Abstract
Glucocorticoids (GCs) regulate distinct physiological processes in the developing fetus, in particular accelerating organ maturation that enables the fetus to survive outside the womb. In preterm birth, the developing fetus does not receive sufficient exposure to endogenous GCs in utero for proper organ development predisposing the neonate to complications including intraventricular hemorrhage, respiratory distress syndrome (RDS) and necrotizing enterocolitis (NEC). Synthetic GCs (sGCs) have proven useful in the prevention of these complications since they are able to promote the rapid maturation of underdeveloped organs present in the fetus. While these drugs have proven to be clinically effective in the prevention of IVH, RDS and NEC, they may also trigger adverse developmental side effects. This review will examine the current clinical use of antenatal sGC therapy in preterm birth, their placental metabolism, and their effects on the developing brain.
Keywords: antenatal, glucocorticoids, preterm birth, neurodevelopment
Clinical Use of Antenatal Synthetic Glucocorticoid Therapy
Glucocorticoids (GCs) regulate distinct physiological processes in the developing fetus, in particular accelerating organ maturation that enables the fetus to survive outside the womb 1–4. In preterm birth, the developing fetus does not receive sufficient exposure to endogenous GCs in utero for proper organ development predisposing the neonate to complications including intraventricular hemorrhage (IVH), respiratory distress syndrome (RDS) and necrotizing enterocolitis (NEC). Synthetic GCs (sGCs) have proven useful in the prevention of these complications since they are able to promote the rapid maturation of underdeveloped organs present in the fetus 1-4. While these drugs have proven to be clinically effective in the prevention of IVH, RDS and NEC, they may also trigger adverse developmental side effects 5. This review will examine the current clinical use of antenatal sGC therapy in preterm birth, their placental metabolism, and their effects on the developing brain.
Current clinical guidelines indicate that mothers at risk of premature delivery before 34 weeks of gestation are candidates for antenatal sGC therapy, as administration does not demonstrate significant reductions in morbidity or mortality after this time 1,4,6. One exception to this recommendation is in the case of suspected pulmonary insufficiency 6. Recommended treatment courses include two doses of 12mg betamethasone administered intramuscularly 24 hours apart or four doses of 6mg dexamethasone administered intramuscularly every 12 hours. This treatment is not standardized across the United States and treatment courses may be administered multiple times 6, despite recent evidence that suggests that multiple doses of antenatal sGC do not significantly improve clinical outcomes 7. In fact, the Multiple Courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5 study) indicated that multiple courses of antenatal sGC may have an increased risk for neurodevelopmental and neuropsychiatric conditions at the age of five years providing evidence against the use of multiple courses 7.
While antenatal sGC therapy remains the standard of care in the antenatal prevention of IVH, RDS and NEC, enthusiasm for postnatal sGC therapy for treatment or prevention of bronchopulmonary dysplasia (BPD) has diminished due to a number of studies demonstrating negative effects on brain development in preterm infants without a significant long-term improvement in pulmonary health 8,9. Specifically, postnatal sGC treatment has been found to increase the risk for cerebral palsy 8,10–13.
While detrimental side effects have been observed after multiple courses of antenatal sGC therapy and postnatal sGC therapy, it is widely accepted that single dose sGC therapy does not have significant side effects. However a 2014 review highlighted that even a single course of antenatal sGC can result in an 18% reduction in birth weight, a 9% decrease in head circumference, 6% decrease in body length, as well as placental abnormalities when compared with both preterm and term unexposed infant 14. Additionally relevant are the particular outcomes based on the timing of sGC administration prior to delivery. While the maximal benefit occurs after 24 hours but within 7 days of maternal administration, it is not yet clear if longer exposure in utero may add to or detract from detrimental neurodevelopmental effects 14.
The benefits of antenatal sGCs, if administered properly, provide significant improvements in infant health, as highlighted by two recent analyses 10,15. In fact, a single course of antenatal sGC therapy improved most neurodevelopmental outcomes studied in infants born prior to 34 weeks gestation 10. A large study recently published by the Eunice Kennedy Shriver NICHD Neonatal Research Network which examined very early gestation births revealed a significant reduction in death and neurodevelopmental disability at 23, 24 and 25 weeks gestation, but not prior to 23 weeks 16. This evidence suggests that antenatal sGC therapy is most beneficial to the infant at the limits of viability. This NICHD study also found a significant reduction in death, IVH, periventricular leukomalacia (PVL) and NEC.
While antenatal sGC therapy is indispensable in the prevention of IVH, RDS and NEC associated with preterm birth, it remains unclear as to the proper or optimal timing or dosage of these medications. Exposure to sGCs in utero at critical developmental stages can lead to alterations in the function of many organ systems that extend into adult life. The consequences of this altered “fetal organ programming” are poorly characterized in humans, but it is well documented in animal models that antenatal exposure to sGCs can alter the function of the adult hypothalamic-pituitary-adrenal (HPA) axis, glucose homeostasis, and the cardiovascular system 2,3,17,18. In addition, the long-term effects of sGC exposure at different developmental stages of fetal growth are not well known. This is particularly true for infants whose in utero exposure is lengthened by failure to delivery prematurely. Of particular concern are the approximately 70% of pregnant women who are given sGC for threatened preterm labor but do not deliver within 7 days, the accepted time of maximal benefit 17. While antenatal sGC therapy is administered routinely, there is growing evidence for incomplete benefit, particularly in certain subpopulations of infants, and for sex specific benefits, further expressing the need for individualized therapy in the clinical setting. Specifically, understudied variables that may affect the response to antenatal sGC therapy include birth weight, multiple gestation, race and sex.
