Abstract
The impact of spice powders on physical, mechanical, thermal and barrier properties, and on storage stability, of whey protein isolate (WPI)-based films was determined. Films with added spices were prepared from casting solution containing 10 % (w/w) heat-denatured WPI, glycerol (WPI:glycerol of 3:2 w/w), sodium chloride (0.4 g/100 g solution), garlic and pepper powders (≤3 g each/100 g solution). Water activity (aw) of all films was 0.53–0.57. Addition of spice powders increased thickness, darkness and yellowness of the WPI films. Films with added spices had lower tensile strength (TS), percent elongation (%E), and melting enthalpy of WPI matrices, but possessed higher water vapor permeability (WVP) than WPI film without sodium chloride and spices. The WPI film containing highest amount of garlic powder and lowest amount of pepper powder was selected for storage tests at 25–45 °C. Storage for up to 49 days resulted in reduced aw and %E, increased TS, and color changes at 35 and 45 °C, with few changes at 25 °C. However, film WVP and OP were not affected by storage conditions after 7 days storage. Active ingredients decreased over time with up to 81 % allicin and 37 % piperine retained in the film matrix after 47 days storage.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2259-z) contains supplementary material, which is available to authorized users.
Keywords: Edible film, Whey protein isolate, Spice powder, Storage stability
Introduction
Research studies on edible packaging materials have been conducted to serve consumer demands for safe, convenient and/or healthy food products with prolonged shelf life as well as sustainability awareness. For instance, edible films have been improved to efficiently protect food products via their selective barrier properties, provide additional advantages including controlled release of active ingredients, and increase in mechanical integrity of foods with minute environmental impact (Janjarasskul and Krochta 2010). Among biopolymers used to fabricate edible films, whey protein has gained more interest since it provides the film with distinct properties. Edible film from whey protein isolate (WPI) is transparent, heat-sealable, and possesses good oxygen barrier as well as tensile properties at low to medium relative humidity (RH) conditions (McHugh and Krochta 1994). Moreover, it is tasteless and odorless, which can be applied as a carrier for food additives. Previous studies reported that WPI film could efficiently serve as a carrier for antioxidants and antimicrobial agents (Campos et al. 2011; Han and Krochta 2007; Murillo-Martínez et al. 2013).
Spices are not only important food ingredients that impart subtle flavors, but also good sources of active compounds with antioxidant and/or antimicrobial activities. Incorporation of spices into edible films has generally been performed by adding their essential oils into the film casting solution. By this way, high concentration of active compounds can be added, yielding the films with acceptable antioxidant and/or antimicrobial activities (Campos et al. 2011; Sánchez-González et al. 2011). However, the resulting films are likely to provide strong aroma of the essential oils. When applying to specific food product, the film containing essential oil can negatively influence organoleptic properties, and eventually consumer acceptance, of that food product. Moreover, high dose of the active compounds in essential oil-incorporated edible films may induce health-related problems regarding their toxicity and/or allergenicity (Sánchez-González et al. 2011). The facile volatility of essential oils renders themselves to promptly act as antimicrobial agents and/or antioxidants. However, the essential oil incorporated edible films should be readily used upon casting process to prevent exhaustion of their finite bioactive capacity. These critical disadvantages could hinder the commercial applications of these essential oil based bioactive films. Alternatively, addition of spice powder into the film casting solution can alleviate those problems. There have been very few studies in this area. Kechichian et al. (2010) prepared the starch-based edible film containing spice powders, including cinnamon powder, clove powder and red pepper powder, as natural antimicrobial ingredients. The spice powders were added up to 0.3 g/100 g of film casting solution. It was found that spice powders altered mechanical and barrier properties of the edible films. The films containing spice powders tended to have lower tensile strength (TS) and percent elongation (%E) but possess higher water vapor permeability (WVP). However, none of those films provided satisfactory results on extending shelf life of white bread.
More research is required to help develop edible film incorporated with spice powders. The developed film may not only be used for shelf life extension but also utilized for culinary purposes. The spice film can be applied as a convenient delivering system of pre-measured spices for instant marination or a serving portion (e.g. edible wrapping) to enhance food flavor and taste as well as offer product novelty and variety. In this study, garlic (Allium sativum) and pepper (Piper nigrum L.) powders, versatile seasoning for everyday meals, were selected as representatives of spice powders. WPI film was selected as an edible film base. The effect of the incorporated spice powders on physical, mechanical, thermal and barrier properties of the WPI film was investigated. Changes in the film properties and concentration of the active ingredients in both spices during storage under different temperatures were also monitored. This data set could be used to determine storage stability of the developed film.
