Abstract
Objective
Combining 18F-FDG PET with whole-body MR for paediatric cancer staging is practically feasible if imaging protocols can be streamlined. We compared 18F-FDG PET/STIR with accelerated 18F-FDG PET/FSPGR for whole-body tumour imaging in children and young adults.
Methods
Thirty-three children and young adults (17.5±5.5 years, range 10–30 years) with malignant lymphoma or sarcoma underwent a 18F-FDG PET staging exam, followed by ferumoxytol-enhanced STIR and FSPGR whole-body MR. 18F-FDG PET scans were fused with MR data and the number and location of tumours on each integrated exam was determined. Histopathology and follow-up imaging served as standard of reference. The agreement of each MR sequence with the reference and the whole-body imaging times were compared using Cohen’s kappa coefficient and student t-test, respectively.
Results
Comparing 18F-FDG PET/FSPGR to 18F-FDG PET/STIR, sensitivities were 99.3% for both, specificities were statistically equivalent, 99.8% versus 99.9% and the agreement with the reference based on Cohen’s kappa coefficient was also statistically equivalent, 0.989 versus 0.992. However, the total scan-time for accelerated FSPGR of 19.8±5.3 minutes was significantly shorter compared to 29.0±7.6 minutes for STIR (p=0.001).
Conclusion
18F-FDG PET/FSPGR demonstrated equivalent sensitivities and specificities for cancer staging compared to 18F-FDG PET/STIR, but could be acquired with shorter acquisition time.
Keywords: Whole-body PET/MR imaging, PET/CT, Iron Oxide Nanoparticles, STIR, Paediatric Cancer
Introduction
Accurate staging of malignant tumours in children and adolescents is critical to estimate prognosis and initiate appropriate cancer treatment [1–3]. 18F-FDG PET/CT exams are the current staging procedure of choice for the majority of paediatric patients with lymphomas and for some patients with sarcomas [4]. However, 18F-FDG PET/CT exams are associated with considerable radiation exposure [5–7]. We recently reported that radiation-free whole-body MRI scans provide equivalent information for cancer staging compared to 18F-FDG PET/CT [8]. While further studies are underway to determine the value of whole-body MRI for treatment monitoring, 18F-FDG PET/MR studies can provide an interim alternative for tumour re-staging through an imaging process that exposes young patients to significantly less radiation than PET/CT and includes metabolic and anatomic information for therapy response assessment [9, 10]. A recent study reported that co-registration of 18F-FDG PET data with MR scans instead of CT scans allowed for whole-body staging of children with 80% reduced radiation exposure. However, previous PET/MR whole-body staging protocols for children were limited by using T2-weighted sequences for anatomical co-registration of PET data, which led to significantly increased scan times compared to a PET/CT exam [11]. Although PET/MRI offers improved soft tissue contrast compared to CT [12–15] and allows for primary tumour and whole-body staging in one session [16], two potential concerns have previously inhibited implementation of faster T1-weighted sequences: (1) Inability to delineate vessels over the duration of a whole-body scan due to contrast extravasation and (2) misaligned PET data co-registration on breath-hold scans after deep inspiration.
We propose to solve these problems by using the blood pool agent ferumoxytol for long-lasting vascular enhancement and end-expiration breath-hold T1-weighted sequences for co-registration with 18F-FDG PET data. Ferumoxytol is an FDA-approved iron supplement composed of ultra-small super-paramagnetic iron oxide nanoparticles which can be used “off label” as a contrast agent for MR imaging [17–20]. The goal of our study was to compare the time-efficiency, cost-efficiency and diagnostic accuracy of fused 18F-FDG PET/STIR and 18F-FDG PET/FSPGR exams for whole-body tumour staging. Through our analysis, we have concluded that ferumoxytol-enhanced breath-hold T1-weighted fast spoiled gradient recalled echo (FSPGR) scans substantially expedite acquisition of anatomical background information, improve diagnostic cost-efficiency, and provide equal imaging specificity and sensitivity for whole-body PET/MR staging of paediatric patients and young adults. This reduction in image acquisition time makes the 18F-FDG PET/FSPGR exam a clinically feasible, radiation-free staging alternative for diagnosing children and young adults with cancer.
Materials and Methods
Patients
This prospective, non-randomized study was approved by the institutional review board (IRB = local ethical committee) and the Cancer Center at our institution and written informed consent was obtained from all individual participants and their parents (in case of minors) included in the study. We investigated 33 patients with malignant lymphomas (n = 27) and malignant sarcomas (n = 6) including 24 males and 9 females with a mean age of 17.5 ± 5.5 years (range 10 to 30 years) as shown in Table 1 with age, gender and tumour type of all patients. Twenty one of the 33 patients were part of a previously reported study on whole-body diffusion-weighted MRI. To provide accelerated cancer staging, the goal of our current study was to compare the sensitivities and specificities of two different PET/MR techniques for cancer staging, namely 18FFDG PET/FSPGR and 18F-FDG PET/STIR sequences. Inclusion criteria comprised (1) age between 8 and 30 years (2) newly diagnosed lymphoma or sarcoma and (3) scheduled or completed 18F-FDG PET/CT. All tumours were evaluated before start of therapy. The lower age threshold of eight years was chosen to avoid the need of sedation or anaesthesia. Exclusion criteria comprised (1) MR-incompatible metal implants, (2) claustrophobia (3) hemosiderosis/hemochromatosis and (4) history of allergies against iron products or any history of severe anaphylactic reactions.
Table 1.
