Abstract
Background/Objectives
Determine the prevalence of muscle weakness using the two 2014 Foundation for the National Institutes of Health (FNIH) Sarcopenia Project criteria and its relationship to physical limitations, basic and instrumental activities of daily living (ADL).
Subjects/Methods
We performed a cross-sectional analysis of community-dwelling adults from the Health and Retirement Study 2006-2008 and identified a subsample of 5,092 adults aged ≥60 years with grip strength data. Self-reported physical limitations, basic and instrumental activities of daily living (ADL) were assessed. Criteria for grip strength (GS) (men<26kg; women <16kg), and GS adjusted for body mass index (GS/BMI) (men <1.0; women <0.56) were applied to the sample. We determined prevalence of muscle weakness in each sex. Multivariable logistic regression was used to calculate the association of physical limitations, basic and instrument ADLs with weakness definitions e in each sex.
Results
Mean age was 72.1 years (54.9% female).Mean GS was 38.3 and 22.9 kg and mean BMI was 29 kg/m2, respectively in males and females. Weakness prevalence using GS and GS:BMI definitions were 7.8 and 15.2 (p<0.001), respectively in males, and 11.4 and 13.3% (p=0.04), in females. Overall prevalence of physical limitations, basic and instrumental ADL limitations was 52.9%, 28.1%, and 35.9%. In those with weakness, prevalence of physical limitations, basic ADL and instrumental ADL was 78.5%, 42.3% and 65.3% using the GS definition, and 79.7%, 40.7%, and 58.8% using the GS/BMI definition. GS and the GS/BMI definitions of weakness were strongly associated with physical limitations (OR 2.19 [95%CI:1.67-2.87] and 2.52 [2.01-3.17]), basic ADL (OR 1.59 [1.22-2.07] and 1.66 [1.32-2.07]), and instrumental ADLs (OR 1.98 [1.28-2.54] and 1.78 [1.44-2.20]).
Conclusions
The new FNIH guidelines for weakness are associated with higher prevalence of physical limitations basic and instrumental ADL impairments as compared to individuals without weakness
Keywords: obesity, diagnostic accuracy, body mass index, body fat, epidemiology, Sarcopenia, Elderly, weakness epidemiology, Health & Retirement Study
INTRODUCTION
Age-related muscle loss begins in the third to fourth decade of life1. Though some loss of muscle and strength, termed sarcopenia, is a normal part of aging, clinically significant sarcopenia associated with functional impairment is thought to be a cornerstone in the process of frailty and disability1. While weakness was initially considered to be a direct consequence of the loss of muscle mass 2, it may also be mediated through loss muscle strength 3, 4. Emerging evidence suggests there may be two distinct subgroups of persons with weakness: one due to low appendicular muscle mass, and the other due to reduced strength with intact muscle mass5.
There have been considerable discrepancies in the definitions of sarcopenia in the literature 6, 7 largely due to the different etiologies of clinical weakness. Recently, the Foundations for the National Institutes of Health (FNIH) published criteria for sarcopenia specifically for use in clinical practice5 to identify individuals that consisted of using muscle strength and muscle mass. This group based their cutoffs on a classification and regression tree analysis using grip strength to predict slow gait speed, a clinical measure known to strongly predict incident disability and mortality8. Grip strength cutpoints were based on eight pooled studies in men and women9. Our goal was to a) illustrate the prevalence of clinically defined weakness in a representative population, by applying these new recommendations to a wave of subjects from the Health and Retirement Study; b) observe the degree of impairment in those fulfilling such cutoffs; and c) describe the association between the FNIH cutoffs and physical limitations, basic and instrumental ADL impairment.
METHODS
The Health and Retirement Study (HRS) is a nationally representative survey of community-dwelling adults aged 50 years and older conducted by the University of Michigan. The initial HRS sample was drawn in 1992 from a multi-stage, clustered area probability design of households, with individuals with birth years between 1931-1941. Data from the 2006 and 2008 waves were combined for this particular cross-sectional analysis10. All eligible respondents consenting to enhanced face-to-face interviews were included in the analysis. Study design and sampling procedures are available online (http://hrsonline.isr.umich.edu). The study has been funded by the National Institutes on Aging, and due to the de-identified nature of the data, the local Institutional Review Boards of our institutions have exempted this study from review purposes.