Demographic Variables in the Response to Antenatal sGC Therapy
As previously discussed, antenatal sGC therapy is given to all mothers at risk of premature delivery before 34 weeks gestation. Interestingly, there is no difference in the dosage, timing or specific sGC used in antenatal sGC therapy despite variability in birth weight, multiple gestation, ethnicity, sex or specific genetic polymorphisms, even though significant differences have been observed in clinical outcomes 19–22. With ideal administration of sGC, one recent study suggests that nearly 40% of infants still present with RDS in the neonatal period 23. In order to improve clinical outcomes, it is essential to understand the variables that affect the response to antenatal sGC therapy. This section will outline a number of observations that highlight demographic variables, which need to be assessed in order to individualize and optimize antenatal sGC therapy.
A recent study looking at antenatal sGC therapy in infants with a birth weight of less than 1500 grams (growth-restricted fetuses) demonstrated no significant short or long-term benefit between the exposed and unexposed infants. This suggests that antenatal sGC may not be beneficial for all populations of infants 19. In fact, RDS risk was increased in lower gestational age infants, and this was independent of whether or not a completed course of antenatal sGC therapy was achieved, suggesting that these infants may be needlessly exposed to potent therapeutics. As mentioned previously, there is no significant benefit of antenatal sGC therapy if administered prior to 23 weeks gestation 16. Because of the adverse developmental side effects, it is important to consider whether the benefits outweigh the risks in certain sub-populations of infants.
Multiple-gestation studies have been performed looking at benefit of single course of antenatal sGC therapy. They have consistently found that multiple-gestations respond as singletons, but one study suggested that this was only true if the duration of antenatal sGC therapy optimally occurred between 2 and 7 days after maternal administration 20. In addition, very low birth weight multiple gestation infants exposed to ACS may respond as well as their singleton counterparts 24, but it is not clear if this applies to exposure out of the optimal window for delivery.
Differential outcomes in patients exposed to sGC in utero could result from individual genetic variations in steroid metabolism or pulmonary gene specific single nucleotide polymorphisms (SNPs). In small for gestational age infants, these SNPs have been associated with differences in the reduction in RDS 25. Clinical data suggests that the effect of antenatal sGC therapy is limited in intrauterine growth restricted fetuses (IUGR) and that ‘it remains unclear if ACS therapy is beneficial due to heterogeneous populations and treatment regimes’ 26.
Studies evaluating the influence of maternal ethnicity have also been performed and have reported that maternal ethnicity is ‘independently associated with neonatal respiratory outcomes’ after exposure to antenatal sGC therapy 21. Also, a recent report showed that when controlling for gestational age as well as size, Caucasian infants have significantly higher incidence of RDS than infants who were not Caucasian 27.
In addition to differences in outcome based on ethnicity, a number of studies report different clinical outcomes of antenatal sGC therapy based on fetal sex. One particular study examined VLBW infants and found that ‘compared with girls, VLBW male newborns are at greater risk to develop IVH and severe IVH but not PVL’ 28. However, an earlier study found that fetal sex in intrauterine growth restricted (IUGR) fetuses did not account for different clinical outcomes of sGC exposure 29. Additionally, ‘the association of male sex with IVH or severe IVH is stronger with higher birthweights’ 28. Roberge, et al found that the ‘use of betamethasone was associated with a significant decrease in the rate of RDS in males, but dexamethasone was not. Conversely, dexamethasone use was significantly beneficial in females, but betamethasone was not,’ suggesting differential pharmacogenetics based on sex specificity 22. Lim, et al, confirmed that with VLBW infants, male sex was associated with worsened outcomes in their particular study population 30.
One potential explanation for the observed differences in sex is a recent study looking at placental oxidation state, which found that in steroid exposed infants, there was a male specific imbalance, possibly contributing to ‘processes underlying oxygen radical diseases of the newborn, conditions known to exhibit a male excess’ 16. Stark, et al have also shown a sex specific effect on antenatal sGC therapy and microvascular blood flow and endothelium dependent vasodilation, specifically within 72 hours of exposure 16. Supportive of a sex specific alteration in oxidation state, a recent study by Vento, et al, demonstrated a significant reduction in oxidative stress and better clinical outcomes in female compared to male infants, independent of antenatal sGC therapy 31. Other findings included ‘a reduction in postnatal oxidative stress derived conditions,’ again influenced by timing of sGC administration and female sex 31. These studies highlight the need for individualized dosing based on fetal sex and further highlight the need for studies evaluating the dosing as well as the specific sGC (i.e. dexamethasone and betamethasone), which may affect response to antenatal sGC therapy in certain subpopulations of infants.
Genetic polymorphisms of the glucocorticoid receptor (GR) also contribute to differences observed in outcomes after antenatal sGC therapy. A 2012 study highlighted the difficulty that females face when exposed to sGC therapy showing that there is a significantly increased stress reactivity in term born children, with females faring worse than males 18. A recent study looking at sex specific effects of antenatal sGC exposure and genetic variations leading to differing sensitivity of the GR found that particular genetic variations were associated with IQ and behavior modifications in the young adult, who was exposed to sGC antenatally 32. Additionally, GR and mineralocorticoid receptor (MR) polymorphisms interacted together to produce intellectual differences in those exposed 32.