Patient information: patient age, gender and tumour type
| No. | Gender | Age | Diagnosis |
|---|---|---|---|
| 1 | Male | 10 | Hodgkin’s Lymphoma, stage IIIA |
| 2 | Female | 10 | Hodgkin’s Lymphoma, mixed cellularity, stage III BS |
| 3 | Male | 10 | Osteosarcoma |
| 4 | Male | 11 | Hodgkin’s Lymphoma, stage IIB |
| 5 | Female | 11 | diffuse large B-Cell lymphoma |
| 6 | Female | 13 | Hodgkin Lymphoma, nodular sclerosis subtype, stage IVA |
| 7 | Male | 13 | Burkitt Leukemia |
| 8 | Male | 13 | Hodgkin’s Lymphoma, stage IV BE |
| 9 | Male | 13 | Hodgkin’s Lymphoma, mixed cellularity, stage III BS |
| 10 | Male | 13 | Anaplastic Large Cell Lymphoma, stage III |
| 11 | Male | 14 | NK/T-cell lymphoma |
| 12 | Female | 15 | Hodgkin’s Lymphoma, stage IIIAS |
| 13 | Male | 15 | Hepatosplenic gamma-delta T-cell Lymphoma with associated hemophagocytosis |
| 14 | Male | 15 | Hodgkin Lymphoma with bullky mediastinal disease, stage IVBS |
| 15 | Male | 16 | Hodgkin’s Lymphoma, stage IIIB |
| 16 | Male | 16 | Hodgkin’s Lymphoma, stage IIB |
| 17 | Male | 16 | Hodgkin’s Lymphoma, stage IIIA |
| 18 | Female | 16 | Hodgkin’s lymphoma |
| 19 | Male | 18 | nodular lymphocyte predominant Hodgkin’s lymphoma, stage IIIA |
| 20 | Male | 18 | Osteosarcoma |
| 21 | Female | 19 | classical Hodgkin’s lymphoma, stage IIIA |
| 22 | Female | 19 | Ewing Sarcoma |
| 23 | Male | 20 | Hodgkin’s lymphoma |
| 24 | Male | 20 | Osteosarcoma |
| 25 | Male | 20 | Hodgkin’s lymphoma |
| 26 | Male | 21 | B lympoblastic leukemia |
| 27 | Female | 23 | Ewing Sarcoma |
| 28 | Male | 24 | Hodgkin’s Lymphoma |
| 29 | Male | 25 | Hodgkin’s lymphoma |
| 30 | Male | 26 | Ewings Sarcoma |
| 31 | Male | 26 | Hodgkin’s lymphoma |
| 32 | Male | 29 | grade 1–2 follicular lymphoma, FLIPI 2/5, stage IVAXS |
| 33 | Female | 30 | Hodgkin’s Lymphoma, stage IA |
18F-FDG PET/CT
All patients underwent clinical standard 18F-FDG PET/CT scans for tumour staging on a Discovery LS 690 PET/CT scanner (GE Healthcare). The patients fasted at least six hours before the 18F-FDG scan and blood glucose levels were confirmed to be less than 150 mg/dl at the time of the 18F-FDG injection. Approximately 60 min after injection of 0.33 GBq ± 0.12 GBq 18F-FDG, an emission PET was acquired of the whole-body. PET images were attenuation corrected by low dose CT data, reconstructed with a standard iterative algorithm and reformatted into axial, coronal, and sagittal views.
Whole-body MR Imaging
MR imaging was performed on a 3T Discovery 750 magnetic resonance scanner (GE Healthcare). We obtained investigational new drug approval from the FDA (IND111,154) to use the FDA-approved iron supplement ferumoxytol (Feraheme™, Fe5874 O8752-C11719 H18682 O9933 Na414; AMAG Pharmaceuticals, Inc.) [21] “off-label” as a contrast agent for MR imaging in children and young adults. Ferumoxytol is composed of semi-synthetic carbohydrate-coated super paramagnetic iron oxide nanoparticles with a molecular weight of 731 kDa, a hydrodynamic particle diameter of 30 nm [22], and a long plasma half-life of 15 hours. Due to their super-paramagnetic properties, ferumoxytol nanoparticles give positive contrast on T1-weighted MR images and negative contrast on T2-weighted MR images [23]. All patients received an intravenous injection of ferumoxytol at a dose of 5 mg Fe/kg body weight before their MRI scan over a minimum of 15 minutes. [24] A personalized approach was used to ensure minimal scan time for every patient. Before the scan, each patient practiced guided breath-holding and we recorded their maximum breath-hold interval, which ranged between 20–35 seconds. The minimal field-of-view (FOV) that encompassed the patient was used, which ranged from 20–50 cm. Repetition times (TR) and echo times (TE) were adjusted to ensure a constant signal-to-noise ratio for all patients. Breath-hold fat-saturated T1-weighted fast spoiled gradient recalled echo (FSPGR) sequences were obtained with a flip angle of 15°, a TR of 7–12 ms, a TE of 1–3 ms and an acceleration factor of 2. This parallel imaging acceleration required Array Spatial Sensitivity Encoding Technique (ASSET) calibration. Short TI inversion recovery (STIR) sequences were obtained with a TR of 4000–8000 ms and a TE of 50–60 ms. All sequences were obtained with a single acquisition, a bandwidth of 31 kHz, a slice thickness of 4–5 mm, and an image matrix of 256×192. Voxel dimensions amongst our acquisitions did not exceed 2.5 mm in-plane by 5 mm through-plane, and were sufficiently small to detect lymph nodes that met the clinical criterion for radiological diagnosis (15 mm mediastinal and 10 mm superficial lymph nodes).
To generate whole-body images that integrate the functional and anatomic information of the acquired 18F-FDG and whole-body MRI scans, we post-processed our images using OsiriX software [25]. 18F-FDG PET images were colour-rendered by applying the ‘PET’ CLUT followed by fusion with gray-scale ferumoxytol-enhanced anatomical STIR and T1-weighted FSPGR images.
