult. Truly blinding physicians to target selection and randomizing patients would be challenging and even unethical without more definitive data on the value of LUTS in target selection at this point. An additional limitation is the lack of urodynamic investigations in these patients, which, unfortunately, could not be performed in this study. Urodynamics provide data that would appear less susceptible to bias than questionnaire scores. Questionnaire scores are subject to inherent patient response bias as patients can develop coping mechanisms to minimize bothersome symptoms. Also, improvement in motor symptoms post DBS could influence responses about urologic changes post surgery as they become more mobile and are more easily able to get to the bathroom. Finally, the sample size, while large for some DBS studies, is small to make meaningful comparisons among targets.
Conclusion
We found that DBS overall improved LUTS QOL scores post surgery. In the STN target, QOL scores were also significantly improved. Other survey scores demonstrated no definitive changes in the overall cohort or when analyzed individually.- Comparison of the changes post surgery between targets was limited by the lack of blinded randomization and sample size in this pilot trial. A larger more in depth trial could uncover differences as there were some suggestive trends in the current data. To our knowledge, no prior study has directly investigated the effect of pallidal DBS in Parkinson’s disease on LUTS let alone compared it to the STN target. These results suggest that there could be a difference in the effect of STN vs. GPi-DBS on LUTS, and emphasizes the need for more extensive, randomized studies looking at the nonmotor features of Parkinson’s disease like LUTS in both the GPi and STN target.
Acknowledgments
Financial Support: There was no direct financial support for this project. However, this project was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences, which provided the use of Redcap, an online database, for research. Some of the work was done using the CranialVault atlas demonstrating lead localization was supported by NIH R01 EB006136 and NIH R01 NS095291 09.
Footnotes
Authorship Statement: All authors met criteria for authorship with all contributing to the conception of the project; design of the analysis; interpretation of the data; and drafting and writing of the manuscript. Drs. Mock, Osborn and Tolleson collected and analyzed the data as well as prepared the manuscript with important intellectual input from Drs. Brown, Reynolds and Dmochowski. Mr. Turchan, Dr. Pallavaram and Mr. Rodriguez assisted with statistical support and lead localization. All authors discussed the results and approved the final manuscript.
Conflicts of interest: Dr. Tolleson has done consulting work for Medtronic. Dr. Pallavaram is a stockholder in Neurotargeting, LLC that licenses the software suite from Vanderbilt University that was used for lead localization. Drs. Mock, Osborn, Brown, Reynolds, Dmochowski and Mr. Turchan and Mr. Rodriguez have no conflicts of interest to disclose.
References
- 1.Zesiewicz TA, Sullivan KL, Arnulf I, Chaudhuri KR, Morgan JC, Gronseth GS, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74(11):924–931. doi: 10.1212/WNL.0b013e3181d55f24. [DOI] [PubMed] [Google Scholar]
- 2.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59(3):408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25(15):2493–2500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- 4.Sung VW, Nicholas AP. Nonmotor symptoms in Parkinson’s disease: expanding the view of Parkinson’s disease beyond a pure motor, pure dopaminergic problem. Neurol Clin. 2013 Aug;31(3 Suppl):S1–S16. doi: 10.1016/j.ncl.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Lim SY, Lang AE. The nonmotor symptoms of Parkinson’s disease--an overview. Mov Disord. 2010;(Suppl 1(25)):S123–S130. doi: 10.1002/mds.22786. [DOI] [PubMed] [Google Scholar]
- 6.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52(5):784–788. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 8.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro JM, Pahwa R, Lyons KE. The impact of bilateral subthalamic stimulation on non-motor symptoms of Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(8):606–609. doi: 10.1016/j.parkreldis.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Reich M, Ray Chaudhuri K, Ashkan K, Hulse N, Costello A, Moriarty J, et al. Changes in the non-motor symptom scale in Parkinson’s disease after deep brain stimulation. Basal Ganglia. 2011;1(3):131–133. [Google Scholar]