In summary, there are many variables, which need to be taken into account when choosing the optimal course of antenatal sGC therapy. This is of particular importance because clinical outcomes are significantly affected by differences in birth weight, multiple-gestation, ethnicity, sex and genetic polymorphisms, especially those in GR and MR. In addition, there are multiple sGCs to consider including dexamethasone and betamethasone, and although not as well studied, it appears that timing of antenatal sGC therapy also affects clinical outcomes. Taken together, these elements construct a complicated clinical conundrum which indicates that more work is needed to determine how best to individualize therapy to achieve the most beneficial response to antenatal sGC therapy.
GC Metabolism and Signaling in Pregnancy
Typically, endogenous GC levels in fetal circulation increase rapidly late in gestation to promote the development of fetal organs including the lungs, kidney and brain, utilizing the receptor for cortisol, GR, which is expressed throughout gestation, a finding confirmed in studies from our lab in mice 2,3,33. Throughout development, the placenta tightly regulates fetal GC levels, while the HPA axis regulates maternal GC. The HPA axis regulates levels of cortisol through the secretion of corticotrophin releasing hormone (CRH) from the paraventricular nucleus (PVN) of the hypothalamus and vasopressin from the posterior pituitary gland, which in turn triggers the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland. ACTH stimulates secretion of cortisol from the adrenal glands. Circulating cortisol provides negative feedback by activating GR and mineralocorticoid receptor (MR) in the hippocampus and GR in the hypothalamus and anterior pituitary gland to maintain homeostasis 2,3,34.
Dysregulation of negative feedback within the HPA axis leads to increased systemic levels of GC, which can lead to adverse effects including disorders of mental and metabolic health. Specifically, exposure to high levels of GC or sGC in utero have been observed to lead to an increased risk for disorders of HPA axis function later in life, as well as cardiovascular, reproductive and metabolic abnormalities 2,3,5,35. A recent study examined antenatal betamethasone administration and demonstrated that antenatal sGC therapy induces immediate hormonal alterations in the fetus36. Depending on the nature of the exposure, HPA function may demonstrate increased or decreased reactivity to GC 2,3,37–39. Neural stem/progenitor cells (NSPCs) are especially sensitive to increased levels of GCs, and recent evidence suggests the extent of exposure may have life-long effects on NSPCs, which ultimately shape brain development and behavior 39–45. It is important to recognize that many of the effects of GCs in the developing brain are necessary for proper development and have positive effects including NSPC proliferation, differentiation and survival 2,3,39.
In human gestation, levels of GC greatly increase starting around week 12 of gestation in maternal circulation due an increase in the release of CRH from the maternal hypothalamus. During the third trimester, the concentration of GCs in maternal circulation continue to increase due to secretion of CRH by the placenta. While cortisol suppresses the secretion of CRH from the hypothalamus, it increases the secretion of CRH from the placenta, creating a positive feedback loop that stops only after birth 34. This physiologic increase in GC is necessary for the maturation of fetal organs before birth in term born infants. This is the basis for antenatal sGC therapy, as infants born at an early gestational age will not have adequate exposure to GC leading up to parturition, and thus, organ maturation will lag behind.
Maternal cortisol reaching the fetus is regulated through the activity of placental enzymes that degrade endogenous glucocorticoids. Specifically, the enzyme 11ß-hydroxysteroid dehydrogenase type-2 (HSD11B2) is responsible for the conversion of cortisol to its inactive metabolite cortisone 34. The placenta effectively separates the maternal and fetal HPA axes during gestation. A tightly regulated decrease in placental expression of HSD11B2 takes place around 38-40 weeks of gestation, which allows maternal cortisol to enter fetal circulation late in gestation 46. Proper timing of this event is crucial since it results in the final maturation of fetal organs in preparation for life outside the womb.
Antenatally administered sGC take the place of maternal cortisol in promoting organ development in preterm birth, but it is important to note that there are differences in their pharmacologic properties. The two most common sGCs administered to pregnant females are betamethasone and dexamethasone, which are fluorinated corticosteroids. Not only are they 25 times more potent than cortisol, but they are also resistant to metabolism by HSD11B2 47. Therefore, they are freely able to cross the placenta and enter fetal circulation independent of the tightly regulated decrease in HSD11B2 late in pregnancy. Because sGC are often administered prior to the endogenous cortisol surge, and freely pass through the placenta unchanged, they trigger GR signaling in the developing fetus which not only prevent negative consequences of preterm birth but also potentially increase risks of metabolic disease, heart conditions, and neuropsychiatric disorders in adulthood 2,3,28. It is also important to note that betamethasone and dexamethasone have demonstrated differences in terms of clinical outcome, although they have striking similarity at a chemical level. Betamethasone has demonstrated a greater decrease in mortality and fewer side effects compared to dexamethasone, making betamethasone the current drug of choice for antenatal sGC therapy 47.
Cerebral Cortical Development
While there are clear benefits for sGC administration to reduce the risk of IVH and the subsequent neurological abnormalities that arise, recent evidence indicates that sGC alter neurogenesis leading to longer term changes in stress and anxiety related behaviors later in life. The majority of cells in the developing brain are derived from neural stem/progenitor cells (NSPCs) that line the developing ventricles and arise in a precisely timed spatial pattern. The timing of neurogenesis and gliogenesis are tightly controlled and regulated such that neurogenesis occurs before birth in humans, followed by the subsequent formation of oligodendrocytes and astrocytes 48–52. Human and mice NSPCs express GR from early developmental stages and have been shown to respond to GC stimulation in-vitro indicating that the developing brain is poised to respond to premature GC administration and activate downstream signaling 33,53. Since GCs have been shown to have anti-proliferative effects on NSPCs 51,54, GC exposure is likely to have effects on neurogenesis and gliogenesis.
