Abstract
The aims of this work were to construct corn protein hydrolysate (CPH)-based curcumin nanoparticles (Cur NPs) and to compare the colloidal stability, bioaccessibility and antioxidant activity of the Cur NPs stabilized CPH and sodium caseinate (NaCas) respectively. The results indicated that Cur solubility could be considerably improved after the Cur NPs fabrication. The spectroscopy results demonstrated that the solubilization of Cur should be attributed to its complexation with CPH or NaCas. The Cur NPs exhibited good colloidal stability after 1 week’s storage but showed smaller (40 nm) size in CPH than in NaCas (100 nm). After lyophilization, the Cur NPs powders showed good rehydration properties and chemical stability, and compared with NaCas, the size of Cur NPs stabilized by CPH was still smaller. Additionally, the Cur NPs exhibited higher chemical stability against the temperature compared with free Cur, and the CPH could protect Cur from degradation more efficiently. Comparing with NaCas, the Cur NPs stabilized by CPH exhibited better bioaccessibility and antioxidant activity. This study demonstrated that CPH may be better than NaCas in Cur NPs fabrication and it opens up the possibility of using hydrophobic protein hydrolysate to construct the NPs delivery system.
Keywords: Corn protein hydrolysate, Curcumin, Nanoparticles, Bioaccessibility, Antioxidant activity
Introduction
The increasing focus on health and wellness among the consumers leads to the emergence of a specialized category of food products, namely the functional foods. The development of functional foods relies on the enrichment and fortification of food produced by incorporation of bioactive ingredients, such as polyphenols, phytosterols, vitamins, minerals, functional lipids, and so on (Patel and Velikov 2011). However, many of these bioactive ingredients could not be simply introduced into food stuff in pure form due to their limited physicochemical and biological properties, such as poor water solubility, chemical or physical unstable in processing or under storage conditions (Wan et al. 2015). Therefore, the modern food industry is facing a stiff challenge of devising novel techniques for delivering functional ingredients without compromising the product functionalities.
Food-grade delivery system is given increasing attentions due to their protection and controlled release behaviour. Among these food-grade materials, food proteins are a versatile group of biopolymers that have high nutritional value along with considerable functional properties, including emulsification, gelation, foaming, and their applications as ingredients in food industry (Foegeding and Davis 2011; Lam and Nickerson 2013). Their chemical and structural versatility make them appropriate candidates for the delivery of bioactive ingredients. Milk proteins have been reported to be successful in fabricating nanovehicles for the delivery of hydrophobic nutraceuticals, such as vitamin D and ω-3 polyunsaturated fatty acids in casein micelles, curcumin and resveratrol in β-lactoglobulin nanovehicles (Li et al. 2007; Semo et al. 2007; Zimet et al. 2011). In contrast to delivery vehicles using hydrophilic animal proteins, plant proteins such as soybean protein, zein and gliadin also have been used as carriers for bioactive compounds (Chen et al. 2015a, b; Joye et al. 2015). Generally, the solubility and chemical stability of the hydrophobic bioactive substances, such as curcumin and riboflavin, could be greatly improved by this delivery system (Chen and Subirade 2006; Pan et al. 2013; Zimet et al. 2011).
Structuring food-grade delivery system with protein fragments or hydrolysate is a new attempt. Zein hydrolysate exhibited excellent amphiphilic and self-assembly properties in water solution, and it was successfully used as a nano-delivery vehicle for curcumin (Wang et al. 2015). The NPs fabricated by the complexation of zein hydrolysate and curcumin had a monodisperse size distribution (<50 nm), and the size is considerably smaller than the delivery systems fabricated by native proteins. Additionally, α-lactalbumin hydrolysate was also found the self-assembling properties, and it was successfully applied as a delivery vehicle for iron (Wang et al. 2014a, b). Now, we found that CPH also could be utilized as a novel nano-vehicle for curcumin delivery.
Compared with the native proteins, protein fragments or hydrolysates may turn out to be a better vehicle to deliver water-insoluble bioactive compounds, because the protein hydrolysates generally have smaller size and better environmental tolerance (e.g. digestive enzymes, temperature, pH and saline ions). Additionally, protein hydrolysates themselves have many beneficial effects on the bodies. However, there was no report on the comparation of native proteins and protein fragments in the applications of delivery vehicles. In fact, studying this issue could pave the way for many important applications, such as nutrients fortification in clear drinks and drug delivery.
Curcumin (Cur), a natural polyphenol found in the rhizomes of curcuma longa (turmeric), exhibits anti-inflammatory, antineoplastic, antioxidant, and chemopreventive activities (Prasad et al. 2014). Several previous clinical trials have addressed the pharmacokinetics, safety, and efficacy of Cur in humans (Dhillon et al. 2008). However, despite extensive researches and developments, the poor water solubility of Cur, due to its hydrophobic property and preferential interaction with lipid membranes, remains a major barrier in its bioavailability and clinical efficacy (Anand et al. 2007). To increase its solubility and bioavailability, many attempts have been made through design and development of nano-sized delivery systems for Cur, including liposomes, polymeric NPs and micelles, conjugates, peptide/protein carriers, cyclodextrins, solid dispersions, lipid NPs and emulsions (Naksuriya et al. 2014). In this study, we show the possibility of using CPH to stabilize Cur NPs, and to compare the colloidal stability, bioaccessibility and antioxidant activity of the Cur NPs stabilized by CPH and NaCas respectively.
Materials and methods
Raw materials
Corn gluten meal (CGM) was kindly granted by corn developing Co., Ltd (Zhucheng, China). The protein content of CGM was 52.9 ± 1.1 %, determined by Kjeldahl method (N × 6.25, wet basis). Alcalase 2.4L (endoproteinase from Bacillus licheniformis, 2.4 AU per g) was obtained from Novozymes North America Inc. (Franklinton, NC). Curcumin (∼98 % purity, from Curcuma longa) was purchased from Sigma-Aldrich (St Louis, MO). Sodium caseinate (>90 %) was obtained from Meryer Chemical Technology Co., Ltd (Shanghai, China). All other chemicals used were of analytical grade.