Data Analysis
In order to compare fused 18F-FDG PET/STIR and 18F-FDG PET/FSPGR exams, all images were interpreted by consensus between an experienced radiologist and an experienced nuclear medicine physician. 18F-FDG PET/STIR and 18F-FDG PET/FSPGR scans of each patient were evaluated in random order in separate sessions, which were separated by at least two months in order to avoid recall bias. In order to further minimize recall bias, the reviewers were blinded to histopathology, staging outcome, disease history and other imaging findings. Histopathology, as well as clinical and imaging follow up for at least 6 months served as the standard of reference. All primary tumours were confirmed by histopathology. Sixty two regions assessed in each patient (n = 33) are specified as:
-
Nodal regions (N1–33):
Head/neck (H1–5): Waldeyer’s ring, bilateral cervical, and bilateral supraclavicular
Chest (C1–11): bilateral infraclavicular, prevascular, aortopulmonary, paratracheal, pretracheal, subcarinal, posterior mediastinal, bilateral hilar, and retrocrural
Axilla/extremities (Ax1–2): bilateral axillary
Abdomen (Ab1–7): gastrohepatic, periportal, aortocaval, retrocrural, mesenteric, retroperitoneal, and paraaortic
Pelvis (P1–8): bilateral common iliac, bilateral internal iliac, bilateral external iliac, and bilateral inguinal
Extra-nodal regions (E1–10): bilateral pleura, bilateral lung, bilateral breast, myocardium, spleen, liver, and bowel
Bone regions (B1–19): cervical spine, bilateral clavicle, bilateral scapula, bilateral rib, thoracic spine, bilateral humerus, sternum, bilateral pelvis, bilateral femur, tibia, sacrum, lumbar spine, and coccyx
Based on regional analysis, sensitivities, specificities, positive predictive values, negative predictive values and diagnostic accuracies were calculated for 18F-FDG PET/STIR and efficient 18F-FDG PET/FSPGR exams. The Cohen’s kappa statistic was evaluated to determine the level of agreement between the 18F-FDG PET/STIR and 18F-FDG PET/FSPGR methods and the standard of reference. Cohen kappa coefficients of 1 or 0 indicate complete agreement or no more agreement than can be expected to occur by chance alone, respectively.
In addition, whole-body image acquisition times were recorded for each patient as: a) the MR scan-time per single anatomical site, the total whole-body scan-time as well as b) the combined time for coil placement, localizer sequence, ASSET calibration and whole-body MR sequence. Mean acquisition times for 18F-FDG PET/STIR and 18F-FDG PET/FSPGR scans were compared with a student’s t-test with a significance threshold of 0.05.
Results
The reviewers successfully detected 285 malignant lesions in 2046 anatomical regions (62 regions in 33 patients). Ferumoxytol-enhanced 18F-FDG PET/FSPGR exams provided a similar visual representation of pediatric tumors compared with 18F-FDG PET/STIR exams as shown for a 25-year-old patient with Hodgkin’s lymphoma with mediastinal lymph node (Fig. 1).
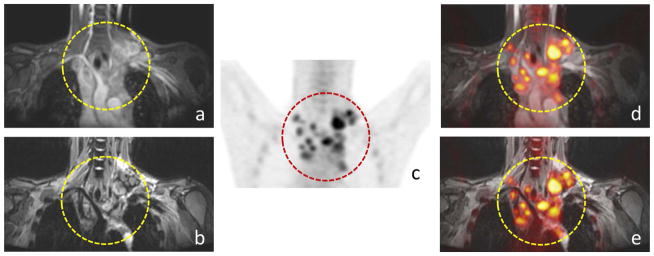
Fig. 1.
Comparison of ferumoxytol-enhanced 18F-FDG PET/STIR and 18F-FDG PET/FSPGR exams for the detection of lymph nodes in a 25-year-old patient with Hodgkin’s lymphoma: Positive mediastinal lymph nodes are noted (circle) on coronal (a) FSPGR, (b) STIR, (c) 18F-FDG PET as well as (d) fused 18F-FDG PET/FSPGR, (e) PET/STIR exams.
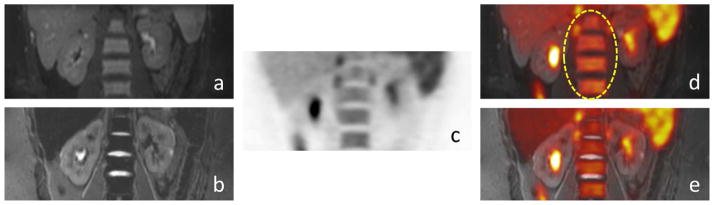
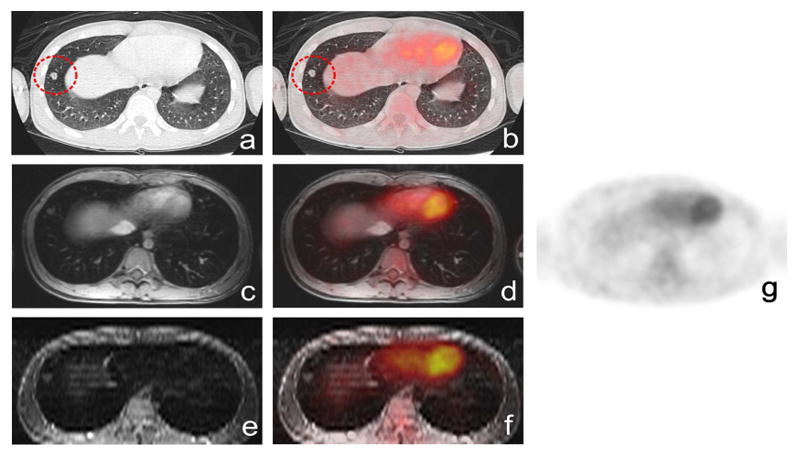
18F-FDG PET/FSPGR detected 286 lesions, including 205 nodal lesions, 58 bone lesions and 23 extra-nodal lesions. 18F-FDG PET/STIR identified 285 lesions, including 205 nodal lesions, 57 bone lesions and 23 extra-nodal lesions. 18F-FDG PET/FSPGR revealed 3 false positive findings, 2 false negative, 283 true positive and 1380 true negative lesions. 18F-FDG PET/STIR diagnosed 2 false positive and 2 false negative lesions, 283 true positive and 1381 true negative lesions. False negative findings included one bone lesion and one lung nodule as shown in Fig. 2. False positive lesions included physiological bowel uptake on PET (n = 1), inflammatory lymph nodes (n = 1) and hypercellular hematopoietic marrow (n = 1). False positive lesions on 18F-FDG PET/FSPGR, but not 18F-FDG PET/STIR included hypermetabolic hematopoietic bone marrow on 18F-FDG PET, which showed normal homogenous ferumoxytol-enhancement on STIR images and was confirmed as normal bone marrow by bone marrow biopsy (Fig. 3).
Fig. 2.