There were 10,615 participants aged ≥60 who consented to the enhanced face to face interview. Respondents were excluded if grip strength, or BMI were missing (n=5,523). Our final analytical sample consisted of 5,092 subjects. We included persons of all races and ethnicities. Years of education and smoking status were obtained by self-report questionnaire. We created a co-morbidity index ranging from 0 to 8, which was meant to reflect self-reported health conditions, including, cancer, diabetes, heart failure, hypertension, lung disease, arthritis, myocardial infarction, and stroke11. Physical activity was assessed using a questionnaire during the HRS interview, details of which are described online. Subjects were classified as being physically active if they engaged in moderate or vigorous physical activity at least once per week.
Measures
Weight was measured using a Healthometer 830KL (Medstock, Australia) scale and rounded to the half pound, and converted to kilograms. HRS did not measure subjects with a weight >136.4kg, as this exceeded the capacity of the scale. Height was measured using a stadiometer with the respondent standing without shoes or socks and their heels and shoulders touching the wall. Body mass index (BMI) was calculated as weight (in kilograms) divided by height, in meters squared. Waist circumference (WC) was assessed using a tape measure at the level of the umbilicus while standing. The measure was recorded at the end of exhalation after a deep breath. High WC was determined using cutoffs of 88 cm and 102 cm in females and men, respectively12. Walking speed was used as a marker in the FNIH criteria. In HRS, it was measured in meters per second and measured as respondents walked a 98.5 inch course two times (there and back). Participants were instructed to wear appropriate footwear (low or no heal), and the interviewer walked to the side and slightly behind the respondent. The measure could be completed with a walking aid. The interview used a stop watch to time the respondent.
Grip strength (GS) was measured using a Smedley spring-type hand dynamometer (TTM, Tokyo, Japan). In right handed individuals, values from the dominant hand were used. In the case of ambidextrous and left-handed individuals, the higher of the values from either hand was used13. All measurements were taken with the subject in a standing position, with their arm at their side at a 90° angle. Two measurements were taken with each hand and averaged across trials. The highest average score from either hand was used. We classified subjects as having weakness based on the 2014 FNIH Sarcopenia Project guidelines5. A GS<26kg and <16kg were considered as having weakness in men and women, respectively. As this consensus group suggested two independent definitions, we also classified subjects as having weakness if their GS divided by BMI ratio (GS/BMI) was <1.0 and <0.56 in males and females, respectively.
Functional impairment was assessed using self-reported questionnaires classifying the subject’s ability in terms of physical limitations, basic activities of daily living (ADL) and instrumental ADL. Physical limitations were defined by the inability or difficulty in performing two or more of the following tasks, as described in our previous publication11: walking several blocks, walking 1 block, sitting 2 hours, getting up from a chair, climbing stairs, climbing one flight of stairs, stooping, reaching arms, pulling or pushing large objects, lifting weights or picking up a dime. Individuals with one or more difficulties in bathing, dressing, eating, toileting, or getting out of bed were considered to have an ADL limitation. Lastly, subjects reporting difficulties with at least one of the following were considered to have an instrumental ADL limitation: managing money, preparing meals, requiring help with house or yard work, using the phone or taking medications.
Statistical Analysis
Continuous variables are presented as means (standard errors), and categorical values as counts (%). T-tests compared baseline characteristics between sexes for continuous variables, and chi-square for categorical variables. Subjects were classified as having weakness using the aforementioned definitions. Overall and sex-specific prevalence estimates of weakness are presented for both FNIH definitions, and a t-test compared the mean differences between them. We stratified these results by ethnicity (white, black and other), and by three age categories (60-69.9 years, 70-79.9 years, and ≥80 years). Prevalence of overall and sex-specific physical impairments (physical limitations, basic and instrumental ADL limitations) were determined for the overall cohort, and by ethnicity and age-group. The prevalence of limitations by weakness status was evaluated using each definition.
The primary aim of this study was to determine the association of the two FNIH definitions of weakness (GS and GS/BMI) and physical limitations, basic ADL limitations, and Instrumental ADL limitations. Overall and sex-specific unadjusted and adjusted logistic regression models were constructed to ascertain the association of each definition with degree of impairment. We adjusted for age, race, number of years of schooling completed, smoking status, co-morbidity, and physical activity status. For the overall cohort, we adjusted for sex as well. Odds ratios and 95% confidence intervals are presented. We determined the relationship between walking speed in those with and without weakness based on each respective sarcopenia definition, stratified by sex, ethnicity and age group. All data was managed and processed using SAS software version 9.3 (SAS Institute, Cary, NC), and results were weighted using HRS respondent-level weights for physical measures which includes adjustments for sample selection probability and non-response. A p-value <0.05 and confidence intervals excluding 1.0 were considered statistically significant.