Materials and methods
Materials and chemicals
Whey protein isolate BiPRO® (97.0 g protein/100 g) was obtained from Davisco Foods International, Inc. (Minnesota, USA). Garlic (Allium sativum) and white pepper (Piper nigrum L.) powders were supplied from Artchit International Pepper and Spice Co., Ltd. (Thailand). Iodized salt (NaCl) was supplied from Thai Refined Salt Co., Ltd. (Thailand). Glycerol (99.5 % purity) and magnesium nitrate were purchased from QRëC Chemical Co., Ltd. (Thailand). 4-mercaptopyridine (4-MP) and piperine standard were purchased from Sigma-Aldrich (Missouri, USA). Sodium phosphate, Ethylenediaminetetraacetic acid (EDTA) and chloroform were supplied from Ajax Finechem (Australia), QRëC (New Zealand), and RCI Labscan (Thailand), respectively.
Film preparation and storage
Film casting solution containing WPI (10 %, w/w), glycerol (WPI:glycerol of 3:2 w/w), salt and spice powders was prepared by dissolving WPI in distilled water and heating the solution at 90°C for 30 min in a water bath to induce partial denaturation of whey protein. The solution was cooled down to room temperature before adding glycerol and mixed well. Spice powders were then added and stirred for 2 min using magnetic stirrer, then salt was added and dissolved under constant stirring (~15–30 s). Amounts of salt and spice powders added into 100 g of the WPI-glycerol solution was varied as indicated in Table 1. Film casting solution containing well-suspended spice powders was quickly poured onto 15 cm × 15 cm acrylic plates (20 mL/plate). Cast films were dried in a tray dryer at 50 °C for 15 h. The films were conditioned in a hygrostat containing saturated solution of magnesium nitrate at 25 °C for at least 48 h prior to determination of film properties. Equilibrium RH provided by the saturated solution at 25 °C was approximately 53 % (Greenspan 1977).
Table 1.
Amount of salt and spice powders added into 100 g of solution containing WPI and glycerol used for edible film preparation
| Formulation | Pepper powder (g) | Garlic powder (g) | Salt (g) | Total (g) |
|---|---|---|---|---|
| G1 | 0.6 | 1.0 | 0.4 | 2.0 |
| G2 | 0.6 | 2.0 | 0.4 | 3.0 |
| G3 | 0.6 | 3.0 | 0.4 | 4.0 |
| P1 | 1.0 | 0.6 | 0.4 | 2.0 |
| P2 | 2.0 | 0.6 | 0.4 | 3.0 |
| P3 | 3.0 | 0.6 | 0.4 | 4.0 |
For the storage test, the film samples were stored in hygrostats under controlled RH using magnesium nitrate solution as previously indicated. The hygrostats were placed in incubators at 25, 35 and 45 °C, attaining equilibrium RH of approximately 53, 50 and 47 %, respectively (Greenspan 1977), for up to 49 days.
Determination of the film morphology
Surface morphology of the film samples was investigated by stereomicroscope (SMZ-1000, Nikon Corp., Japan) equipped with 10× eyepiece lens, Plan Apo 1× WD-70 objective lens and digital camera (DXM1200F, Nikon Corp., Japan). Intermediate magnification was set as 1×. Images were captured via Nikon ACT-1 software, version 2.6.3.0 (Nikon Corp., Japan).
Determination of film properties
Physical properties
Film thickness was measured by a digital micrometer (Model ID-C112, Mitutoyo MFG Co. Ltd., Japan). Color of the films, in CIELAB system, was measured by a Chroma meter (Model CR-400, Minolta Co., Ltd., Japan). The total color difference (ΔE) was calculated using Eq. 1:
| 1 |
For ΔE calculation, the control film () was the WPI film without salt and spice powders in order to evaluate effects of the additives (salt and spice powders), whereas the film at 0 day storage was the control for determining storage stability of the films.
Mechanical properties
Tensile strength, TS and elongation, %E were determined by Instron universal testing machine (Model 5565, Instron, Massachusetts, USA) using the standard method, ASTM D882 (ASTM 1997). The test films were cut into 1 cm × 12 cm strips. The initial distance between the grips was 50 mm. The cross-head speed was 5.0 mm/s and the load cell was 5 kg.