- 11.Sakakibara R, Tateno F, Kishi M, Tsuyuzaki Y, Uchiyama T, Yamamoto T. Pathophysiology of bladder dysfunction in Parkinson’s disease. Neurobiol Dis. 2012;46(3):565–571. doi: 10.1016/j.nbd.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Winge K, Werdelin LM, Nielsen KK, Stimpel H. Effects of dopaminergic treatment on bladder function in Parkinson’s disease. Neurourol Urodyn. 2004;23(7):689–696. doi: 10.1002/nau.20054. [DOI] [PubMed] [Google Scholar]
- 13.Badri AV, Purohit RS, Skenazy J, Weiss JP, Blaivas JG. A review of lower urinary tract symptoms in patients with Parkinson’s disease. Curr Urol Rep. 2014;15(9):435–443. doi: 10.1007/s11934-014-0435-0. [DOI] [PubMed] [Google Scholar]
- 14.Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, et al. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain. 2008;131(Pt 1):132–145. doi: 10.1093/brain/awm254. [DOI] [PubMed] [Google Scholar]
- 15.Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, et al. Subthalamic stimulation modulates cortical control of urinary bladder in Parkinson’s disease. Brain. 2006;129:3366–3375. doi: 10.1093/brain/awl302. [DOI] [PubMed] [Google Scholar]
- 16.Finazzi-Agro E, Peppe A, D’amico A, Petta F, Mazzone P, Stanzione P, et al. Effects of subthalamic nucleus stimulation on urodynamic findings in patients with Parkinson’s disease. J Urol. 2003;169(4):1388–1391. doi: 10.1097/01.ju.0000055520.88377.dc. [DOI] [PubMed] [Google Scholar]
- 17.Seif C, Herzog J, van der Horst C, Schrader B, Volkmann J, Deuschl G, et al. Effect of subthalamic deep brain stimulation on the function of the urinary bladder. Ann Neurol. 2004;55(1):118–120. doi: 10.1002/ana.10806. [DOI] [PubMed] [Google Scholar]
- 18.Winge K, Nielsen KK, Stimpel H, Lokkegaard A, Jensen SR, Werdelin L. Lower urinary tract symptoms and bladder control in advanced Parkinson’s disease: effects of deep brain stimulation in the subthalamic nucleus. Mov Disord. 2007;22(2):220–225. doi: 10.1002/mds.21253. [DOI] [PubMed] [Google Scholar]
- 19.Mordasini L, Kessler TM, Kiss B, Schüpbach M, Pollo C, Kaelin-Lang A. Bladder function in patients with dystonia undergoing deep brain stimulation. Parkinsonism Relat Disord. 2014;20(9):1015–1017. doi: 10.1016/j.parkreldis.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 21.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128(Pt 10):2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 23.Konrad PE, Neimat JS, Yu H, Kao CC, Remple MS, D’Haese PF, et al. Customized, miniature rapid-prototype stereotactic frames for use in deep brain stimulator surgery: initial clinical methodology and experience from 263 patients from 2002 to 2008. Stereotact Funct Neurosurg. 2011;89(1):34–41. doi: 10.1159/000322276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord. 2006;21(S14):S259–S283. doi: 10.1002/mds.20960. [DOI] [PubMed] [Google Scholar]
- 25.Barry MJ, Fowler FJ, Jr, O’Leary MP, Brushewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 26.Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–574. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 27.Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM):a 5-year review of research and clinical experience. Int J Impot Res. 2005;17(4):307–319. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- 28.D’Haese P, Pallavaram S, Kao C, Neimat JS, Konrad PE, Dawant BM. Effect of data normalization on the creation of neuro-probabilistic atlases. Stereotact Funct Neurosurg. 2013;91:148–152. doi: 10.1159/000345268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pallavaram S, Dawant BM, Koyama T, Yu H, Neimat J, Konrad PE, et al. Validation of a fully automatic method for the routine selection of the anterior and posterior commissures in magnetic resonance images. Stereotact Funct Neurosurg. 2009;87:148–154. doi: 10.1159/000209295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thobois S, Mertens P, Guenot M, Hermier M, Mollion H, Bouvard M, et al. Subthalamic nucleus stimulation in Parkinson’s disease: clinical evaluation of 18 patients. J Neurol. 2002;249(5):529–534. doi: 10.1007/s004150200059. [DOI] [PubMed] [Google Scholar]
- 31.Romito LM, Raja M, Daniele A, Contarino MF, Bentivoglio AR, Barbier A, et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17(6):1371–1374. doi: 10.1002/mds.10265. [DOI] [PubMed] [Google Scholar]
- 32.Halbig TD, Tse W, Frisina PG, Baker BR, Hollander E, Shapiro H, et al. Subthalamic deep brain stimulation and impulse control in Parkinson’s disease. Eur J Neurol. 2009;16(4):493–497. doi: 10.1111/j.1468-1331.2008.02509.x. [DOI] [PubMed] [Google Scholar]
- 33.Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Multicenter Wensing C Advanced Parkinson’s Disease Deep Brain Stimulation Group. Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23(3):416–421. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]