RESULTS
Baseline characteristics are shown in Table 1. Differences were observed in most characteristics between males and females. Grip strength (GS) and walking speed differed between sexes (p<0.001), as were the mean number of physical limitations. Table 2 represents the prevalence of weakness based on FNIH criteria of reduced GS and reduced GS:BMI ratios in the overall cohort, by ethnicity and by age group. Overall prevalence rates using GS were higher in females than in males (11.4 vs. 7.8%; p<0.001) but higher in males (15.2 vs. 13.3%; p=0.05) using GS:BMI. This was generally seen across ethnic and age categories. Stratifying by sex, prevalence rates were generally higher using GS:BMI than GS. Notably, the prevalence of weakness increased with age.
Table 1.
Characteristics of Subjects of HRS Aged ≥60 Years
| Overall N=5092 |
Men N=2195 |
Women N=2897 |
p-value | |
|---|---|---|---|---|
| Age, years | 72.1 (7.9) | 71.9 (7.5) | 72.1 (8.1) | .37 |
| Education, years | 12.4 (3.1) | 12.6 (3.3) | 12.3 (2.9) | <.001 |
| Race | ||||
| White | 4308 (84.6) | 1901 (86.6) | 2407 (83.1) | <.001 |
| Black | 619 (12.2) | 272 (10.1) | 397 (13.7) | <.001 |
| Other | 165 (3.2) | 72 (3.3) | 93 (3.2) | .10 |
| Current Smoker (%) | 582 (11.4) | 259 (11.8) | 323 (11.2) | <.001 |
| Number of Comorbiditiesd | 2.02 (1.21) | 2.0 (1.3) | 2.1 (1.2) | <.01 |
| Grip Strength, kg | 29.5 (10.6) | 38.3 (9.1) | 22.9 (5.8) | <.001 |
| Walking Speed, m/sa | 2.46 (0.86) | 2.61 (0.85) | 2.34 (0.84) | <.001 |
| Waist Circumference, cm | 100.3 (14.0) | 104.7 (11.5) | 96.9 (14.7) | <.001 |
| High Waist Circumference (%)b | 3229 (63.4) | 1200 (54.7) | 2029 (70.0) | <.001 |
| Body Mass Index, kg/m2 | 28.7 (5.5) | 28.9 (4.7) | 28.7 (6.0) | .20 |
| Obesity, BMI≥30kg/m2 | 1849 (36.7) | 790 (36.1) | 1059 (37.2) | <.001 |
| Mean number of Physical | <.001 | |||
| Limitations | 2.5 (2.5) | 1.9 (2.26) | 2.9 (2.6) | |
| Physically Activec | 3062 (60.2) | 1451 (66.2) | 1611 (55.7) | <.001 |
All values represented are means (standard deviation) or counts (percentages)
Walking speed was available for 3,849 participants
≥88cm in females; ≥102cm in males
Physically Active was defined as participation in moderate/vigorous activity at least 1 per week.
Comorbidities (hypertension, diabetes, lung disease,stroke,cancer, MI,CHF and arthritis)
Table 2.
Prevalence of FNIH Weakness in Health & Retirement Survey by Definition in Age≥60 years
| GS | GS:BMI | GS | Vs | GS:B MI |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Overall | Males | Females | p- value |
Overall | Males | Females | p-value | p-value overall |
Male | Femal es |
|
| Overall Cohort | 503 (9.9) | 172 (7.8) | 331 (11.4) | <.001 | 719 (14.1) | 334 (15.2) | 385 (13.3) | .05 | <.001 | <.001 | .04 |
| Ethnicity | |||||||||||
| White | 438 (10.2) | 147 (7.7) | 291 (12.1) | <.001 | 601 (14.0) | 286 (15.0) | 315 (13.1) | .07 | <.001 | <.001 | .33 |
| Black | 48 (7.8) | 17 (7.8) | 31 (7.8) | .95 | 87 (14.1) | 34 (15.3) | 53 (13.4) | .50 | <.01 | <.01 | .02 |
| Other | 17 (10.3) | 8 (11.1) | 9 (9.7) | .76 | 31 (18.8) | 14 (19.4) | 17 (18.3) | .85 | .04 | .20 | .12 |
| Age Group | |||||||||||
| 60-69 years | 80 (3.6) | 28 (2.9) | 52 (4.1) | .13 | 201 (9.0) | 89 (9.3) | 112 (8.9) | .73 | <.001 | <.001 | <.001 |
| 70-79 years | 173 (9.0) | 60 (7.1) | 113 (10.6) | .01 | 291 (15.2) | 142 (16.7) | 149 (14.0) | .09 | <.001 | <.001 | .03 |
| 80 years | 250 (26.3) | 84 (21.8() | 166 (29.4) | .01 | 227 (23.9) | 103 (26.7) | 124 (22.0) | .09 | .29 | .16 | .04 |
All values are represented as counts (weighted percentages). p-value compares the prevalence within each subgroup between Prevalence using GS vs. Prevalence using GS/BMI
FNIH – Foundation for the National Institutes of Health; GS - Grip Strength; GS/BMI – Grip Strength divided by BMI.