Preparation of corn protein concentrates
CGM is usually difficult to utilize directly, because it contains plenty of crude fat and pigment, and has an intense odor. Hence, the purification and concentration process are needed. Corn protein concentrates (CPC) was prepared by the following process. Briefly, defatting operation was carried out by subcritical extraction equipment (CBE-100L, Henan Province Subcritical Extraction Equipment Engineering Research Centre, China) with the butane as extraction agent (solid/solvent ratio = 1: 10). The defatted CGM was dispersed in distilled water (10 %, w/v), and shearing dispersion procedure was carried out by a high-shear dispersion homogenizer (IKA LABOR-PILOT 2000/4) for 10 min. Then, thermal stable amylase was added to the dispersion (0.5 %, w/v), incubating at pH 6.0 and 85 °C for 2 h. After neutralization with 1 M NaOH, the suspension was subjected to filtration via filtration equipment (F2.5-A-0.3M, Hyflux Ltd, Shanghai). The retentate was diluted with twofold volume water, and the filtration process was run again. Finally, the retentate was spray dried by a mini spray drier (B-29, BÜCHI Co Ltd.), and the pale yellow CPC (protein ~85 %) was recovered.
Preparation of CPH
CPC suspension in deionized water (5 % w/v) was hydrolyzed with Alcalase at 50 °C. The ratio of enzyme to protein substrate was 2:100 (mL/g). The pH of the suspension was adjusted to 9.0 before hydrolysis was initiated, and maintained at 9.0 by continuously dropwise adding 1 M NaOH. The enzymolysis was terminated when pH didn’t drop in 5 min. After hydrolysis, the pH was brought to 7.0 using 1 M HCl, and the solution was then heated at 95 °C for 5 min to inactivate the enzyme. Then the hydrolysate was centrifuged at 10,000 r/min, 25 °C for 20 min. The supernatant was dialyzed (100 Da cutoff) over night against deionized water and finally freeze-dried (Dura-Dry MP freeze-dryer, FTS Systems, Inc., Ridge, NY). The prepared CPH was stored at 4 °C before use.
Fabrication of Cur NPs
0.25 mL stock solution of Cur (3 mg/mL in ethanol) was added into 4.75 mL of CPH solution (2.5 mg/mL). The mixture was centrifuged at 10,000×g, 4 °C for 20 min to pellet the unbound Cur, and the supernatants containing Cur NPs were preserved in a light-resistant container at 4 °C for determination. As the contrasts, pure PBS and NaCas (2.5 mg/mL) instead CPH were also prepared at the same conditions.
Water solubility determination of Cur
The water solubilization of Cur was confirmed by UV–Vis spectra (UV2300, ECHCOMP). Before the determination, freshly prepared Cur NPs were diluted 10 times with deionized water. As a contrast, pure CPH with the same concentration was also tested. To quantify the water solubility of Cur, the methodology according to Ma et al. (2008) was adopted with some modifications. Briefly, 100 μL of freshly prepared Cur dispersion was added to 1.90 mL methanol in a tube. After a violent shaking, each sample was centrifuged at 10,000 rpm, 4 °C for 10 min, and the extracted supernatant was used for Cur quantification by the absorbance at 420 nm. The solubility of Cur in pure PBS, CPH and NaCas solutions was quantified by the Cur standard curve (Y = 7.2766 X + 0.0333, R2 = 0.9994).
X-ray diffraction (XRD)
The XRD patterns of the lyophilized samples were characterized using an XRD powder X-ray diffractometer (D8 ADVANCE, Bruker) with Cu Kα radiation at a wavelength of 0.15418 nm. Measurements were performed at 40 kV and 40 mA. The 2 Theta angle was set from 2° to 75°, and the scanning rate was 2°/min.
Size distribution and surface charges determination
The changes of size distributions and surface charges of the Cur NPs were measured by a commercial dynamic light scattering and micro-electrophoresis device (Malvern Instruments Co. Ltd., Worcestershire, UK). The freshly prepared Cur NPs in CPH and NaCas solutions were stored at 4 °C under light-resistant condition for 1 week. The size distributions based on intensity were determined at different time intervals. Zeta potential was reported on the basis of three replicates. As a contrast, free Cur in pure PBS was also determined at the same conditions.
Solubility and stability of the Cur NPs powder
The freshly prepared Cur NPs in CPH and NaCas were lyophilized under light-resistant condition by covering with foil. As a control, free Cur dispersion prepared in pure PBS was also performed. The lyophilized powders were dissolved in methanol (0.5 mg/mL) respectively, and centrifuged at 10,000 rpm for 10 min. The supernate was analysed by UV spectrum to confirm the chemical stability of Cur after the lyophilization. The Cur NPs powders were also dispersed in deionized water and the particle size distributions were measured by DLS.
Chemical stability measurement
The freshly prepared Cur NPs dispersions were incubated at 4 °C (light-resistant), 25 and 75 °C respectively. As a contrast, free Cur was also dispersed in PBS by the same method. At specified time points, samples (100 μL) were taken out and added to 1.9 mL of methanol for quantitative analysis of Cur by Cur standard curve, as described above. The results were represented by the percentage of the absorbance at certain time points with respect to the initial value.