False negative lung nodule in a 16-year-old patient with lymphoma on axial (a) CT shows a lung nodule close to the diaphragm (circle), (b) 18F-FDG PET/CT shows no significant FDG update, (c) FSPGR, (d) 18F-FDG PET/FSPGR, (e) STIR, (f) 18F-FDG PET/STIR exam show equivalent information, (g) 18F-FDG PET.
Fig. 3.
False positive, biopsy proven normal hypercellular hematopoietic marrow in a 15-year-old patient with lymphoma: (a) coronal ferumoxytol-enhanced FSPGR(b) coronal STIR scans show homogenous negative (dark) ferumoxytol-enhancement, confirming normal marrow, (c) hypermetabolic hematopoietic bone marrow on 18F-FDG PET and (d) on coronal 18F-FDG PET/FSPGR (ellipse), (e) hypermetabolic hematopoietic bone marrow on fused 18F-FDG-PET/STIR exams.
Sensitivity and specificity were 99.3% and 99.8% for 18F-FDG PET/FSPGR and 99.3% and 99.9% for 18F-FDG PET/STIR imaging, respectively. The positive predictive value was 99.0% for 18F-FDG PET/FSPGR and 99.3% for 18F-FDG PET/STIR (Table 2). Based on Cohen’s kappa coefficient, the agreement with the standard of reference was 0.989 for 18F-FDG PET/FSPGR and 0.992 for 18F-FDG PET/STIR.
Table 2.
Comparison of 18F-FDG PET/STIR and for 18F-FDG PET/FSPGR for the detection of malignant lesions in children and young adults: Analysis based on 285 lesions at 2046 anatomical sites of 62 regions (nodal, extra-nodal and bone) assessed in 33 patients.
| Regions | # of regions | Procedure | # of lesions | True Positive | False Positive | True Negative | False Negative | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nodal | Head/Neck | 5 | PET/FSPGR | 50 | 50 | 0 | 50 | 0 | 100 | 100 | 100 | 100 | 100 |
| PET/STIR | 50 | 50 | 0 | 50 | 0 | 100 | 100 | 100 | 100 | 100 | |||
| Chest | 11 | PET/FSPGR | 82 | 82 | 0 | 249 | 0 | 100 | 100 | 100 | 100 | 100 | |
| PET/STIR | 82 | 82 | 0 | 249 | 0 | 100 | 100 | 100 | 100 | 100 | |||
| Axilla | 2 | PET/FSPGR | 18 | 18 | 0 | 35 | 0 | 100 | 100 | 100 | 100 | 100 | |
| PET/STIR | 18 | 18 | 0 | 35 | 0 | 100 | 100 | 100 | 100 | 100 | |||
| Abdominal | 7 | PET/FSPGR | 30 | 30 | 0 | 177 | 0 | 100 | 100 | 100 | 100 | 100 | |
| PET/STIR | 30 | 30 | 0 | 177 | 0 | 100 | 100 | 100 | 100 | 100 | |||
| Pelvic | 8 | PET/FSPGR | 25 | 24 | 1 | 200 | 0 | 100 | 99.5 | 96.0 | 100 | 99.6 | |
| PET/STIR | 25 | 24 | 1 | 200 | 0 | 100 | 99.5 | 96.0 | 100 | 99.6 | |||
| Extra-nodal | 10 | PET/FSPGR | 23 | 22 | 1 | 266 | 1 | 95.7 | 99.6 | 95.7 | 99.6 | 99.3 | |
| PET/STIR | 23 | 22 | 1 | 266 | 1 | 95.7 | 99.6 | 95.7 | 99.6 | 99.3 | |||
| Bone | 19 | PET/FSPGR | 58 | 57 | 1 | 402 | 1 | 98.3 | 99.8 | 98.3 | 99.8 | 99.6 | |
| PET/STIR | 57 | 57 | 0 | 403 | 1 | 98.3 | 100 | 100 | 99.8 | 99.8 | |||
| Sum of all regions | 62 | PET/FSPGR | 286 | 283 | 3 | 1380 | 2 | 99.3 | 99.8 | 99.0 | 99.9 | 99.7 | |
| PET/STIR | 285 | 283 | 2 | 1381 | 2 | 99.3 | 99.9 | 99.3 | 99.9 | 99.8 | |||
PPV: Positive Predictive Value, NPV: Negative Predictive Value
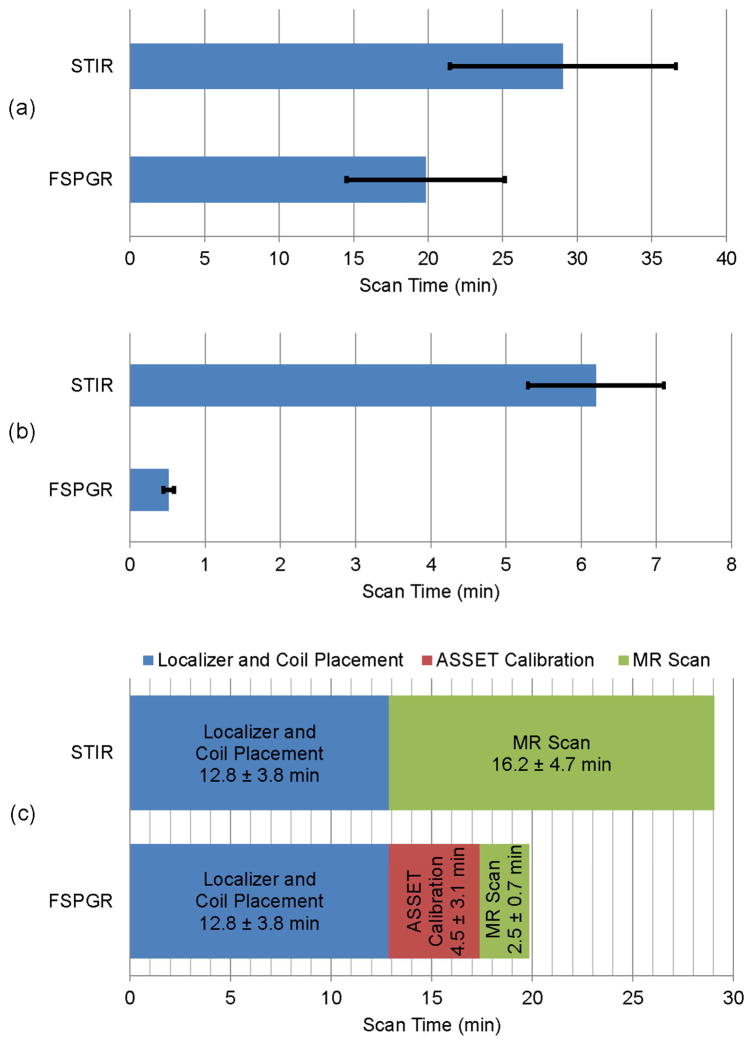
The imaging time for the whole-body scan was significantly shorter using accelerated FSPGR sequences (19.8 ± 5.3 minutes with pure MR scan-time of 2.5 ± 0.7 minutes) as opposed to STIR images (29.0 ± 7.6 minutes with pure MR scan-time of 16.2 ± 4.7 minutes; p = 0.001). Fig. 4(a) shows the total scan-time for FSPGR and STIR scans. Fig. 4(b) demonstrates that the scan-time per anatomical site for accelerated FSPGR (0.52 ± 0.07 minutes) is considerably shorter than STIR (6.2 ± 0.9 minutes), p < 0.001. In addition, the time for localizer, coil placement, ASSET calibration and pure MR scan are illustrated in Fig. 4(c).