Grip Strength cutoffs are <16kg in females and < 26kg in males, and GS/BMI men <1.0; women <0.56. GS/BMI represents grip strength (in kilograms) divided by body mass index.
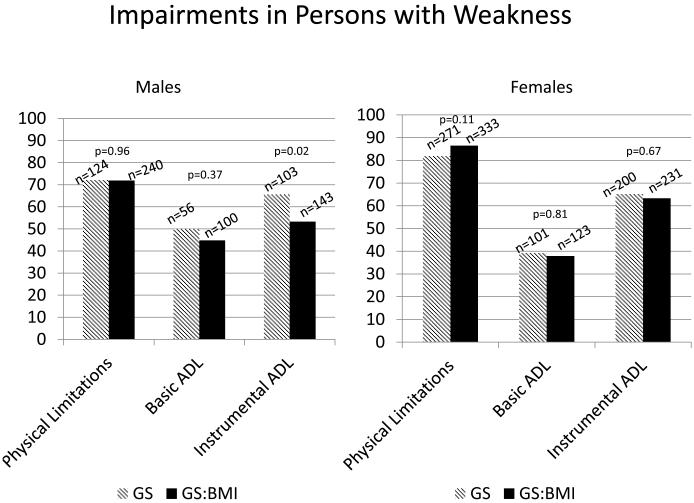
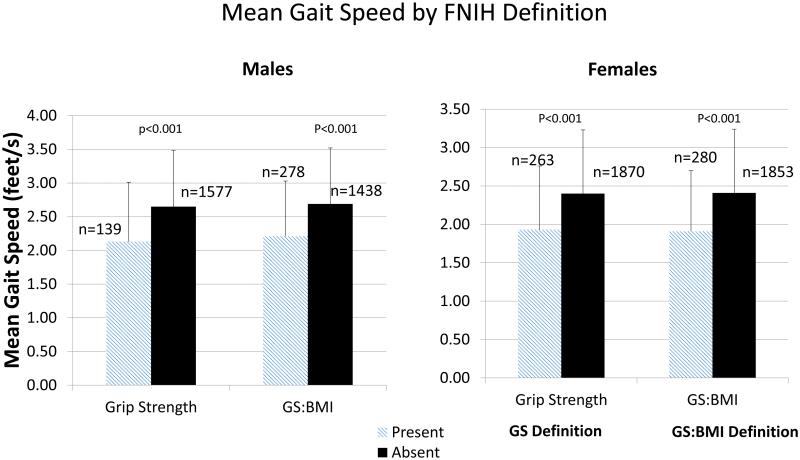
We determined the prevalence of physical limitations, basic ADL and Instrumental ADL limitations by overall cohort, sex, ethnicity and by age group. The prevalence of physical limitations in men was 44.7% and in females was 59% (Table 3). Higher prevalence rates of physical limitations, basic ADL, instrumental ADL were observed in blacks and by increasing age. Figure 1 represents the prevalence of limitations in men and women in those with weakness, depending on the definition used. The prevalence rates in both sexes suggest that rates of physical limitations are highest in those with weakness (irrespective of definition) followed by instrumental ADL and basic ADL impairments. Mean walking speed was uniformly lower in those with weakness, irrespective of the FNIH definition, sex, ethnicity and age (Figure 2 and Appendix 1). Analysis of individuals excluded in this study were less likely to be white, had higher comorbidity burden, higher WC and higher mean number of physical limitations (Appendix 2). Full details of sub-analyses can be seen in Appendix 3. We additionally present crude data on the relationship between BMI, grip strength and limitations by sex in Appendix 4. A bimodal relationship occurs between BMI and limitations with higher rates in underweight and obese individuals, while the relationship between GS and BMI appears more linear.