The orderly production of cell types in the mouse cerebral cortex largely reflects the development of the human cortex 55 (Figure 1). At the onset of neurogenesis in the mouse cortex, neuroepithelial cells divide to form RGCs, which are distinguished by their expression of Pax6 and are located in the ventricular zone (VZ), adjacent to the lateral ventricles 49,56. RGCs can divide symmetrically to give rise to two RGCs or asymmetrically to give rise to an RGC (inner RGC or iRGC) and to cells in the second proliferative population in the subventricular zone (SVZ) located above the VZ. In both human and mice the second proliferative population in the SVZ consists of two distinct cell types, outer RGC cells (oRGCs) and intermediate progenitor cells (IPCs), although the relative proportions of each cell type differs between humans and lower species. IPCs and oRGCs divide to give rise to the majority of neurons in the cortex 49,57–59. The cells born from progenitors in the VZ and SVZ ultimately migrate and give rise to the exquisitely organized six layers of neurons that populate the cortical plate. Essential to the development of the individual layers of the cortex is temporal patterning, which is controlled by intrinsic processes, intracellular communication and environmental influences 60,61. Throughout brain development, neurons create connections through the process of synaptogenesis, which is essential for the establishment of cortical architecture, and this continues postnatally 49.
Figure 1. Cerebral cortical development.
The cellular organization of the human and mouse cerebral cortex follow a similar developmental pattern. VZ: ventricular zone, SVZ: subventricular zone, IZ: intermediate zone, SP: subplate, CP: cortical plate, MZ: marginal zone. (I-VI: invidual layers of the cortex). On the upper portion of the figure, the timeline for antenatal synthetic glucocorticoid (sGC) administration is depicted. Different cell types are represented by different colors. (Progression of human architectural organization adapted from: Volpe, J Neurology of the Newborn, Fifth ed. p. 83; Mrzljak L, Uylings HBM, Kostovic I, et al: Prenatal development of neurons in the human prefrontal cortex. I. A qualitative Golgi study, J Comp Neurol 271:355-386, 1988)
In addition to neurons, glial cell types, including astrocytes and oligodendrocytes (OLGs), populate the cortex and account for the majority of cells in the brain 49. These cells are not only responsible for maintaining homeostasis but also play an essential role in cortical development 49,62,63. Disturbing proliferation, differentiation, migration or maturation of neuronal or glial cell types could lead to changes in not only the number of cells that are formed but also changes in cortical architecture and synaptic connectivity.
While there are many similarities between the development of the murine and human cortex, there are significant differences in the relative timing of cell fate decisions (Figure 1). The neural tube of humans closes around embryonic day (E) 30 and is composed of neuroepithelial cells 50,64. These cells divide symmetrically to form the VZ around E33, beginning the process of neurogenesis very early in gestation 50,65. As RCGs begin to appear from neuroepithelial cells within the VZ, they divide and migrate forming cells in the SVZ. In humans, as well as other primates, precursors within the SVZ begins to demonstrate neuronal and glial fate specificity early in development and organize into inner and outer sublayers divided by tangentially oriented fibers around gestational week 20 (GW20) 50,66. By GW26, past the earliest time point of sGC administration, the human VZ is only one layer thick while cells in the SVZ are still actively proliferating 50,67,68. Migration of neurons to the CP is complete by the third trimester, and the six layers of the cortical plate are identifiable by the seventh month of human gestation 50,69.
The divisions of the SVZ are much more pronounced in humans than rodents and the outer SVZ of humans contains an extraordinary number of RGCs, which give rise to a substantial number of both progenitor cells and neurons. In addition, RGCs and IPCs within human SVZ have a greater proliferative capacity than in mice. The difference between the SVZ of humans most likely accounts for the expansion of the primate cortex 49,70–72. Another unique characteristic of the human SVZ is that it is able to give rise to interneurons, whereas all mouse interneurons in the cortex are thought to migrate from the basal telencephalon 50.
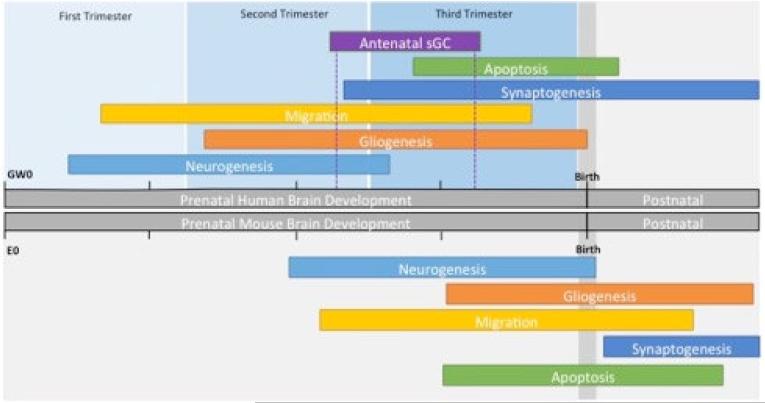
In addition to anatomical differences, the timing of neurogenesis, gliogenesis, migration, synaptogenesis and apoptosis are not identical in humans and mice, but follow the developmental timeline 51,73. Human neurogenesis begins around gestational week GW 5-6 and continues rapidly until around the time of 26 weeks gestation 49,51. This is followed by gliogenesis, which begins around GW 13 and continues late into the fetal growth period 49,51. Human neural migration occurs mainly between GW18-36, with the majority of apoptosis and pruning occurring between GW34-41 49,51. Mouse neurogenesis begins around E11-13 and continues rapidly until around the time of birth at E20-21, followed by gliogenesis, beginning around E12 and continuing into the early postnatal period 49,51. In the mouse, neural migration begins at approximately E14, while the majority of apoptosis and pruning are between E18-P21 40,42,51,74. These dates are depicted graphically in Figure 2.