In vitro digestion
An in vitro model that simulated sequential gastric and intestinal digestion was applied to evaluate the influence of digestion on release properties of Cur (free, or NPs in CPH and NaCas). The process was carried out according to the methodology by Chen et al. (2015a, b) with slight modifications. In brief, 5 mL of freshly prepared Cur NPs in CPH or NaCas solution was well mixed with 20 mL of 0.1 M HCl (pH 1.5), and preincubated in a shaker (at 37 °C) at a rate of 100 rpm for 10 min. If necessary, the pH of the mixture was adjusted to 1.5 with 1.0 M HCl. Then, 5 mg of pepsin powder was added and well mixed to start the simulated gastric digestion. After 60 min, the pH of the pepsin-digests was immediately adjusted to 7.0 with 4 M NaOH, and 25 mg of bile extract was added and well dispersed in the shaker for 10 min. Last, 10 mg of pancreatin powder was added to start the intestinal digestion (60–180 min). After 180 min, 500 μL of the final digest dispersion was also collected and centrifuged as above to determine the Cur content in the supernate. The residual ratios of Cur in the supernatant of digest dispersions were represented by the percentage of the Cur content in supernate at 180 min with respect to the total amount of Cur (745 μg and 720 μg for CPH and NaCas solutions respectively). For free Cur (control), the Cur dispersion was prepared by mixing 500 μL of Cur ethanol solution (3.0 mg/mL) with 9.5 mL PBS, and then subject to the same digestion process as above. Additionally, the in vitro digestion without enzyme addition was also carried out using the above procedure to investigate the effect of enzymes on release properties of Cur.
Antioxidant activity assay
Total antioxidant activity
The total antioxidant activity of NPs was evaluated by the ABTS assay, and the protocol was adopted from Pan et al. (2013) with slight modifications. Briefly, ABTS (6.2 mM) was dissolved in deionized water. Potassium persulfate was dissolved at an overall concentration of 5 mM in the ABTS solution and was allowed to stand in the dark at room temperature for 12 h before use. The ABTS radical solution was diluted with 10 mM PBS to an absorbance of 0.70 ± 0.02 at 734 nm and equilibrated at 30 °C. The free Cur in EtOH and the Cur NPs in CPH and NaCas were diluted to a series of concentrations (0.5–10 μg/mL) with PBS. 500 μL of the diluted samples were added to 1.5 mL of ABTS radical solution, and the absorbance reading was taken at 30 °C exactly 1 min after initial mixing for up to 6 min. The PBS was used as a blank in each run. The scavenging capability of the test samples was determined using the formula:
where As and Ac are the absorbance of test (samples) and control (PBS).
DPPH scavenging activity
The methodology of DPPH scavenging activity measurement was carried out following Yen et al. (2010) with modifications. Briefly, free Cur in DMSO and Cur NPs in CPH and NaCas were prepared at multiple concentrations (10–50 μg/mL). 2.0 mL of DPPH (0.2 mM in ethanol) was mixed with equivalent aliquots of different samples and subsequently incubated (in darkness) at room temperature. After 30 min the absorbance was measured at 517 nm using a UV spectrometer. As the blanks, 2.0 mL of pure ethanol without DPPH but with equivalent aliquots of different samples was also tested. Additionally, the mixture of equivoluminal DPPH and deionized water was also run as the control. Each sample was tested in triplicate. The inhibition percentage of the samples was calculated using the formula:
where A s, A b and A c are the absorbance of sample, blank and control.
Statistical analysis