Table 3.
Prevalence of Limitations in Health & Retirement Study in Age ≥60 years
| Definition | Physicala
n=5092 |
Overall Basicb n=2998 |
Instrumentalc
n=4873 |
Physicala
N=2195 |
Males Basicb N=1086 |
Instrumentalc
N=2080 |
Physicala
N=2897 |
Females Basicb N=1912 |
Instrumentalc
N=2793 |
|---|---|---|---|---|---|---|---|---|---|
| Overall Cohort | 2691 (52.9) | 837 (28.1) | 1740 (35.9) | 981 (44.7) | 336 (31.0) | 609 (29.5) | 1710 (59.0) | 501 (26.5) | 1131 (40.7) |
| Ethnicity | |||||||||
| White | 2238 (52.0) | 650 (26.2) | 1434 (35.0) | 853 (44.9) | 284 (30.4) | 517 (29.0) | 1385 (57.5) | 366 (23.7) | 917 (39.7) |
| Black | 372 (60.1) | 158 (38.7) | 258 (43.4) | 102 (46.0) | 46 (39.3) | 78 (36.5) | 270 (68.0) | 112 (38.5) | 180 (47.2) |
| Other | 81 (49.1) | 29 (31.9) | 48 (30.0) | 26 (36.1) | 6 (18.8) | 14 (20.6) | 55 (59.1) | 23 (39.0) | 34 (37.0) |
| Age Group | |||||||||
| 60-69 years | 1019 (45.8) | 286 (25.1) | 547 (25.6) | 356 (37.1) | 101 (26.6) | 186 (20.4) | 513 (50.5) | 127 (21.2) | 273 (27.7) |
| 70-79 years | 1009 (52.6) | 318 (26.9) | 674 (36.7) | 394 (46.4) | 138 (29.6) | 250 (31.2) | 499 (55.6) | 132 (22.4) | 345 (39.7) |
| 80+ years | 663 (69.8) | 233 (35.6) | 519 (59.0) | 231 (59.8) | 97 (40.9) | 173 (48.9) | 432 (76.6) | 136 (32.6) | 346 (65.9) |
All values are represented as counts (weighted percentages)
ADL – Activities of Daily Living; IADL – Instrumental Activities of Daily Living
Physical Limitations were defined by inability or difficulty in performing two or more of the following tasks: walking several blocks, walking 1 block, sitting 2 hours, getting up from chair, climbing stairs climbing one flight of stairs, stooping, reaching arms, pulling/pushing large objects, lifting weights and picking up a dime
Basic ADL impairments are defined as difficulty or inability with at least 1: bathing, dressing, eating, toileting or getting out of bed
Instrumental ADL impairments are defined as difficulty or inability with at least 1: preparing meals, managing money or needing help with house or yard work, using the phone or taking medication.
Figure 1.
Prevalence of Impairments (Physical Limitations, Basic Activities of Daily Living and Instrumental Activities of Daily Living) in subjects with weakness aged ≥60years in the Health and Retirement Survey 2006-2008, based on the Foundation for the National Institutes on Health Sarcopenia Project Guidelines
p-values represent the differences between Grip strength and grip strength:body mass index definitions.
Figure 2.
Mean Walking Speed in Subjects with and without Sarcopenia aged ≥60years in the Health and Retirement Survey 2006-2008, based on the Foundation for the National Institutes on Health Sarcopenia Project Guidelines. Error bars represent standard deviations
P-values represent the differences between individuals with and without weakness (depending on the definition used)
Univariate and multivariate analyses are shown in Table 4. The strength of association across all limitations (physical, instrumental and basic ADL) was higher in those with sarcopenia. The strengths of association were higher in females in physical limitations, lower in instrumental ADL. Minimal differences were observed between sexes in basic ADLs. We observed reduced odd ratios after adjusting for all of our covariates across all models.
Table 4.