Fig. 4.
(a) Total scan-time of whole-body for FSPGR and STIR sequences, p = 0.001 indicates statistically significant difference based on a student’s t-test. (b) MR scan-time per single anatomical site (such as chest, abdomen, pelvis or extremities) for FSPGR and STIR sequences, p < 0.001 indicates statistically significant difference based on a student’s t-test,(c) Acquisition time for coil placement, localizer sequence, ASSET calibration and whole-body MR sequence. Data are displayed as means of 33 imaging studies and standard deviation.
The imaging cost in our institution for whole-body MR scan is $627 per hour plus $70 per exam, thus accelerated FSPGR sequences costs on average 19.8 min × $627/hour + $70 = $276.91 which is less than STIR that costs on average 29.0 min × $627/hour + $70 = $373.05.
Discussion
Different sequences are typically used in whole-body MR imaging for monitoring tumours in children and young adults, including STIR, T2-weighted fast spin-echo sequences, diffusion-weighted images (DWI), and gadolinium-enhanced T1-weighted gradient echo sequences. STIR has been the most commonly used sequence in whole-body MR imaging and can be performed in less than 30 minutes [26–28]. Our data show that ferumoxytol-enhanced 18F-FDG PET/FSPGR exams provide equal sensitivity and specificity compared to 18F-FDG PET/STIR exams with improved time- and cost-efficiency. PET/MR combines the high anatomical resolution and soft tissue contrast of MRI with the high sensitivity of PET, thereby overcoming the limited sensitivity of traditional MRI approaches and concerns regarding radiation exposure of paediatric patients from PET/CT scans [29–32]. Through these benefits, patient quality of life (QoL) is purportedly enhanced by providing equivalent diagnostic information with reduced patient dose.
We acquired MR images after administration of the iron supplement ferumoxytol, in order to achieve long lasting blood pool enhancement of vessels throughout the duration of the whole body scan. [8]. Perhaps one could compare this to adding a diagnostic CT to a PET/CT, which was traditionally done with low dose scans for attenuation correction only. Ferumoxytol is a “blood pool agent” and has a blood half-life of 15 hours in humans, which allows for long lasting vessel enhancement on MR images [33]. Therefore, it is not crucial for the quality of the imaging scan to administer the contrast agent while the patient is in the scanner or to start a post-contrast scan immediately after contrast media administration. This is a convenient feature for clinical applications in paediatric patients, where timing of contrast agent injections and scanning cannot always be reliably planned.
Integrated PET/MR scanners are expensive and may not be affordable for many paediatric imaging centres. Our data also show that image registration of sequentially acquired 18F-FDG PET and MR scans is a feasible alternative. The proposed breath-hold T1-weighted SPGR sequence can be equally used to expedite whole-body MRI data acquisition of sequential or integrated PET/MR technologies. We utilized OsiriX for image data fusion which allows for image co-registration within less than 500 microns [34]. Others have confirmed the feasibility of this approach [35–37]. Using sequential imaging approaches takes advantage of CT-based attenuation correction of PET data, while integrated scans require MR-based attenuation correction. Our ongoing studies on an integrated PET/MR scanner confirm that the same approach described here can be used for fused ferumoxytol-enhanced 18F-FDG PET/MR scans.
Both ferumoxytol-enhanced T1-weighted FSPGR and STIR sequences have some limitations due to specific artefacts: On STIR images, ghosting artefacts from breath motion in the single-average acquisition was noted. On the T1-weighted FSPGR images during breath hold, cardiac motion artefacts were apparent, but more confined than the pervasive ghosting from the free breathing acquisition. Using large field-of-views for whole body scanning may lead to incomplete fat saturation at the edge of the coil. Also, the calibration and ASSET scans can in principle cause uncorrected aliasing and reduce SNR, although we did not encounter any major artefacts of this nature.
Of note, we injected ferumoxytol directly before the MRI scans. This was technically convenient and provided strong vascular enhancement, similar to the procedure of a contrast-enhanced CT scan. Our previous studies showed that the vascular enhancement improved tumour delineation compared to unenhanced scans [8]. However, this relatively early post-contrast time point was too early to achieve enhancement of tumours or lymph nodes, which is strongest at 24 hours post injection [38, 39]. Further studies are needed to determine whether the added diagnostic information for lymph node characterization justifies a two-step visit (day one for contrast injection and day 2 for MRI).