Unless otherwise specified, all measurements were carried out in triplicate, and an analysis of variance (ANOVA) of the data was performed using the SPSS 19.0 statistical analysis system. The Duncan Test was used for comparison of mean values among the three treatments using a level of significance of 5 %.
Results and discussion
Solubilization of Cur
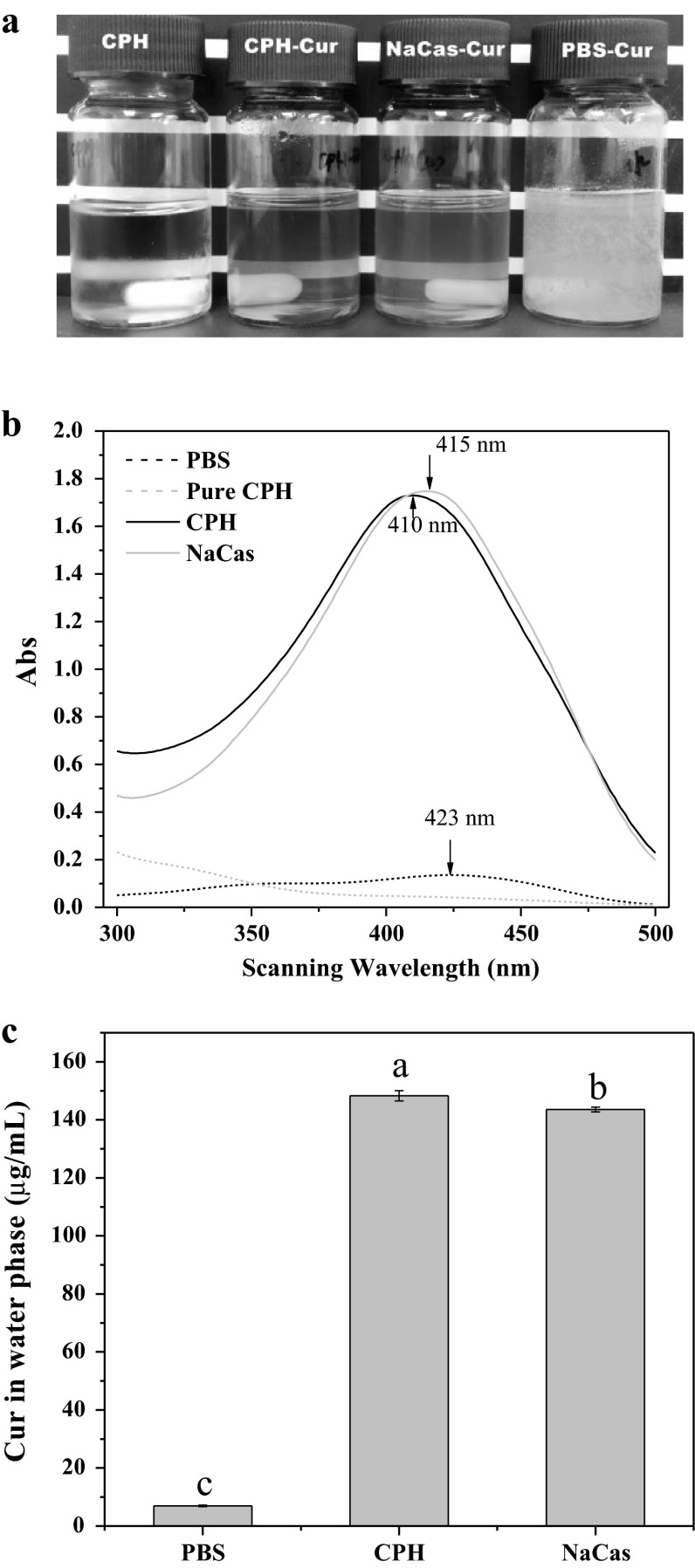
The appearances of Cur in CPH, NaCas and PBS are shown in Fig. 1a. Free Cur in PBS was very turbid due to its poor water solubility, and it could quickly precipitate after a short time standing. However, the appearances of Cur in CPH and NaCas were yellow and highly transparent, and more importantly, they were very stable in long time storage. The solubilizing effectiveness of Cur in CPH and NaCas was further confirmed by UV-spectra scanning (Fig. 1b). Because of poor water solubility, free Cur in PBS only showed a weak absorption peak at 423 nm. However, the absorbance intensity of Cur in CPH and NaCas solutions were considerably increased, and the corresponding maximum absorption wavelengths were all blue shifted (410 nm for CPH and 415 nm for NaCas). These observations suggested that the interactions (hydrophobic or hydrogen bond interactions) between Cur and CPH or NaCas could take place in the fabrication process of Cur NPs. This notion has gained strong support from the previous study, in which binding-induced static quenching between hydrophobin and Nile red or VD3 were confirmed by UV absorbance spectra (Israeli-Lev and Livney 2014). Figure 1c shows that the water solubility of Cur in CPH and NaCas solutions were 148 and 142 μg/mL respectively, suggesting a considerable improvement compared with free Cur in PBS. Furthermore, compared with 11 μg/L of free Cur in water (Kaminaga et al. 2003), the solubility of Cur in CPH and NaCas were enhanced by more than 14,000-folds. Therefore, CPH is better than or comparable with NaCas in the solubilization of Cur.
Fig. 1.
a Appearances of pure CPH solution and Cur in CPH, NaCas and PBS solutions ([CPH] and [NaCas] = 2.5 mg/mL, [Cur] = 0.15 μg/mL). b UV–Vis absorbance spectra of pure CPH (red), free Cur in PBS (black), and Cur in CPH (blue) and NaCas solutions (gray). The arrows indicate the maximum absorbance wavelength of Cur in each solution. c Water solubility of Cur in PBS, CPH and NaCas solutions. Different characters (a–c) on the top of columns represent significant difference among the samples (P < 0.05, n = 3)
Amorphous structure of NPs
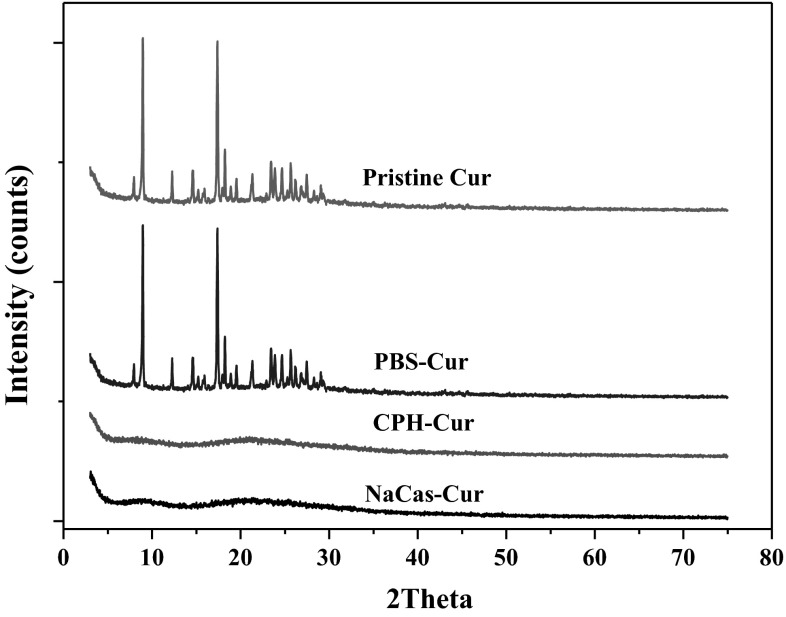
Powder XRD analyses were applied to investigate the crystal transformation of the NPs system (Fig. 2). The frequent peaks on the diffraction patterns implied the highly crystalline structure of the pristine Cur. In contrast, there were no characteristic peaks on the patterns for the lyophilized Cur NPs powders. The result indicated a conversion of Cur from a highly crystalline state to an amorphous one. Additionally, this observation can be well supported by the previous study of Pan et al. (2013), in which the crystal peaks of Cur disappeared after the encapsulation by NaCas. This observation may be explained by the classic nucleation theory. The CPH and NaCas probably suppressed the crystal nucleus formation of Cur during the antisolvent precipitation process, thus promoting the formation of an amorphous complex. Finally, the encapsulated Cur was present in an amorphous state or was dispersed at the molecular level. A similar report on dissolution enhancement of quercetin through nanofabrication was made by Kakran et al. (2011), where the quercetin NPs were no longer present in the crystalline form, but existed in the amorphous state or the monomolecular dispersed state.
Fig. 2.