Barrier properties
Water vapor permeability, WVP was determined according to ASTM E96 (ASTM 1989). Films were mounted on test cups containing 6 mL of water. The cups were placed in cabinets, with forced air circulation, under controlled RH (0 %) using silica gel. Oxygen permeability (OP) was evaluated using a standard method ASTM D3985-05 (ASTM 2002) at 23 °C and 0 % RH using an OX-TRAN® 2/21 ST modular system (MOCON Inc., Minnesota, USA). Film samples were covered with an aluminum foil mask, having a 5 cm2 open testing area.
Thermal properties
Thermal transitions were determined by differential scanning calorimeter (DSC) (Diamond, PerkinElmer Inc., Massachusetts, USA) equipped with Intracooler 2P (PerkinElmer Inc.) and nitrogen gas purge. The film sample (3–9 mg each) was hermetically sealed in a large volume stainless steel pan (60 μL), and heated from 30 to 200 °C, 10 °C/min. An empty stainless steel pan was used as a reference. Onset temperature (To), peak temperature (Tp) and enthalpy (∆H) of the endotherms were determined via Pyris™ version 11 software (PerkinElmer Inc.).
Chemical properties
Water activity (aw) of the films was measured by water activity meter (AquaLab Series 3, Decagon Devices Inc., Washington, USA). Allicin, an important flavor precursor and a major bioactive compound in garlic, was determined according to the method of Miron et al. (2002) and Rahman et al. (2009), with slight modification. Film sample was dried in a desiccator at room temperature (≥48 h) before grinding. The 0.7 g of the ground film was mixed with 5 mL of distilled water and vortexed for 2 min. The mixture was incubated in the dark at room temperature for 10 min before centrifugation at 2400g for 20 min. The supernatant was separated, filtered through 0.45 μm polyamide membrane filter (Sartorius AG, Germany), and used for spectroscopic analysis. To prepare the sample, 200 μL of the extract were mixed with 2.8 mL of 0.12 mM 4-MP solution in pH 7.2 buffer (50 mM sodium phosphate and 2 mM EDTA) and incubated in the dark at room temperature for 30 min. Reagent blank was prepared by mixing 200 μL of distilled water with 2.8 mL of the 4-MP solution. Sample blank consisted of 200 μL of the extract and 2.8 mL of pH 7.2 buffer. Absorbance of the sample, reagent blank and sample blank was measured at 324 nm. Allicin concentration was calculated using Eq. 2:
| 2 |
where C was the allicin concentration (M), ε was molar extinction coefficient of 39,600 M−1 cm−1, b was optical path length (cm), ∆A was calculated from Eq. 3:
| 3 |
where ARB, ASA and ASB represented absorbance of the reagent blank, sample and sample blank, respectively.
Piperine, the alkaloid contributing to the pungency of black pepper, was determined by the modified method of Fagen et al. (1955) and Nisha et al. (2009). The ground film sample (0.7 g) was mixed with chloroform and final volume was adjusted to 25 mL in a volumetric flask. The mixture was incubated in the dark at room temperature for 1 h, and filtered through Whatman #1 filter paper. Absorbance of the supernatant was measured at 345 nm. Piperine concentration was calculated from the standard curve (0–5 ppm piperine standard solutions).
Statistical analysis
All experiments were done in at least triplicate. Data were analyzed by one-way analysis of variance. Differences among means were reported at 95 % confidence level using Duncan’s new multiple range test. SPSS Statistics 17.0 (IBM Corp., New York, USA) was employed for the statistical analysis.
Results and discussion
Film formation and morphology
Preliminary studies indicated that addition of salt and spice powders not only enhanced organoleptic properties of the whey protein film but also increased viscosity of the casting solution. After mixing spice powders and salts with the WPI solution, the casting solution should immediately be poured onto the casting plates to avoid time-dependent viscosity increase and possibly gelation. These could cause difficulty in evenly spreading the solution on the casting plate.