Our proposed approach requires injection of an iron oxide nanoparticle compound. The European radiological community has extensive clinical experience with these compounds and our team has applied various iron oxide nanoparticle compounds as MR contrast agents in phase I–IV clinical trials [40–44]. These agents are generally well tolerated and show excellent safety profiles [45–48]. Considering a ferumoxytol dose of 5 mg/kg and a concentration of 30 mg Fe/ml, we anticipate injecting 250–400 mg Fe in a young adult with an average body weight of 50–80 kg (of note, these are coated iron particles, not free iron). This iron dose is lower than the FDA-approved ferumoxytol dose for anaemia treatment and comparable to the iron dose administered with one blood transfusion. Anaphylaxis or anaphylactoid reactions to ferumoxytol were reported in 0.1–0.2% of exposed adult patients, which is comparable to other MR contrast agents. Ferumoxytol is not excreted via the kidneys and thus, not associated with any risk of nephrogenic sclerosis (a potential adverse event with Gd-chelates) [19, 49, 50]. In addition, ferumoxytol may represent al alternative to the recently reported deposition of Gd-chelates in the brain [51], although no comparative information is available so far regarding ferumoxytol deposition in the brain. Iron oxide nanoparticles are not approved for clinical use in children and applications in the US are done through an investigational new drug (IND) application with the FDA. Safety data in paediatric patients are limited, but have not shown clinically significant side effects so far [52, 53]. The recently renewed safety discussion around iron products does not relate to new toxic effects [24], but has to be taken seriously and has led to a black box warning by the FDA. Our team currently investigates the underlying pathophysiology of anaphylactic reactions to iron oxide nanoparticles, which will hopefully enable preventive actions in the future.
Although recent advances in MRI sequences for pulmonary imaging do allow for detection of pulmonary nodules, there is a potential to miss small pulmonary lesions on MR scans which are more conspicuous with CT. Previous investigators’ findings are in accordance with our results [54, 55]. Future technical improvements may overcome this limitation [56, 57].
Focal and multifocal bone marrow infiltration shows only a minor or no iron oxide uptake, while normal hematopoietic bone marrow shows substantial iron oxide uptake. Therefore, normal and neoplastic marrow can be differentiated on ferumoxytol-enhanced STIR images, which can help to correctly identify false positive, hypermetabolic hematopoietic bone marrow on PET scans. The bone marrow iron enhancement is detectable over a wide range of magnetic field strengths, from 1.5 to 7 Tesla [58].
We recently reported a radiation-free approach for whole-body tumour staging with MRI which relied on fusion of colour-encoded diffusion-weighted (DW) MRI sequences with anatomical FSPGR sequences [8]. To date, clinical decisions rely on metabolic information from 18F-FDG PET scans for monitoring of paediatric lymphomas [59–64]. Thus, while the medical community is in the process of assessing the value of diffusion-weighted sequences and apparent diffusion coefficients (ADC values) for treatment monitoring of paediatric lymphomas, 18F-FDG PET/MR with accelerated acquisition protocols may represent a compromise toward accurate diagnoses with the least possible radiation exposure.
Conclusion
We increased the time- and cost-efficiency of whole-body PET/MR scans by replacing traditional STIR scans with contrast-enhanced, breath-hold T1-weighted FSPGR scans. This efficient imaging approach improved the feasibility of PET/MR as a monitoring option for paediatric tumours, with reduced radiation exposure compared to PET/CT.
Key Points.
Breath-hold FSPGR sequences shorten the data acquisition time for whole-body MR and PET/MR.
Ferumoxytol provides long lasting vascular contrast for whole-body MR and PET/MR.
18F-FDG PET/FSPGR data provided equal sensitivity and specificity for cancer staging compared to 18F-FDG PET/STIR.
Acknowledgments
The scientific guarantor of this publication is Dr. Heike E. Daldrup-Link. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by the Thrasher Fund and the Child Health Research Institute, Stanford University, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health, grant number R01 HD081123A. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported in “Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study”, Lancet Oncol 15: 275–285. Methodology: retrospective, cross sectional study, performed at one institution.
References
- 1.Federman N, Feig SA. PET/CT in evaluating pediatric malignancies: a clinician’s perspective. Journal of Nuclear Medicine. 2007;48:1920–1922. doi: 10.2967/jnumed.107.046045. [DOI] [PubMed] [Google Scholar]
- 2.Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. Journal of Nuclear Medicine. 2007;48:1932–1939. doi: 10.2967/jnumed.107.045286. [DOI] [PubMed] [Google Scholar]
- 3.Kleis M, Daldrup-Link H, Matthay K, Goldsby R, Lu Y, Schuster T, et al. Diagnostic value of PET/CT for the staging and restaging of pediatric tumors. European journal of nuclear medicine and molecular imaging. 2009;36:23–36. doi: 10.1007/s00259-008-0911-1. [DOI] [PubMed] [Google Scholar]
- 4.Tatsumi M, Miller JH, Wahl RL. 18F-FDG PET/CT in evaluating non-CNS pediatric malignancies. J Nucl Med. 2007 Dec;48:1923–31. doi: 10.2967/jnumed.107.044628. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007 Nov 29;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 6.Hall E, Brenner D. Cancer risks from diagnostic radiology. Cancer. 2008;81 doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 7.Robbins E. Radiation risks from imaging studies in children with cancer. Pediatric blood & cancer. 2008;51:453–457. doi: 10.1002/pbc.21599. [DOI] [PubMed] [Google Scholar]
- 8.Klenk C, Gawande R, Uslu L, Khurana A, Qiu D, Quon A, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014 Mar;15:275–85. doi: 10.1016/S1470-2045(14)70021-X. [DOI] [PubMed] [Google Scholar]