X-ray diffraction patterns of pristine Cur, free Cur in PBS and Cur NPs stabilized by CPH and NaCas respectively
Colloidal properties of Cur NPs
The dissolution enhancement of Cur is based on the formation of Cur nanocomplexes, and the Cur NPs could be fabricated by the complexation of Cur with proteins (Chen et al. 2015a, b). Water solubility of Cur in long-term storage is in accordance with the colloidal stability. Generally, smaller size and higher zeta potential imply the prominent colloidal stability. A minimum zeta potential of greater than −60 mV is required for excellent and −30 mV for good physical stability (Kovačević et al. 2014). The zeta potentials of free Cur, Cur NPs in CPH and NaCas were −15, −30, and −37 mV respectively (Fig. 3a). This observation suggested the poor physical stability of free Cur in PBS and the good stability of Cur NPs in CPH and NaCas solutions. The changes of size distributions with storage time further confirmed the physical instability of free Cur (Fig. 3b). Although the free Cur exhibited a monodisperse size distribution with a mean diameter of 100 nm, a bimodal distribution appeared only after 12 h standing, suggesting the quick aggregation of the free Cur particles in PBS. In fact, with the increasing of storage time, obvious precipitation could take place. In contrast, the fresh Cur NPs in CPH and NaCas also showed a narrow size distribution (Fig. 3c, d). But, Cur NPs stabilized by CPH showed a smaller size (~40 nm) compared with that in NaCas (~100 nm). Additionally, the size distributions of the Cur NPs in CPH and NaCas were all unchanged after 7 days storage, confirming the long-term storage stability of the Cur NPs. It should be noted that the size of Cur NPs stabilized by NaCas was obviously smaller than in the previous report (Pan et al. 2013), where the mean diameter of the Cur NPs prepared in NaCas was 168.7 nm. This difference may be attributed to the aggregation of NPs in the spray drying process. Additionally, the average particle size of Cur NPs stabilized by other native proteins (e.g. zein, β-lactoglobulin) was ~100 nm or higher (Patel et al. 2010; Sneharani et al. 2010). Therefore, the size of Cur NPs in CPH is obviously smaller than the native protein-based Cur NPs. The results may be explained by the smaller size of protein fragments than the intact proteins.
Fig. 3.
Zeta potentials of free Cur in PBS, and Cur NPs in CPH and NaCas (a). The time evolutions of the size distribution for free Cur in PBS (b), Cur NPs in CPH (c) and NaCas (d). Different characters (a–c) on the top of columns represent significant difference among the samples (P < 0.05, n = 3)
Rehydration properties of the lyophilized Cur NPs powder
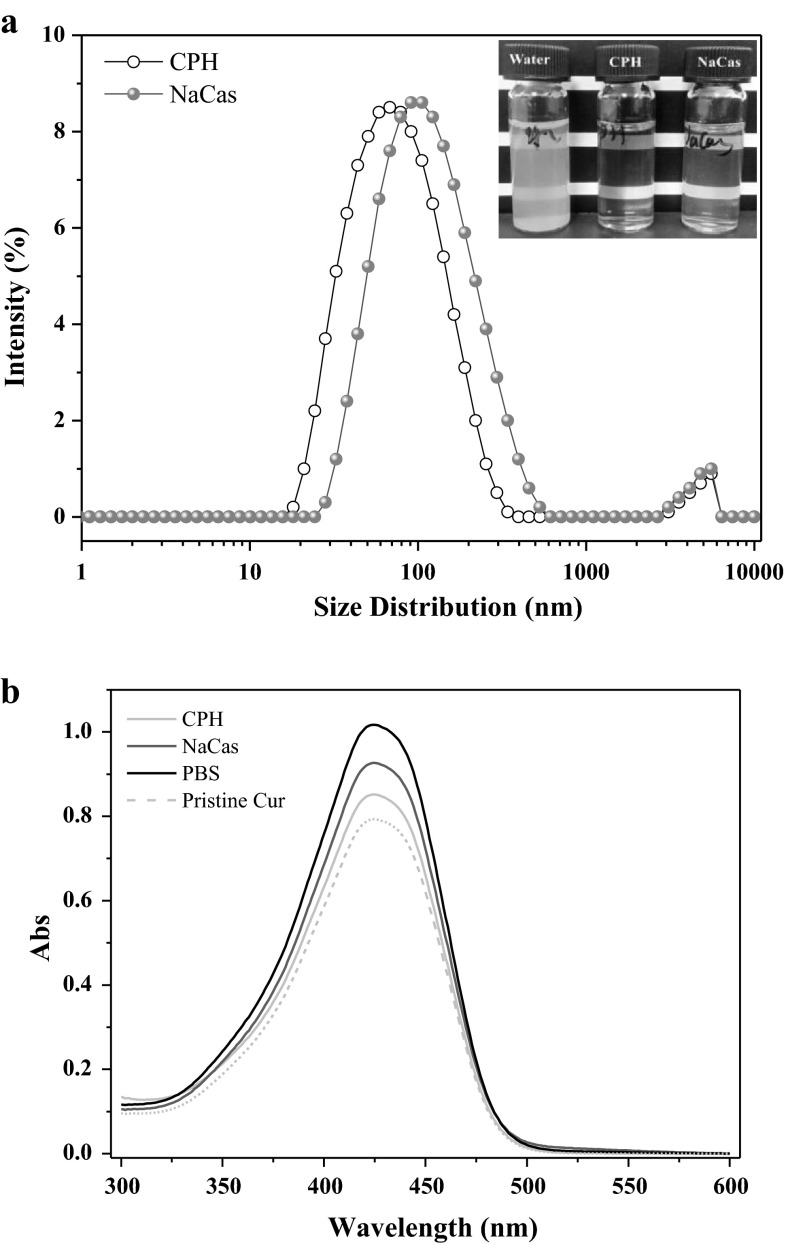
Figure 4 shows the rehydration properties and chemical stability of the lyophilized free Cur and Cur NPs powders. The Cur NPs powders obtained from Cur-CPH and Cur-NaCas complexes all showed good redispersibility in water and the dispersion liquids were very clear and yellow (Fig. 4a). The DLS result showed that the rehydrated Cur NPs still exhibited the monodisperse size distribution, and the average size was 60 and 100 nm for CPH and NaCas stabilized Cur NPs respectively. The UV-spectrum of Cur showed that compared with the fresh pristine Cur, free Cur in PBS, Cur NPs in CPH and NaCas were still chemical stable after the lyophilization treatment (Fig. 4b). These observations suggested the possible applications of Cur NPs powders in functional food ingredients.