Addition of salt to heat-denatured whey protein solution could induce thickening and gelation of the system at ambient temperature. Heat denaturation of whey protein solution at 70–90 °C, 5–60 min, pH ~7, induced the formation of filamentous type structure of the protein aggregates without gelation. After cooling down and adding salt into the solution, electrostatic repulsion between the filamentous-type protein aggregates was shielded, resulting in enhancing protein–protein interactions. Viscosity increase and gelation could be obtained due to increasing number and thickness of the contact points between the filaments (Bryant and McClements 1998). Extent of such changes depends on concentration of salt and protein in the solution. McClements and Keogh (1995) reported the preparation of cold-setting gel by adding 0.2 mol/kg (1.2 % w/w) NaCl into 10 % WPI solution, pH 7, that was heat-denatured at 90 °C, 30 min. In this study, NaCl concentration (0.4 % w/w, Table 1) was much lower than that required for the cold setting of the film casting solution. Note that the main purpose of adding salt was to enhance the flavor and taste of the spiced film, which could extend its application as seasoning carrier in processed foods and cuisine. NaCl concentration was fixed for all film formulas (Table 1). Viscosity of the film casting solution containing both salt and spice powders was much higher than that with salt but did not have spice powders (Supplementary Table 1). Time-dependent increase in viscosity was also found. After leaving for 5 min at ambient temperature, the solution with greater amount of spice powders was more thickened. This might be due to the hydrophilic components in spice powders, including starch and fiber (Pradeep et al. 1993; USDA 2015) which might aid in viscosity increase.
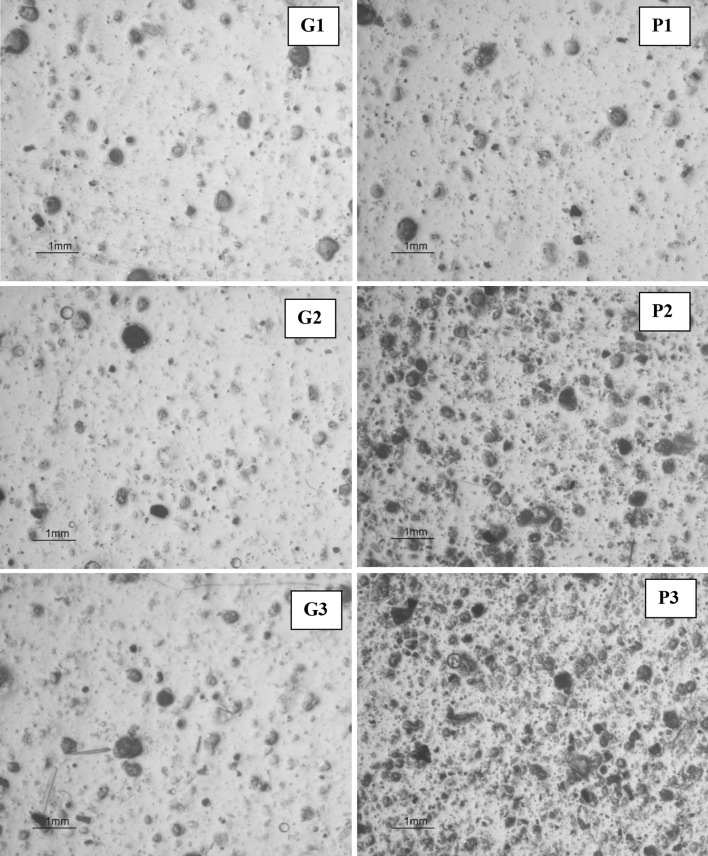
Note that films from P2 and P3 formulas also had distinct appearance. As shown in Fig. 1, formula P2 and P3 contained greater amount of small air bubbles, together with suspended spice particles; whereas morphology of the films from the other formulas was nearly similar with less suspended particles and air bubbles. The difference in morphology could influence the film properties reported in the following section.
Fig. 1.
Morphology of the whey protein films incorporated with different amount of spice powders. Similar magnification was applied for every images
Effects of spice powders on film properties
The aw of all spiced films ranged from 0.53 to 0.57 (data not shown), which were lower than minimum aw required for microbial growth (0.6) (Labuza et al. 1970). Hence, microbial spoilage will not occur if the spiced films are kept under low to moderate humidity environments (e.g. ≤50 % RH). Addition of spice powders greatly affected physical, mechanical and barrier properties of the WPI films (Table 2). The spiced films had higher thickness and b*, with lower L*, compared to the controls (C1 and C2) (p ≤ 0.05). Addition of spice powders enhanced darkness and yellowness to WPI film. At the similar level of spice powder addition (e.g. G1 and P1), pepper powder provided greater impact on film thickness and color parameters than garlic powder. The color difference might be related to the original color of the spice powders, with garlic powder being lighter. Besides, white pepper powder also contained some black particles, which could further decrease L* of the films (Fig. 1). Moreover, ∆E of all spiced films was higher than 6, indicating that the color difference between each formulation of spiced film and the control film could be visibly differentiated (Garcia and Sobral 2005).