Figure 2. Comparison of human and mouse brain development.
The top panel depicts the timing of human brain development. Gestational age is defined by gestational weeks (GW) which is split into the trimesters of human pregnancy. Above, the timing of neurogenesis, gliogenesis, migration, synaptogenesis and apoptosis are depicted by the length of the rectangles. The timing of antennal sGC therapy is depicted similarly. The bottom panel depicts the timing of mouse brain development. Gestational age is defined by embryonic days (E). The major developmental milestones are depicted similarly to the top panel. (Figure created from data from the following sources: Oomen, C. A. et al. 2009; Braun, M., et al. 2003., Jiang, X. et al. 2015; Pressler, R. et al. 2013; Knuesel, I. et al. 2014. O'Rahilly, R. et al. 2006; Rakic, P. 1977; Carpenter, M. K. et al. 2001; Bayatti, N. et al. 2008; Simonati, A. et al. 1999; Rakic, P, 1974; Chan W.Y. et al. 2002.)
While mice serve as a convenient and well-studied model for human physiology and disease, it is important to understand their limitation as a model for studying the development of the cerebral cortex. In the context of brain development after antenatal sGCs therapy, the major differences in cerebral cortical development between species must be taken into account when interpreting outcomes.
GC Signaling in the Developing Brain
Many nuclear receptors, including retinoic acid receptors, thyroid hormone receptors and the glucocorticoid receptor, are expressed in NSPCs in the developing brain and play essential roles in development and metabolism 75. GCs are lipophilic and thus can freely cross the plasma membrane to enter the cytoplasmic compartment of cells. Once in the cytoplasm, GC binds GR, which is bound in a complex consisting of heat shock protein (HSP) 90, HSP70 and immunophilin proteins such as FKBP2. Following the binding of ligand, GR is released from this complex and translocates to the nucleus to regulate the transcription of target genes in the classical “genomic” pathway. GR can also rapidly activate many signaling molecules in the cytoplasm independent of its nuclear action. These non-classical, non-genomic GR signaling pathways are proposed to play a role in various physiological actions of GCs 54,76-78.
In the developing brain, the classical genomic GR signaling pathway is important for the regulation of genes involved in the stabilization of vascular endothelial cells and proliferation of neural stem/progenitor cells (NSPCs) 11,79. The stabilization of vascular endothelial cells by GR likely plays an important role in decreasing the risk of IVH after preterm birth through the stabilization of the delicate vasculature of the developing blood barrier, which is often the primary site of hemorrhage in IVH 11,80,81. In NSPCs, sGC exert dose dependent anti-proliferative effects via various mechanisms 82-91. These anti-proliferative effects could ultimately trigger lasting effects on neural circuitry and contribute to some of the cognitive and behavioral impairments observed in some infants exposed to sGC antenatally 2,3,18,84, 85,92.
GR also influences NSPC proliferation through a non-genomic pathway by regulating the activity of connexin 43 (Cx43) containing gap junctions. Gap junction-dependent spontaneous Ca2+ waves have been demonstrated to be necessary for maintaining NSPC proliferation and for normal neurogenesis in the developing cortex 65,74,76. Cx43 facilitate gap junction intracellular communication (GJIC), which allows adjacent NSPCs in the VZ to communicate, and either proliferate or exit the cell cycle as a group 61. Plasma membrane associated GR activates a mitogen-activated protein kinase-dependent phosphorylation of Cx43 at specific sites, which disrupts GJIC and synchronous cell cycle progression 54. In fact, a one-hour rapid exposure to dexamethasone reduced S-phase progression 54. Furthermore, these rapid actions of GC required the localization of GR in Cav-1 enriched lipid rafts 54. Our lab has shown that Cav-1 is necessary for the phosphorylation of Cx43 as GR dependent phosphorylation of Cx43 was not observed in Cav-1 knockout animals 54. These studies suggest that GR affects NSPC proliferation and differentiation through multiple mechanisms, each of which could provide unique drug targets to modulate antenatal GC action in the developing brain.
We have also found that the GR non-genomic pathway in NSPCs does not act independently, but rather indirectly modulates a genomic transcriptional response 77. To understand whether the non-genomic GC pathway contributed to genomic action, we utilized Cav-1 knockout mice, interfering with non-genomic GR signaling. Our results demonstrated that non-genomic action of lipid-raft associated GR was required for efficient phosphorylation of GR at a specific site, Serine 211. Furthermore, the GR transcriptome was altered in Cav-1 knockout mice, further suggesting that transcriptional output may be altered by Cav-1 interactions 77. Site-specific phosphorylation of GR at serine 211 may contribute to differential transcriptional outcomes of sGC exposure of NSPCs as evidenced by the differential recruitment of phosphorylated GR to select target genes of wild type versus Cav-1 knockout mouse NSPCs. One target gene whose expression and GR recruitment required crosstalk with the non-genomic GR signaling pathway was serum and glucocorticoid-regulated kinase (SGK-1), which regulates NSPC proliferation. Other affected gene networks included those responsible for organ development, axon guidance and protein degradation 77. Pharmacological manipulation of these interacting pathways could provide better control of the diverse transcriptome activated by GCs. These manipulations could help to prevent the negative outcomes associated with sGC treatment, or modulate the GR response in such a way as to eliminate groups of infants who are less responsive to antenatal sGC therapy.