Fig. 4.
a The size distribution of the rehydrated Cur NPs. Inset the appearances of the rehydrated free Cur and Cur NPs dispersion liquids; b UV-spectrum of Cur extracted from free Cur and Cur NPs powders
The chemical stability of Cur NPs
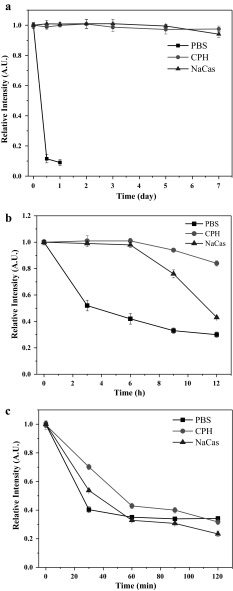
The chemical stability of free Cur and Cur NPs were evaluated by the retention ratio of Cur after storage at different temperatures (Fig. 5). The results showed that free Cur in PBS was very unstable, and only 10 % was retained after 12 h of storage at 4 °C under light-resistant conditions (Fig. 5a). However, for Cur NPs, there were no obvious reductions to be observed under the same conditions. Similar results have been reported by Leung and Kee (2009), where the authors claimed that under the neutral pH condition Cur could degrade by about 90 % within 30 min at room temperature. In ambient conditions, the Cur NPs in CPH and NaCas were still very stable in 6 h storage compared with the free Cur in PBS (Fig. 5b), although an obvious reducing tendency appeared with the storage time increasing. The observation indicated that the chemical stability of Cur could be hugely improved by the encapsulation process. This notion could be strongly supported by a large number of related investigations (Chen et al. 2015a, b; Pan et al. 2013; Prasad et al. 2014; Sneharani et al. 2010), where Cur all exhibited good chemical stability after the NPs fabrication. The chemical stability of Cur at a high temperature (75 °C) was also investigated (Fig. 5c). The result showed that free Cur and Cur NPs were all sensitive to high temperature, and 40, 55 and 70 % of Cur were remained respectively for PBS, NaCas and CPH after 30 min incubation. However, the retention ratios of Cur were all dropped down to 40 % after 2 h incubation.
Fig. 5.
The chemical stability of free Cur and Cur NPs at 4 °C under light resistant (a), ambient (b) and 75 °C (c) conditions
Bioaccessibility of Cur in vitro digestion
The bioaccessibility was calculated according to the amount of Cur transferred to the aqueous phase during the digestion. The sequential processes of in vitro gastric and intestinal digestion (absence and presence of proteases) for free Cur and Cur NPs are shown in Fig. 6. When the enzymes weren’t added during the digestion, for free Cur, only 5 % of Cur was transferred to the aqueous phase, indicating extremely low bioaccessibility. In contrast, the bioaccessible amount of the Cur NPs in CPH and NaCas reached to 77 and 84 % respectively. Obviously, Cur NPs showed a higher bioaccessibility compared with the free Cur. However, when the enzymes were added, the bioaccessible amount of free Cur increased considerably to 33 %. Additionally, the bioaccessibility of Cur NPs also significantly improved in the digestion with enzymes added. This observation should be attributed to the solubilization of bile extract and the enzymes. A similar result has been reported by Chen et al. (2015a, b), in which the bioaccessibility of free Cur was also improved with the presence of sodium cholate and the enzymes. Hence, the result indicated that the bioaccessible amount of Cur is highly depended on the present state of Cur, as well as the presence of the surfactants and enzymes. Interestingly, although the presence of enzymes increased the bioaccessible amount of the CPH stabilized Cur NPs from 77.3 to 81.9 %, the NaCas stabilized Cur NPs decreased from 84.6 to 68.5 %. This observation could be explained by the degradation of NaCas during the digestion. The presence of the enzymes resulted in the destruction of protein structure, and finally the release, aggregation and precipitation of the bonded Cur. Compared with NaCas, CPH was more tolerant to pepsin and pancreatin, suggesting higher stability of the CPH stabilized NPs in the digestion.
Fig. 6.