Table 2.
Physical, mechanical and barrier properties of the whey protein films with different amount of spice powdersa,b
| Formulation | Thickness (mm) | L* | a* | b* | ΔEe | Tensile strength (MPa) | Elongation (%) | Water vapor permeability (g mm/kPa h m2) |
|---|---|---|---|---|---|---|---|---|
| C1c | 0.094a ± 0.002 | 91.42e ± 0.53 | −1.51bc ± 0.01 | 5.12b ± 0.11 | – | 2.60d ± 0.26 | 74.97b ± 25.23 | 3.32a ± 0.23 |
| C2d | 0.146b ± 0.004 | 96.08f ± 0.36 | −0.15d ± 0.04 | 3.82a ± 0.14 | – | 1.25ab ± 0.30 | 101.31c ± 30.65 | 5.01b ± 0.78 |
| G1 | 0.182c ± 0.010 | 88.41d ± 0.11 | −1.57b ± 0.07 | 11.00c ± 0.45 | 6.62 | 1.49abc ± 0.04 | 33.31a ± 4.55 | 7.62c ± 0.12 |
| G2 | 0.184c ± 0.008 | 88.01d ± 0.52 | −1.46c ± 0.05 | 12.85e ± 0.84 | 8.46 | 1.53bc ± 0.15 | 15.28a ± 1.23 | 7.07c ± 0.15 |
| G3 | 0.201d ± 0.008 | 86.89c ± 0.77 | −1.70a ± 0.07 | 14.52f ± 0.47 | 10.44 | 1.47abc ± 0.06 | 9.70a ± 1.04 | 7.20c ± 0.30 |
| P1 | 0.209d ± 0.008 | 87.23c ± 0.28 | −1.49bc ± 0.06 | 12.07d ± 0.50 | 8.12 | 1.59c ± 0.19 | 25.26a ± 2.59 | 8.60d ± 0.14 |
| P2 | 0.246e ± 0.010 | 80.62b ± 0.25 | −0.15d ± 0.04 | 16.73g ± 0.33 | 15.93 | 1.30abc ± 0.05 | 22.08a ± 2.39 | 8.84d ± 0.13 |
| P3 | 0.278f ± 0.002 | 76.33a ± 0.29 | 1.17e ± 0.01 | 20.44h ± 0.18 | 21.67 | 1.20a ± 0.07 | 14.34a ± 2.23 | 10.14e ± 0.72 |
aValues are the averages ± standard deviations
bDifferent superscripts (a, b, c, …) indicate significant differences within the same column (p ≤ 0.05)
cC1 was WPI film without salt and spice powders
dC2 was WPI film with salt but did not contain spice powders
eΔE was calculated based on the color parameters of the C1 film
Spice powder incorporation negatively influenced mechanical and barrier properties of the WPI film (Table 2). TS of the spiced films were lower than those of the C1 film (p ≤ 0.05). Addition of spice powders also decreased %E, in comparison with the controls (C1 and C2) (p ≤ 0.05). The results indicated that whey protein film containing spice powders had lower strength and flexibility. Greater extent of those changes was evidenced in the film added with higher concentration of the spice powders. Moreover, incorporation of spice powders increased WVP of the films (p ≤ 0.05), with pepper powder generating a larger effect. These results were in an agreement with Kechichian et al. (2010), who reported that cassava starch film incorporated with natural antimicrobial ingredients, including cinnamon powder, clove powder and red pepper powder, tended to have lower TS and %E and higher WVP. The effects also depended on type and concentration of those additives. Changes in mechanical and barrier properties of the spiced films could be related to their modified microstructure. Soluble and insoluble biopolymers from spiced powders, together with small air bubbles (Fig. 1), might interrupt intermolecular bonding within the whey protein film matrix, rendering inferior mechanical properties. The resulting heterogeneous structure and loosened matrix of spiced films with smaller degree of cohesive forces might facilitate mass transfer of water molecules. Moreover, protein and carbohydrate were reported as major components in garlic powder and white pepper powder (USDA 2015). Those hydrophilic biopolymers could increase the solubility coefficient, and eventually enhanced WVP of the spiced films.