Association of Impairment (Physical, ADL, IADL) and FNIH Cutoff
| Overall (n=5,092) | Men (n=2,195) | Women (n=2,897) | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Physical | GS | 3.91 [3.06-5.01] | 2.19 [1.67-2.87] | 4.03 [2.72-5.99] | 1.98 [1.28-3.05] | 3.58 [2.61-4.91] | 2.34 [1.66-3.30] |
| Limitations | GS/BMI | 4.44 [3.58,5.52] | 2.52 [2.01,3.17] | 4.02 [3.00,5.39] | 2.14 [1.57,2.91] | 5.86 [4.18,8.22] | 3.59 [2.51,5.13] |
|
| |||||||
| Basic | GS | 2.15 [1.68,2.76] | 1.59 [1.22,2.07] | 1.54 [.96,2.47] | 1.86 [1.17,2.97] | 1.88 [1.38,2.54] | 1.61 [1.16,2.21] |
| ADLs | GS/BMI | 2.22 [1.78,2.75] | 1.66 [1.32,2.07] | 2.52 [1.79-3.56] | 1.67 [1.18,2.37] | 2.01 [1.50,2.65] | 1.59 [1.19,2.13] |
|
| |||||||
| Instrumental | GS | 4.32 [3.45,5.40] | 1.98 [1.25,2.54] | 7.18 [4.86,10.6] | 2.88 [1.83,4.54] | 3.03 [2.31,3.99] | 1.53 [1.13,2.05] |
| ADLs | GS/BMI | 3.47 [2.87,4.19] | 1.78 [1.44,2.20] | 4.27 [3.32,6.67] | 1.97 [1.43,2.71] | 3.13 [2.42,4.03] | 1.79 [.34,2.37] |
Odds ratios with 95% confidence intervals are presented
Models are weighted and adjusted for age, race, number of years of school, smoking status, comorbidities, physical activity status. Overall model also adjusts for sex; There were n=3831 with non-positive weights or weights=0 that dropped out of the model
ADL – activities of daily living; BMI: Body mass index; GS: Grip Strength; IADL – instrumental activities of daily living; PL – physical limitations GS cutoffs men<26kg; women <16kg; GS/BMI men <1.0; women <0.56
DISCUSSION
We demonstrate that the prevalence of clinical weakness is high in a representative population but differ by sex and by FNIH definition used. Our results confirm that the rates of limitations are higher in individuals with weakness, irrespective of the definition used. We also observed that a strong association exists between weakness and limitations, more so in females than in males.
FNIH definitions are meant to identify those at risk for weakness5. The study results were readily apparent using the GS:BMI ratio than the GS alone, paralleling those observed using the FNIH muscle mass cutpoints alone in our other work14. While both definitions have been used to classify sarcopenia, a shift from using muscle mass to muscle strength (using grip strength) has been advocated as the prime determinant for studying sarcopenia. This is in direct contrast to the FNIH consensus suggesting that GS was the best predictor in men, and GS/BMI was better in women in terms of model fit statistics. We believe that GS:BMI accounts for both strength and in part muscle mass and that this measure should be considered in clinical practice for identification of sarcopenia. Previous research has also suggested different associations between strength and mobility across BMI15 requiring the need to adjust for this measure. While BMI traditionally has been used for assessment of obesity, in older adults, it has low sensitivity for measuring fat, and that the measure also accounts for muscle mass. No consistent trends were observed in basic or instrumental ADLs across definitions, likely because these definitions are meant to identify individuals at risk for impairments rather than impairments themselves.
Sex-specific differences have been observed in other studies5, 10, 14, including a mortality analysis in older adults14. Part of this reasoning is due to sex-specific differences of normative population-based data for grip strength15-17. Our data suggests that physical limitations and instrumental ADL in those with classified weakness (irrespective of definition), was markedly higher in females than in males. While purely hypothetical, women tend to gain more fat and have lower absolute muscle mass with age, placing them at a greater risk of developing obesity and lower muscle strength with aging4, 18. Consequences of obesity in women may be more significant than in men because of greater loss of existing lower muscle stores leading to incremental limitations in movement. Further, the female population had high central adiposity which can be associated with higher degrees of functional impairment.