Water solubility of Cur in vitro digestion. Different characters (a–e) on the top of columns represent significant difference among the samples (P < 0.05, n = 3)
Antioxidant activity of Cur NPs
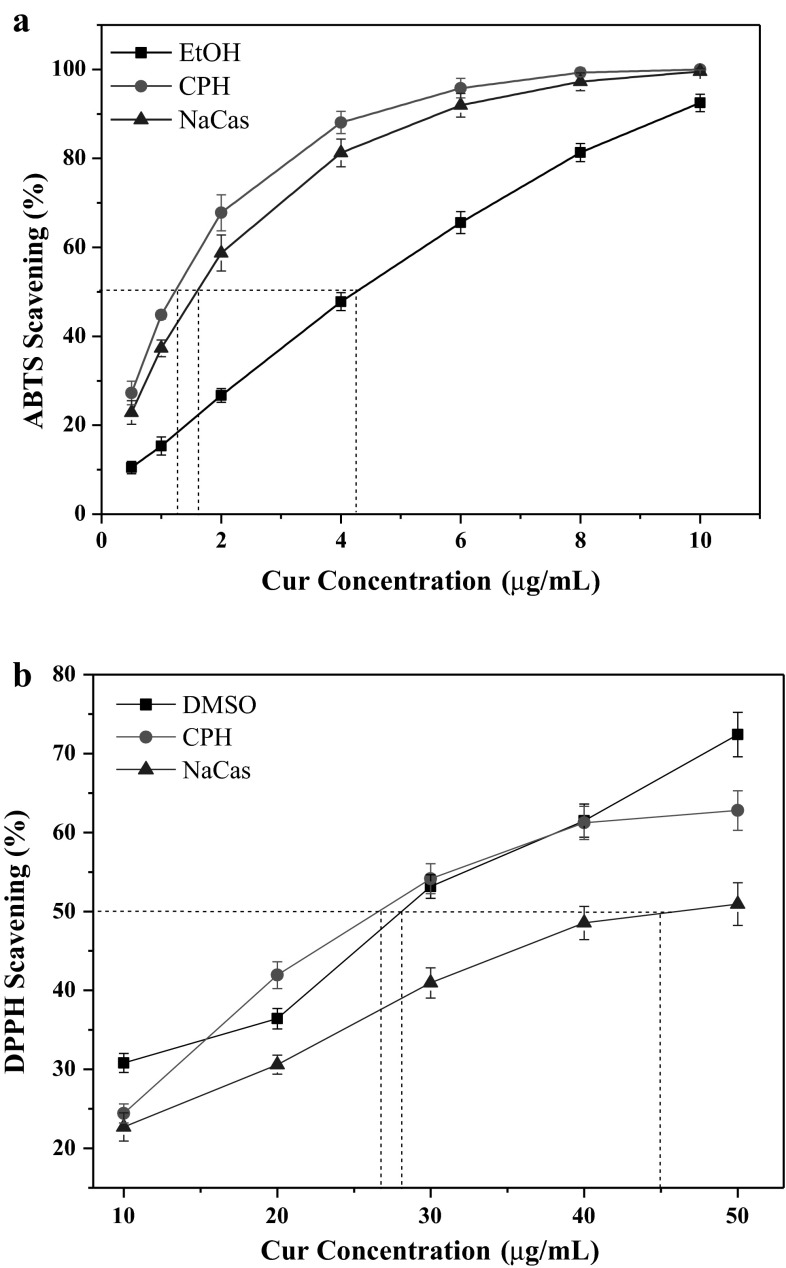
The ABTS scavenging activity of free Cur in EtOH and Cur NPs stabilized by CPH and NaCas are shown in Fig. 7a. For free Cur, the ABTS scavenging activity was highly concentration dependent, and it was in accordance with the first order kinetic model. However, for Cur NPs, the ABTS scavenging activity was stronger than free Cur under the same conditions, and the curves were quite different from that of free Cur. With the concentration increase of the Cur NPs, the ABTS scavenging activity was quickly increased, and the plateaus could be acquired for CPH and NaCas. When the ABTS scavenging activity reaches 50 % (SC50), the needed concentration for free Cur was 4.2 μg/mL, and that for Cur NPs in CPH and NaCas was 1.2 and 1.5 μg/mL respectively. This observation implied that Cur NPs have a higher ABTS scavenging activity compared with free Cur. Figure 7b shows the DPPH scavenging activity of free Cur in DMSO and Cur NPs in CPH and NaCas. With Cur concentration increasing, the DPPH scavenging ratios all increased. Compared with NaCas, CPH stabilized Cur NPs exhibited higher DPPH scavenging activity under the same conditions. The SC50 was 26 and 45 μg/mL respectively for CPH and NaCas and 28 μg/mL for free Cur. This suggested that the DPPH scavenging activity of Cur NPs in CPH was stronger than that in NaCas, and was comparable with that of free Cur in DMSO. Similar results on the improved antioxidant activity of Cur after its complexation with colloidal particles such as β-casein, lecithins and PVP also have been reported (Pan et al. 2013; Takahashi et al. 2009; Yen et al. 2010). These results may be attributed to the homogeneous distribution of NPs in the reaction medium, and the large surface area resulted from the nanometer-sized structures could facilitate the reactions of Cur with free radicals. Additionally, it should be noted that the caseinate, especially the CPH also exhibited strong radical scavenging activity (Kitts 2005; Wang et al. 2014a, b; Zhou et al. 2015; Tang et al. 2010). Hence, the antioxidant activity of CPH also contributed to the present result.
Fig. 7.
ABTS (a) and DPPH (b) scavenging activity of free Cur in PBS and Cur NPs in CPH and NaCas
Conclusions
The water solubility of Cur could be considerably improved by NPs fabrication, which should be attributed to its complexation with CPH or NaCas. The fabricated Cur NPs all showed a good colloidal stability, but the Cur NPs in CPH exhibited smaller size compared with that in NaCas. The obtained Cur NPs powders all showed good rehydration properties and chemical stability. Cur NPs exhibited higher chemical stability compared with free Cur, and CPH could protect Cur from degradation more efficiently. Furthermore, comparing with NaCas, CPH may have higher tolerance to pepsin and pancreatin, which increased the bioaccessibility of Cur NPs. Additionally, Cur NPs exhibited prominent antioxidant activity in ABTS scavenging, and CPH stabilized Cur NPs showed a better DPPH scavenging activity. This study suggested that CPH may be a better material in Cur NPs fabrication, and it opens up the possibility to construct protein hydrolysate-based NPs delivery system.
Acknowledgments
This research was supported by grants from the Chinese National Natural Science Foundation (Serial Numbers: 31130042 and 31371744), the Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201303071), and the Science and Technology Program of Guangdong Province (No. 2013B020311007).