- 9.Doot RK, McDonald ES, Mankoff DA. Role of PET quantitation in the monitoring of cancer response to treatment: review of approaches and human clinical trials. Clinical and Translational Imaging. 2014;2:295–303. doi: 10.1007/s40336-014-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clinical Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatric radiology. 2013;43:860–875. doi: 10.1007/s00247-012-2570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas MI, Becker M, Garibotto V, Heinzer S, Loubeyre P, Gariani J, et al. Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies. Magnetic Resonance Materials in Physics, Biology and Medicine. 2013;26:57–69. doi: 10.1007/s10334-012-0340-9. [DOI] [PubMed] [Google Scholar]
- 13.Yankeelov TE, Peterson TE, Abramson RG, Garcia-Izquierdo D, Arlinghaus LR, Li X, et al. Simultaneous PET–MRI in oncology: a solution looking for a problem? Magnetic resonance imaging. 2012;30:1342–1356. doi: 10.1016/j.mri.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loevner LA, Kim AK, Mikityansky I. PET/CT-MR Imaging in Head and Neck Cancer Including Pitfalls and Physiologic Variations. PET Clinics. 2008;3:335–353. doi: 10.1016/j.cpet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Seiboth L, Van Nostrand D, Wartofsky L, Ousman Y, Jonklaas J, Butler C, et al. Utility of PET/neck MRI digital fusion images in the management of recurrent or persistent thyroid cancer. Thyroid. 2008;18:103–111. doi: 10.1089/thy.2007.0135. [DOI] [PubMed] [Google Scholar]
- 16.Littooij AS, Torigian DA, Kwee TC, de Keizer B, Alavi A, Nievelstein RA. Potential Clinical Applications of PET/Magnetic Resonance Imaging. PET Clinics. 2013;8:367–384. doi: 10.1016/j.cpet.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Landry R, Jacobs PM, Davis R, Shenouda M, Bolton WK. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am J Nephrol. 2005 Jul-Aug;25:400–10. doi: 10.1159/000087212. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Tutton S, Vu AT, Pierchala L, Li BS, Lewis JM, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging. 2005 Jan;21:46–52. doi: 10.1002/jmri.20235. [DOI] [PubMed] [Google Scholar]
- 19.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010 May;85:315–9. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 20.Aghighi M, Golovko D, Ansari C, Marina NM, Pisani L, Kurlander L, et al. Imaging Tumor Necrosis with Ferumoxytol. PLoS ONE. 2015;10:e0142665. doi: 10.1371/journal.pone.0142665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AMAG Pharmaceuticals, Inc. Feraheme (ferumoxytol) package insert. Lexington, MA: 2009. [Google Scholar]
- 22.Balakrishnan VS, Rao M, Kausz AT, Brenner L, Pereira BJ, Frigo TB, et al. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest. 2009 Jun;39:489–96. doi: 10.1111/j.1365-2362.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 23.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, Jacobs PM, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009 Mar;75:465–74. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA; Safety Announcement, Drug Safety Communication, editor. FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol) 2015. [Google Scholar]
- 25.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004 Sep;17:205–16. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira SR, Elias J, Junior, Nogueira-Barbosa MH, Guimarães MD, Marchiori E, Santos MK. Whole-body magnetic resonance imaging in children: state of the art. Radiologia brasileira. 2015;48:111–120. doi: 10.1590/0100-3984.2014.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavhan GB, Babyn PS. Whole-body MR imaging in children: principles, technique, current applications, and future directions. Radiographics. 2011;31:1757–1772. doi: 10.1148/rg.316115523. [DOI] [PubMed] [Google Scholar]
- 28.Kellenberger CJ, Miller SF, Khan M, Gilday DL, Weitzman S, Babyn PS. Initial experience with FSE STIR whole-body MR imaging for staging lymphoma in children. European radiology. 2004;14:1829–1841. doi: 10.1007/s00330-004-2432-z. [DOI] [PubMed] [Google Scholar]
- 29.Balyasnikova S, Lofgren J, de Nijs R, Zamogilnaya Y, Hojgaard L, Fischer BM. PET/MR in oncology: an introduction with focus on MR and future perspectives for hybrid imaging. Am J Nucl Med Mol Imaging. 2012;2:458–74. [PMC free article] [PubMed] [Google Scholar]
- 30.Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez-Moller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012 Jun;53:845–55. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 31.Herzog H, Van Den Hoff J. Combined PET/MR systems: an overview and comparison of currently available options. Q J Nucl Med Mol Imaging. 2012 Jun;56:247–67. [PubMed] [Google Scholar]
- 32.Platzek I, Beuthien-Baumann B, Langner J, Popp M, Schramm G, Ordemann R, et al. PET/MR for therapy response evaluation in malignant lymphoma: initial experience. MAGMA. 2013 Feb;26:49–55. doi: 10.1007/s10334-012-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon GH, von Vopelius-Feldt J, Fu Y, Schlegel J, Pinotek G, Wendland MF, et al. Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Invest Radiol. 2006 Jan;41:45–51. doi: 10.1097/01.rli.0000191367.61306.83. [DOI] [PubMed] [Google Scholar]
- 34.Mandel M, Amorim R, Paiva W, Prudente M, Teixeira MJ, Andrade AFd. 3D Preoperative Planning in the ER with OsiriX®: When There is No Time for Neuronavigation. Sensors. 2013;13:6477–6491. doi: 10.3390/s130506477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen FL, Ladefoged CN, Beyer T, Keller SH, Hansen AE, Højgaard L, et al. Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone. Neuroimage. 2014;84:206–216. doi: 10.1016/j.neuroimage.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 36.Antonica F, Asabella AN, Ferrari C, Rubini D, Notaristefano A, Nicoletti A, et al. Useful diagnostic biometabolic data obtained by PET/CT and MR fusion imaging using open source software. Hellenic journal of nuclear medicine. 2014:50. [PubMed] [Google Scholar]