There are several significant limitations in this study. First, information on chronic illnesses, smoking status, race, and physical limitations were all obtained by self-report, which has the potential for inaccuracies. Second, sampling bias is likely and may impact our results despite the survey’s intention to be a representative sample of older adults. Third, we created a co-morbidity score incorporating the number of disorders, each of which was given an equal weighting. However, we deliberately presented our unadjusted results which suggested that the odds ratios remained significant. Fourth, we analyzed a cross-sectional cohort of persons. Fifth, our cohort was predominantly white, making generalizations to other races very difficult. We presented data on black and other as an exploratory analysis, however, we recognize that drawing definitive conclusions is not possible and should be interpreted with caution. There are ethnic-differences in body composition, which may explain some of the discordance in our results. The FNIH studies were based predominantly on Caucasian subjects, although there were subgroups examined that consisted of African-American and Hispanic origin. Recalibration of cutpoints and definitions may be needed for these groups, paralleling the approach used in the Framingham Risk score or Metabolic Syndrome19. Sixth, we defined physical limitations, basic and instrumental ADL using composite scores in line with our previous analyses11. This could introduce bias in classifying limitations and impact our estimates observed in Table 4. Seventh, this population is a well-functioning, community-dwelling, older adult population that may not be typically representative of those in this age range. Eighth, there may be selection bias based on a survival effect in clinical studies in those over the age of 80 years may be relatively healthier. Lastly, we recognize that there were a number of participants we excluded which could bias our results. However, an attempt was made to compare the characteristics of participants included and those excluded. The use of HRS respondent level weights for the sub-analysis (that contains adjustments for non-response) helped account for the potential bias(http://hrsonline.isr.umich.edu/modules/meta/tracker/desc/PMWeight2004_Description_public.pdf).
While the thresholds focus on weakness and function, those with weakness and obesity were not adequately categorized. In the absence of body composition data, this cannot be determined using this dataset. With over 35% of the older adult population having obesity20, the implications of muscle weakness in this subgroup of should not be overlooked. In both cross-sectional21 and longitudinal studies22, loss of muscle mass and weakness are associated with impairments in function. A major limitation of this current analysis is the inability to formally assess body fat nor the ability to assess muscle mass, a core component of the sarcopenia definition.
Cut-points are based and determined by their referent populations, all of which may have differing physical and functional characteristics. When applying different thresholds for a variable (ie: grip strength), altering a cutoff may dramatically alter prevalence. Further, grip strength is a continuous variable and its relationship with impaired function, disability, and mortality is based on this structure. Dichotomizing GS into low vs. normal may impact those who are slightly above the threshold, yet their long-term risk may modestly be increased versus a counterpart just below this threshold. The potential for overdiagnosis (or underdiagnosis) of a clinical condition is possible.
To our knowledge, this is the first study to apply the FNIH criteria for weakness to the Health and Retirement Study. While sarcopenia (and its components) is generally recognized in the geriatric literature, it is only increasingly being appreciated in the general medical community and in the primary care setting. Identification of individuals at risk for weakness allows for targeted interventions in the care setting and implementation of care planning for those at risk of frailty23. Our results suggest that irrespective of the definition used, the association with impairment is evident. Recognition will allow prevention of the condition and its consequences, in addition to treatment of those with existing functional impairments. Only with standardized measures for identification will evaluation of proposed sets of outcomes, including patient-reported outcomes, utilization and serious injury could be performed. BMI is measured routinely in clinical settings and integrating GS may be more practical and cost-effective than gait speed or other functional measures. However, future studies should determine the balance between diagnostic accuracy and practical incorporation in busy clinical practices.
Supplementary Material
ACKNOWLEDGEMENTS
JA Batsis – conception, design, acquired data, analysis and interpretation, drafted the article, final approval of the version to be published
CM Germain - conception, design, acquired data, analysis and interpretation, revised article critically for important intellectual content, final approval of the version to be published
E Vasquez - conception, design, acquired data, analysis and interpretation, revised article critically for important intellectual content, final approval of the version to be published
SJ Bartels – analysis and interpretation, revised article critically for important intellectual content, final approval of the version to be published
Funding: Funded in part by the Department of Medicine, Geisel School of Medicine at Dartmouth, and the Dartmouth Centers for Health and Aging. Support was also provided in part by the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- ADL
Activities of Daily living
- BMI
Body mass index
- FNIH
Foundation for the National Institute of Health
- GS
grip strength
- HRS
Health and Retirement Study
- WC
waist circumference
Footnotes
FINANCIAL DISCLOSURE
Dr. Batsis receives funding from Health Resources Services Administration (UB4HP19206-01-00) for medical geriatric teaching, the Junior Faculty Career Development Award, the Department of Medicine, Dartmouth-Hitchcock Medical Center, and the Dartmouth Centers for Health and Aging
Dr. Bartels receives funding from the National Institute of Mental Health (K12 HS0217695 (AHRQ), NIMH: T32 MH073553, R01 MH078052, R01 MH089811; R24 MH102794; CDC U48DP005018.