References
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Chen L, Subirade M. Alginate–whey protein granular microspheres as oral delivery vehicles for bioactive compounds. Biomaterials. 2006;27(26):4646–4654. doi: 10.1016/j.biomaterials.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Chen FP, Li BS, Tang CH. Nanocomplexation between curcumin and soy protein isolate: influence on curcumin stability/bioaccessibility and in vitro protein digestibility. J Agric Food Chem. 2015;63(13):3559–3569. doi: 10.1021/acs.jafc.5b00448. [DOI] [PubMed] [Google Scholar]
- Chen FP, Li BS, Tang CH. Nanocomplexation of soy protein isolate with curcumin: influence of ultrasonic treatment. Food Res Int. 2015;75:157–165. doi: 10.1016/j.foodres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Foegeding EA, Davis JP. Food protein functionality: a comprehensive approach. Food Hydrocolloids. 2011;25(8):1853–1864. doi: 10.1016/j.foodhyd.2011.05.008. [DOI] [Google Scholar]
- Israeli-Lev G, Livney YD. Self-assembly of hydrophobin and its co-assembly with hydrophobic nutraceuticals in aqueous solutions: towards application as delivery systems. Food Hydrocolloids. 2014;35:28–35. doi: 10.1016/j.foodhyd.2013.07.026. [DOI] [Google Scholar]
- Joye IJ, Davidov-Pardo G, Ludescher RD, Mcclements DJ. Fluorescence quenching study of resveratrol binding to zein and gliadin: towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015;185:261–267. doi: 10.1016/j.foodchem.2015.03.128. [DOI] [PubMed] [Google Scholar]
- Kakran M, Sahoo N, Li L. Dissolution enhancement of quercetin through nanofabrication, complexation, and solid dispersion. Colloid Surf B. 2011;88(1):121–130. doi: 10.1016/j.colsurfb.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555(2):311–316. doi: 10.1016/S0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- Kitts D. Antioxidant properties of casein-phosphopeptides. Trends Food Sci Technol. 2005;16(12):549–554. doi: 10.1016/j.tifs.2005.08.009. [DOI] [Google Scholar]
- Kovačević AB, Müller RH, Savić SD, Vuleta GM, Keck CM. Solid lipid nanoparticles (SLN) stabilized with polyhydroxy surfactants: preparation, characterization and physical stability investigation. Colloid Surf A. 2014;444(4):15–25. [Google Scholar]
- Lam RSH, Nickerson MT. Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem. 2013;141(2):975–984. doi: 10.1016/j.foodchem.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Leung MH, Kee TW. Effective stabilization of curcumin by association to plasma proteins: human serum albumin and fibrinogen. Langmuir. 2009;25(10):5773–5777. doi: 10.1021/la804215v. [DOI] [PubMed] [Google Scholar]
- Li L, Tajmir-Riahi HA, Subirade M. Interaction of β-lactoglobulin with resveratrol and its biological implications. Biomacromolecules. 2007;9(1):50–56. doi: 10.1021/bm700728k. [DOI] [PubMed] [Google Scholar]
- Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A. 2008;86(2):300–310. doi: 10.1002/jbm.a.31584. [DOI] [PubMed] [Google Scholar]
- Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- Pan K, Zhong Q, Baek SJ. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J Agric Food Chem. 2013;61(25):6036–6043. doi: 10.1021/jf400752a. [DOI] [PubMed] [Google Scholar]
- Patel AR, Velikov KP. Colloidal delivery systems in foods: a general comparison with oral drug delivery. LWT Food Sci Technol. 2011;44(9):1958–1964. doi: 10.1016/j.lwt.2011.04.005. [DOI] [Google Scholar]
- Patel A, Hu Y, Tiwari JK, Velikov KP. Synthesis and characterisation of zein-curcumin colloidal particles. Soft Matter. 2010;6(24):6192–6199. doi: 10.1039/c0sm00800a. [DOI] [Google Scholar]
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Semo E, Kesselman E, Danino D, Livney YD. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocolloids. 2007;21:936–942. doi: 10.1016/j.foodhyd.2006.09.006. [DOI] [Google Scholar]
- Sneharani AH, Karakkat JV, Singh SA, Rao AA. Interaction of curcumin with β-lactoglobulin-stability, spectroscopic analysis, and molecular modeling of the complex. J Agric Food Chem. 2010;58(20):11130–11139. doi: 10.1021/jf102826q. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57(19):9141–9146. doi: 10.1021/jf9013923. [DOI] [PubMed] [Google Scholar]
- Tang X, He Z, Dai Y, Xiong YL, Xie M, Chen J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J Agric Food Chem. 2010;58(1):587–593. doi: 10.1021/jf9028656. [DOI] [PubMed] [Google Scholar]
- Wan ZL, Guo J, Yang XQ. Plant protein-based delivery systems for bioactive ingredients in foods. Food Funct. 2015;6(9):2876–2889. doi: 10.1039/C5FO00050E. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Zheng XQ, Kopparapu NK, Cong WS, Deng YP, et al. Purification and evaluation of a novel antioxidant peptide from corn protein hydrolysate. Process Biochem. 2014;49(9):1562–1569. doi: 10.1016/j.procbio.2014.05.014. [DOI] [Google Scholar]
- Wang X, Ai T, Meng XL, Zhou J, Mao XY. In vitro iron absorption of α-lactalbumin hydrolysate-iron and β-lactoglobulin hydrolysate–iron complexes. J Dairy Sci. 2014;97(5):2559–2566. doi: 10.3168/jds.2013-7461. [DOI] [PubMed] [Google Scholar]
- Wang YH, Wang JM, Yang XQ, Guo J, Lin Y. Amphiphilic zein hydrolysate as a novel nano-delivery vehicle for curcumin. Food Funct. 2015;6(8):2636–2645. doi: 10.1039/C5FO00422E. [DOI] [PubMed] [Google Scholar]
- Yen FL, Wu TH, Tzeng CW. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J Agric Food Chem. 2010;58(12):7376–7382. doi: 10.1021/jf100135h. [DOI] [PubMed] [Google Scholar]
- Zhou C, Hu J, Ma H, Yagoub AEA, Yu X, Owusu J, Ma H, Qin X. Antioxidant peptides from corn gluten meal: orthogonal design evaluation. Food Chem. 2015;187:270–278. doi: 10.1016/j.foodchem.2015.04.092. [DOI] [PubMed] [Google Scholar]
- Zimet P, Rosenberg D, Livney YD. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocolloids. 2011;25(5):1270–1276. doi: 10.1016/j.foodhyd.2010.11.025. [DOI] [Google Scholar]