Conclusion
In conclusion, it cannot be understated that the antenatal administration of synthetic GC dramatically reduces the complications of prematurity and is a major success of neonatal-perinatal medicine. Throughout the developed and developing world, antenatal GC administration improves the lives of infants. However, recent studies suggest that this profound benefit may not be without the potential for detrimental effects on the developing brain and metabolic system. Additionally concerning is that certain groups of infants, based on birth weight, placental insufficiency, sex and race, may in fact respond differently to sGC in the clinical setting. For infants who incompletely respond, it becomes unclear if the dosing strategies are sufficient to account for genetic differences between fetuses. Future research should focus on expanding the clinical guidelines to account for differences in the fetus. Personalized fetal medicine, taking into account not only the phenotype of the fetus, but perhaps the genetic makeup of the fetus, and adjusting clinical protocols for these differences may lead to improved outcomes for infants born prematurely.
There is extensive evidence in animal models that antenatal exposure to sGC influences the developing brain. Evidence for adverse neurological outcomes is accumulating in humans exposed to sGC in-utero but born at term. However, what is clear is that the developing brain is primed to respond prematurely to sGC and that the timing of sGC exposure elicits a physiologic response in the developing fetus, which may not be reversible, as suggested by studies on fetal programming as well as magnetic resonance imaging studies of term born infants. In the pregnant female, the placenta acts as an intermediary between mother and fetus. Throughout much of gestation, this intermediary acts to tightly control the exposure of endogenously produced maternal GC to the fetus. Late in gestation, immediately prior to parturition, this placental control is loosened and fetal GCs rapidly rise. With sGC exposure, this intermediary is bypassed, and direct fetal exposure to GC occurs. It is this premature exposure and binding to GR that account for the substantial benefits that newborns experience after birth. However, bypassing the placental control of GC exposure leads to altered neurodevelopmental timing. Continued research is needed on this important topic to develop personalized fetal sGC therapeutic approaches. In summary, the future of antenatal sGC therapy must include pathways for the individual fetus, as opposed to a population based approach.
Acknowledgements
These studies were supported by funding from an ACS grant (RSG-09-054-0 1-GMC, APM), G. Harold and Leila Y. Mathers Charitable Foundation Award (APM), a Nuclear Receptor Signaling Atlas (NURSA) Data Resource Project from an NIH U24 Grant DK097748 (APM, DD), NIH T32-HD071834-01A1 (AR), NIH K12 HD052892-09 (AR), Magee-Womens Clinical Trainee Research Award (AR), and the University of Pittsburgh Medical Center Department of Pediatrics Endowed Instructorship (AR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth (Review). Cochrane Libr. 2013 doi:10.1002/14651858.CD004454.pub2.Copyright. [Google Scholar]
- 2.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: Outcomes. Nat. Rev. Endocrinol. 2014;10:391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 3.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 2014;10:403–11. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- 4.Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane database Syst. Rev. 2013;8:CD006764. doi: 10.1002/14651858.CD006764.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Khulan B, Drake AJ. Glucocorticoids as mediators of developmental programming effects. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:689–700. doi: 10.1016/j.beem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Miracle X, et al. Guideline for the use of antenatal corticosteroids for fetal maturation. J. Perinat. Med. 2008;36:191–196. doi: 10.1515/JPM.2008.032. [DOI] [PubMed] [Google Scholar]
- 7.Asztalos E, et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5). BMC Pregnancy Childbirth. 2014;14:272. doi: 10.1186/1471-2393-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole CH, et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. N. Engl. J. Med. 1999;340:1005–10. doi: 10.1056/NEJM199904013401304. [DOI] [PubMed] [Google Scholar]
- 10.Sotiriadis A, et al. Neurodevelopmental Outcome After a Single Course of Antenatal Steroids in Children Born Preterm: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2015;125:1385–96. doi: 10.1097/AOG.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 11.Vinukonda G, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41:1766–1773. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zia MTK, et al. Postnatal glucocorticoid-induced hypomyelination, gliosis, and neurologic deficits are dose-dependent, preparation-specific, and reversible. Exp. Neurol. 2015;263:200–13. doi: 10.1016/j.expneurol.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaeb SN, Stonestreet BS. Postnatal glucocorticoid-induced hypomyelination, gliosis, and neurologic deficits are dose-dependent, preparation-specific, and reversible. Exp. Neurol. 2016;278:1–3. doi: 10.1016/j.expneurol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Audette MC, Challis JRG, Jones RL, Sibley CP, Matthews SG. Synthetic glucocorticoid reduces human placental system a transport in women treated with antenatal therapy. J. Clin. Endocrinol. Metab. 2014;99:E2226–33. doi: 10.1210/jc.2014-2157. [DOI] [PubMed] [Google Scholar]
- 15.Young JM, et al. Associations of Perinatal Clinical and Magnetic Resonance Imaging Measures with Developmental Outcomes in Children Born Very Preterm. J. Pediatr. 2015 doi: 10.1016/j.jpeds.2015.11.044. doi:10.1016/j.jpeds.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Carlo WA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly BA, et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics. 2012;129:e1282–90. doi: 10.1542/peds.2011-3175. [DOI] [PubMed] [Google Scholar]