- 37.Matthew DJ, Linda CT, Leena K, Yasser MMM, Nandita G-T. Co-registration of sequential multidetector computed tomography studies for the evaluation of surgical instrumentation following resection of spinal tumors. Case reports in radiology. 2011;2011 doi: 10.1155/2011/676410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daldrup-Link HE, Golovko D, Ruffell B, DeNardo DG, Castaneda R, Ansari C, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clinical Cancer Research. 2011;17:5695–5704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barentsz JO, Fütterer JJ, Takahashi S. Use of ultrasmall superparamagnetic iron oxide in lymph node MR imaging in prostate cancer patients. European journal of radiology. 2007;63:369–372. doi: 10.1016/j.ejrad.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 40.Daldrup-Link HE, Kaiser A, Link TM, Settles M, Helbich T, Werner M, et al. Comparison between gadopentetate and feruglose (Clariscan)-enhanced MR-mammography: preliminary clinical experience. Acad Radiol. 2002 Aug;9(Suppl 2):S343–7. doi: 10.1016/s1076-6332(03)80225-8. [DOI] [PubMed] [Google Scholar]
- 41.Daldrup-Link HE, Rummeny EJ, Ihssen B, Kienast J, Link TM. Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin’s lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. Eur Radiol. 2002 Jun;12:1557–66. doi: 10.1007/s00330-001-1270-5. [DOI] [PubMed] [Google Scholar]
- 42.Daldrup-Link HE, Brasch RC. Macromolecular contrast agents for MR mammography: current status. Eur Radiol. 2003 Feb;13:354–65. doi: 10.1007/s00330-002-1719-1. [DOI] [PubMed] [Google Scholar]
- 43.Daldrup-Link HE, Kaiser A, Helbich T, Werner M, Bjornerud A, Link TM, et al. Macromolecular contrast medium (feruglose) versus small molecular contrast medium (gadopentetate) enhanced magnetic resonance imaging: differentiation of benign and malignant breast lesions. Acad Radiol. 2003 Nov;10:1237–46. doi: 10.1016/s1076-6332(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 44.Daldrup-Link HE, Mohanty A, Cuenod C, Pichler B, Link T. New perspectives on bone marrow contrast agents and molecular imaging. Semin Musculoskelet Radiol. 2009 Jun;13:145–56. doi: 10.1055/s-0029-1220885. [DOI] [PubMed] [Google Scholar]
- 45.Daldrup-Link HE, Rydland J, Helbich TH, Bjornerud A, Turetschek K, Kvistad KA, et al. Quantification of breast tumor microvascular permeability with feruglose-enhanced MR imaging: initial phase II multicenter trial. Radiology. 2003 Dec;229:885–92. doi: 10.1148/radiol.2293021045. [DOI] [PubMed] [Google Scholar]
- 46.Simon G, Link TM, Wortler K, Doebereiner F, Schulte-Frohlinde E, Daldrup-Link H, et al. Detection of hepatocellular carcinoma: comparison of Gd-DTPA- and ferumoxides-enhanced MR imaging. Eur Radiol. 2005 May;15:895–903. doi: 10.1007/s00330-005-2669-1. [DOI] [PubMed] [Google Scholar]
- 47.Metz S, Lohr S, Settles M, Beer A, Woertler K, Rummeny EJ, et al. Ferumoxtran-10-enhanced MR imaging of the bone marrow before and after conditioning therapy in patients with non-Hodgkin lymphomas. Eur Radiol. 2006 Mar;16:598–607. doi: 10.1007/s00330-005-0045-9. [DOI] [PubMed] [Google Scholar]
- 48.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008 Aug;19:1599–605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coyne DW. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin Pharmacother. 2009 Oct;10:2563–8. doi: 10.1517/14656560903224998. [DOI] [PubMed] [Google Scholar]
- 50.Schwenk MH. Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy. 2010 Jan;30:70–9. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 51.Schlaudecker JD, Bernheisel CR. Gadolinium-associated nephrogenic systemic fibrosis. American family physician. 2009;80:711–4. [PubMed] [Google Scholar]
- 52.Hassan N, Cahill J, Rajasekaran S, Kovey K. Ferumoxytol infusion in pediatric patients with gastrointestinal disorders: first case series. Ann Pharmacother. 2011 Dec;45:e63. doi: 10.1345/aph.1Q283. [DOI] [PubMed] [Google Scholar]
- 53.Muehe AM, Feng D, von Eyben R, Luna-Fineman S, Link MP, Muthig T, et al. Safety Report of Ferumoxytol for Magnetic Resonance Imaging in Children and Young Adults. Investigative radiology. 2015 doi: 10.1097/RLI.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hochhegger B, Marchiori E, Irion K, Moreira J, Zanetti G. MRI in assessment of lung cancer. Thorax. 2011 Apr;66:357. doi: 10.1136/thx.2011.159111. [DOI] [PubMed] [Google Scholar]
- 55.Chandarana H, Heacock L, Rakheja R, DeMello LR, Bonavita J, Block TK, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology. 2013 Sep;268:874–81. doi: 10.1148/radiol.13130620. [DOI] [PubMed] [Google Scholar]
- 56.Sommer G, Koenigkam-Santos M, Biederer J, Puderbach M. Role of MRI for detection and characterization of pulmonary nodules. Der Radiologe. 2014 doi: 10.1007/s00117-013-2604-4. [DOI] [PubMed] [Google Scholar]
- 57.Kurihara Y, Matsuoka S, Yamashiro T, Fujikawa A, Matsushita S, Yagihashi K, et al. MRI of Pulmonary Nodules. American Journal of Roentgenology. 2014;202:W210–W216. doi: 10.2214/AJR.13.11618. [DOI] [PubMed] [Google Scholar]
- 58.Daldrup-Link HE, Rummeny EJ, Ihssen B, Kienast J, Link TM. Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin’s lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. European radiology. 2002;12:1557–1566. doi: 10.1007/s00330-001-1270-5. [DOI] [PubMed] [Google Scholar]
- 59.Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. Journal of Nuclear Medicine. 2007;48:1626–1632. doi: 10.2967/jnumed.107.042093. [DOI] [PubMed] [Google Scholar]
- 60.Haioun C, Itti E, Rahmouni A, Brice P, Rain J-D, Belhadj K, et al. [18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–1381. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 61.Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiologic clinics of North America. 2005;43:189–204. doi: 10.1016/j.rcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Hoekstra C, Paglianiti I, Hoekstra O, Smit E, Postmus P, Teule G, et al. Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. European journal of nuclear medicine. 2000;27:731–743. doi: 10.1007/s002590050570. [DOI] [PubMed] [Google Scholar]
- 63.Kumar R, Maillard I, Schuster SJ, Alavi A. Utility of fluorodeoxyglucose-PET imaging in the management of patients with Hodgkin’s and non-Hodgkin’s lymphomas. Radiologic Clinics of North America. 2004;42:1083–1100. doi: 10.1016/j.rcl.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Allen-Auerbach M, de Vos S, Czernin J. The Impact of Fluorodeoxyglucose–Positron Emission Tomography in Primary Staging and Patient Management in Lymphoma Patients. Radiologic Clinics of North America. 2008;46:199–211. doi: 10.1016/j.rcl.2008.03.004. [DOI] [PubMed] [Google Scholar]