Dr. Vásquez and Germain declare that they have no conflicts of interest.
CONFLICTS OF INTEREST
none
Work presented at the International Conference on Frailty and Sarcopenia 2015, Boston, MA
REFERENCES
- 1.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12(7):427–432. doi: 10.1007/BF02982703. e-pub ahead of print 2008/07/11; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roubenoff R, Dallal GE, Wilson PW. Predicting body fatness: the body mass index vs estimation by bioelectrical impedance. Am. J. Public Health. 1995;85(5):726–728. doi: 10.2105/ajph.85.5.726. e-pub ahead of print 1995/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A. Biol. Sci. Med. Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. e-pub ahead of print 2006/02/04. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J. Am. Geriatr. Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 5.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A. Biol. Sci. Med. Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. e-pub ahead of print 2014/04/17; doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey - J. Am. Geriatr. Soc. 2013;61(6):974–980. doi: 10.1111/jgs.12260. e-pub ahead of print 2013/05/08; doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. e-pub ahead of print 2011/01/06; doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alley DE, Shardell MD, Peters KW, McLean RR, Dam TT, Kenny AM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. A. Biol. Sci. Med. Sci. 2014;69(5):559–566. doi: 10.1093/gerona/glu011. e-pub ahead of print 2014/04/17; doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germain CM, Vasquez E, Batsis JA. Physical Activity, Central Adiposity and Functional Limitations in Community Dwelling Older Adults. Journal of Geriatric Physical Therapy. 2015 doi: 10.1519/JPT.0000000000000051. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain CM, Vasquez E, Batsis JA. Physical Activity, Central Adiposity, and Functional Limitations in Community-Dwelling Older Adults. J Geriatr Phys Ther. 2015 doi: 10.1519/JPT.0000000000000051. e-pub ahead of print 2015/03/21; doi: 10.1519/jpt.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin. Pharmacol. Ther. 2007;82(5):509–524. doi: 10.1038/sj.clpt.6100355. e-pub ahead of print 2007/09/14; doi: 10.1038/sj.clpt.6100355. [DOI] [PubMed] [Google Scholar]
- 13.Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiotherapy. 2006;92(1):11–15. doi: 10.1016/j.physio.2005.05.003. [Google Scholar]
- 14.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014;68(9):1001–1007. doi: 10.1038/ejcn.2014.117. e-pub ahead of print 2014/06/26; doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 15.Sallinen J, Stenholm S, Rantanen T, Heliovaara M, Sainio P, Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J. Am. Geriatr. Soc. 2010;58(9):1721–1726. doi: 10.1111/j.1532-5415.2010.03035.x. e-pub ahead of print 2010/09/25; doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desrosiers J, Bravo G, Hebert R, Dutil E. Normative data for grip strength of elderly men and women. Am. J. Occup. Ther. 1995;49(7):637–644. doi: 10.5014/ajot.49.7.637. e-pub ahead of print 1995/07/01. [DOI] [PubMed] [Google Scholar]
- 17.Keevil VL, Luben R, Dalzell N, Hayat S, Sayer AA, Wareham NJ, et al. J Nutr Health Aging. 2015;19(1):3–11. doi: 10.1007/s12603-014-0492-6. e-pub ahead of print 2015/01/07; doi: 10.1007/s12603-014-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clin Exp Res. 2007;19(4):277–283. doi: 10.1007/BF03324702. e-pub ahead of print 2007/08/30. [DOI] [PubMed] [Google Scholar]
- 19.Batsis JA, Lopez-Jimenez F. Cardiovascular risk assessment--from individual risk prediction to estimation of global risk and change in risk in the population. BMC Med. 2010;8(29) doi: 10.1186/1741-7015-8-29. e-pub ahead of print 2010/05/27; doi: 10.1186/1741-7015-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, - JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. e-pub ahead of print 2014/02/27; doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 22.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) Study. Am. J. Clin. Nutr. 2009;89(6):1895–1900. doi: 10.3945/ajcn.2008.26950. e-pub ahead of print 2009/04/1; doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 23.Reuben DB. Medical care for the final years of life: "When you're 83, it's not going to be 20 years". JAMA. 2009;302(24):2686–2694. doi: 10.1001/jama.2009.1871. e-pub ahead of print 2009/12/31; doi: 10.1001/jama.2009.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.