- 18.Alexander N, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J. Clin. Endocrinol. Metab. 2012;97:3538–44. doi: 10.1210/jc.2012-1970. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa H, et al. The Effects of Antenatal Corticosteroids on Short- and Long-Term Outcomes in Small-for-Gestational-Age Infants. Int. J. Med. Sci. 2015;12:295–300. doi: 10.7150/ijms.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuk J-Y, et al. Optimal time interval between a single course of antenatal corticosteroids and delivery for reduction of respiratory distress syndrome in preterm twins. Am. J. Obstet. Gynecol. 2013;209:256, e1–7. doi: 10.1016/j.ajog.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Haas DM, Sischy AC, McCullough W, Simsiman AJ. Maternal ethnicity influences on neonatal respiratory outcomes after antenatal corticosteroid use for anticipated preterm delivery. J. Matern. Fetal. Neonatal Med. 2011;24:516–20. doi: 10.3109/14767058.2010.506228. [DOI] [PubMed] [Google Scholar]
- 22.Roberge S, et al. Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: systematic review and meta-analysis. J. Obstet. Gynaecol. Canada JOGC = J. d'obstétrique gynécologie du Canada JOGC. 2011;33:216–26. doi: 10.1016/s1701-2163(16)34822-8. [DOI] [PubMed] [Google Scholar]
- 23.Claire L, Vieux R. Efficacy of antenatal corticosteroids according to maternal and perinatal factors: a retrospective cohort study. Am. J. Perinatol. 2015;32:1070–7. doi: 10.1055/s-0035-1548537. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto LN, Hornung RW, Lindsell CJ, Brewer DE, Donovan EF. Effects of antenatal glucocorticoids on outcomes of very low birth weight multifetal gestations. Am. J. Obstet. Gynecol. 2002;187:804–10. doi: 10.1067/mob.2002.125891. [DOI] [PubMed] [Google Scholar]
- 25.Borowski KS, et al. Neonatal Genetic Variation in Steroid Metabolism and Key Respiratory Function Genes and Perinatal Outcomes in Single and Multiple Courses of Corticosteroids. Am. J. Perinatol. 2015;32:1126–32. doi: 10.1055/s-0035-1549217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrance HL, Derks JB, Scherjon SA, Wijnberger LD, Visser GHA. Is antenatal steroid treatment effective in preterm IUGR fetuses? Acta Obstet. Gynecol. Scand. 2009;88:1068–73. doi: 10.1080/00016340903176784. [DOI] [PubMed] [Google Scholar]
- 27.Kavvadia V, Greenough A, Dimitriou G, Hooper R. Influence of ethnic origin on respiratory distress syndrome in very premature infants. Arch. Dis. Child. Fetal Neonatal Ed. 1998;78:F25–8. doi: 10.1136/fn.78.1.f25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed MA, Aly H. Male gender is associated with intraventricular hemorrhage. Pediatrics. 2010;125:e333–9. doi: 10.1542/peds.2008-3369. [DOI] [PubMed] [Google Scholar]
- 29.Quiñones JN, Stamilio DM, Coassolo KM, Macones GA, Odibo AO. Is fetal gender associated with adverse perinatal outcome in intrauterine growth restriction (IUGR)? Am. J. Obstet. Gynecol. 2005;193:1233–7. doi: 10.1016/j.ajog.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 30.Lim JW, Chung S-H, Kang DR, Kim C-R. Risk Factors for Cause-specific Mortality of Very-Low-Birth-Weight Infants in the Korean Neonatal Network. J. Korean Med. Sci. 2015;30(Suppl 1):S35–44. doi: 10.3346/jkms.2015.30.S1.S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vento M, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid. Redox Signal. 2009;11:2945–55. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 32.van der Voorn B, Wit JM, van der Pal SM, Rotteveel J, Finken MJJ. Antenatal glucocorticoid treatment and polymorphisms of the glucocorticoid and mineralocorticoid receptors are associated with IQ and behavior in young adults born very preterm. J. Clin. Endocrinol. Metab. 2015;100:500–7. doi: 10.1210/jc.2014-2843. [DOI] [PubMed] [Google Scholar]
- 33.Tsiarli M. a, Paula Monaghan a, Defranco DB. Differential subcellular localization of the glucocorticoid receptor in distinct neural stem and progenitor populations of the mouse telencephalon in vivo. Brain Res. 2013;1523:10–27. doi: 10.1016/j.brainres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togher KL, et al. Epigenetic regulation of the placental HSD11B2 barrier and its role as a critical regulator of fetal development. Epigenetics. 2014;9:816–22. doi: 10.4161/epi.28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker DJP, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34:841–5. doi: 10.1016/j.placenta.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 36.Sifianou P, Thanou V, Karga H. Metabolic and hormonal effects of antenatal betamethasone after 35 weeks of gestation. J. Pediatr. Pharmacol. Ther. 20:138–43. doi: 10.5863/1551-6776-20.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 39.Koutmani Y, Karalis KP. Neural stem cells respond to stress hormones: distinguishing beneficial from detrimental stress. Front. Physiol. 2015;6:77. doi: 10.3389/fphys.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oomen CA, et al. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4:e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suri D, et al. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol. Psychiatry. 2013;73:658–66. doi: 10.1016/j.biopsych.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–87. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- 43.Damsted SK, Born AP, Paulson OB, Uldall P. Exogenous glucocorticoids and adverse cerebral effects in children. Eur. J. Paediatr. Neurol. 2011;15:465–77. doi: 10.1016/j.ejpn.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Noorlander CW, De Graan PNE, Middeldorp J, Van Beers JJBC, Visser GHA. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J. Comp. Neurol. 2006;499:924–32. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- 45.Spinillo A, et al. Two-year infant neurodevelopmental outcome after single or multiple antenatal courses of corticosteroids to prevent complications of prematurity. Am. J. Obstet. Gynecol. 2004;191:217–24. doi: 10.1016/j.ajog.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Murphy VE, Clifton VL. Alterations in Human Placental 11β-hydroxysteroid Dehydrogenase Type 1 and 2 with Gestational Age and Labour. Placenta. 2003;24:739–744. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 47.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am. J. Obstet. Gynecol. 2004;190:878–881. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 48.Fox MD, et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X, Nardelli J. Cellular and molecular introduction to brain development. Neurobiol. Dis. 2015 doi: 10.1016/j.nbd.2015.07.007. doi:10.1016/j.nbd.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 51.Pressler R, Auvin S. Comparison of brain maturation among species: An example in translational research suggesting the possible use of bumetanide in newborn. Front. Neurol. 2013 Apr 1;4 doi: 10.3389/fneur.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson S, Vanderhaeghen P. Cortical neurogenesis from pluripotent stem cells: complexity emerging from simplicity. 2015:151–157. doi: 10.1016/j.conb.2014.03.012. doi:10.1016/j.conb.2014.03.012.Cortical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ninomiya E, et al. Glucocorticoids promote neural progenitor cell proliferation derived from human induced pluripotent stem cells. Springerplus. 2014;3:527. doi: 10.1186/2193-1801-3-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samarasinghe R. a., et al. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc. Natl. Acad. Sci. 2011;108:16657–16662. doi: 10.1073/pnas.1102821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molnár Z. Evolution of cerebral cortical development. Brain. Behav. Evol. 2011;78:94–107. doi: 10.1159/000327325. [DOI] [PubMed] [Google Scholar]
- 56.Asami M, et al. The role of Pax6 in regulating the orientation and mode of cell division of progenitors in the mouse cerebral cortex. Development. 2011;138:5067–78. doi: 10.1242/dev.074591. [DOI] [PubMed] [Google Scholar]
- 57.Campbell K, Götz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–8. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 58.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 59.Hartfuss E, Galli R, Heins N, Götz M. Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 60.van den Ameele J, Tiberi L, Vanderhaeghen P, Espuny-Camacho I. Thinking out of the dish: what to learn about cortical development using pluripotent stem cells. Trends Neurosci. 2014;37:334–342. doi: 10.1016/j.tins.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Cheng A, et al. Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev. Biol. 2004;272:203–216. doi: 10.1016/j.ydbio.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Ohtaka-Maruyama C, Okado H. Molecular Pathways Underlying Projection Neuron Production and Migration during Cerebral Cortical Development. Front. Neurosci. 2015;9:447. doi: 10.3389/fnins.2015.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–22. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 64.O'Rahilly RR, Müller F. The Embryonic Human Brain: An Atlas Of Developmental Stages. John Wiley & Sons; 2006. at < https://books.google.com/books?hl=en&lr=&id=-7A2JXzVOJwC&pgis=1>. [Google Scholar]
- 65.Rakic P. Prenatal development of the visual system in rhesus monkey. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1977;278:245–60. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- 66.Bayatti N, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex. 2008;18:1536–48. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simonati A, Tosati C, Rosso T, Piazzola E, Rizzuto N. Cell proliferation and death: morphological evidence during corticogenesis in the developing human brain. Microsc. Res. Tech. 1999;45:341–52. doi: 10.1002/(SICI)1097-0029(19990615)45:6<341::AID-JEMT2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 68.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–7. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 69.Chan WY, et al. Normal and Abnormal Development of the Human Cerebral Cortex. Neuroembryology. 2002;1:78–90. [Google Scholar]
- 70.Betizeau M, et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron. 2013;80:442–57. doi: 10.1016/j.neuron.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 71.Dehay C, Kennedy H, Kosik KS. The outer subventricular zone and primate-specific cortical complexification. Neuron. 2015;85:683–94. doi: 10.1016/j.neuron.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 72.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–9. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 73.Knuesel I, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014;10:643–60. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 74.Uhlén P, Fritz N, Smedler E, Malmersjö S, Kanatani S. Calcium signaling in neocortical development. Dev. Neurobiol. 2015;75:360–8. doi: 10.1002/dneu.22273. [DOI] [PubMed] [Google Scholar]
- 75.Androutsellis-Theotokis A, Chrousos GP, McKay RD, DeCherney AH, Kino T. Expression profiles of the nuclear receptors and their transcriptional coregulators during differentiation of neural stem cells. Horm. Metab. 2013;45:159–68. doi: 10.1055/s-0032-1321789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodriguez L, et al. Reduced phosphatidylinositol 4,5-bisphosphate synthesis impairs inner ear Ca2+ signaling and high-frequency hearing acquisition. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14013–8. doi: 10.1073/pnas.1211869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peffer ME, et al. Caveolin-1 Regulates Genomic Action of the Glucocorticoid Receptor in Neural Stem Cells. Mol. Cell. Biol. 2014;34:2611–2623. doi: 10.1128/MCB.01121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]