Thermal properties of the spiced films were shown in Table 3. For each film sample, an endothermic transition with To and Tp fell within the range of 155–157 and 176–180 °C, respectively. An endotherm with To around 146–156 °C, detected from WPI-based films, was presumed to represent melting of the whey protein matrix and indicate heat sealability of the films (Hernandez-Izquierdo 2007; Hernandez-Izquierdo et al. 2008; Janjarasskul et al. 2011; Leuangsukrerk et al. 2014). Addition of spice powders did not influence To and Tp of the endotherm (p > 0.05), but decreased ΔH, compared to the control (p ≤ 0.05). The latter result could support the hypothesis regarding modified microstructure of the spiced films, as previously discussed. As biopolymers from spice powders inhibited protein–protein interactions in the film matrix, whey protein networks were weakened, requiring less thermal energy (ΔH) to disrupt the networks. The concentration-dependent effect on ΔH reduction can be noted among P1–P3 formulas.
Table 3.
Thermal properties of the whey protein films with different ratios of spice powdersa,b
| Formulation | Tnso (°C) | Tnsp (°C) | ΔH (J/g film, wet basis) |
|---|---|---|---|
| Controlc | 155.67 ± 2.04 | 175.56 ± 1.48 | 19.94d ± 2.38 |
| G1 | 156.85 ± 0.04 | 178.86 ± 0.53 | 13.69bc ± 0.55 |
| G2 | 156.94 ± 0.22 | 179.74 ± 2.31 | 15.68c ± 1.54 |
| G3 | 155.36 ± 0.24 | 178.13 ± 1.15 | 13.61bc ± 1.64 |
| P1 | 156.20 ± 0.70 | 177.63 ± 3.27 | 13.51bc ± 0.74 |
| P2 | 156.41 ± 0.43 | 177.10 ± 2.76 | 11.65b ± 0.95 |
| P3 | 156.52 ± 0.59 | 177.64 ± 3.16 | 8.71a ± 1.63 |
aValues are the averages ± standard deviations
bDifferent superscripts (a, b, c, …) indicate significant differences within the same column (p ≤ 0.05)
cControl was WPI film without salt and spice powders
nsNo significant differences within the same column (p > 0.05)
For the storage stability study, formulations with highest concentration of spice powders, G3 and P3, were considered as representatives. In this way, changes in the active ingredients, allicin and piperine, during storage could be better detected. However, the P3 film had greater extent of structural inhomogeneity, resulting in lower TS and higher WVP than G3 film. Therefore, only the G3 film was selected for the storage study.
Changes in properties of spiced film during storage
Storage time and temperature affected aw of the spiced films (Fig. 2). Loss of free water still occurred during storage under controlled RH. The changes of aw at 25 °C was relatively smaller than those at 35 and 45 °C. Dehydration of the spiced film (≥24 days) could be attributed to the loss of plasticizer, resulting in decreasing water-holding capacity of the films. The moisture loss and migration of plasticizer out of biopolymer films have been reported (Anker et al. 2001; Hernández-Muñoz et al. 2004). Plasticizers are low molecular weight molecules that help improve film flexibility by reducing cohesive forces in polymer films. They do not chemically react, but weakly interact with polymers to decrease intermolecular forces and chain-to-chain interactions. Small plasticizer molecules, e.g. glycerol, were reported to have high plasticizer efficiency but possess low permanence due to the high diffusion rate in polymers and high volatility (Sothornvit and Krochta 2005). The migration rate of glycerol and water attraction out of the polymer matrix was accelerated by storage temperature.
Fig. 2.

Water activity and total color difference (ΔE) of the G3 formula spiced films stored at 25–45 °C. Error bar represents a standard deviation
Stability of the film color during storage was also monitored (Fig. 2). Slight changes in ∆E values of the samples stored at 25 °C indicated its color stability throughout prolonged storage. On the other hand, ΔE values of the films stored at 35 and 45 °C gradually increased over time. The discoloration of WPI-based films at elevated temperatures was reported to mainly associate with Maillard browning of whey proteins and traces of reducing sugars (Miller et al. 1997; Trezza and Krochta 2000). Nonetheless, ΔE values of all stored films were below 6, the threshold value detectable by the human eye (Garcia and Sobral 2005). This could indicate that films aged up to 49 days were not visibly different from freshly-prepared samples.
Effects of storage time and temperature on barrier properties of the spiced films were illustrated in Fig. 3. WVP fluctuated within a narrow range (2–4 g mm/kPa h m2) during storage at any given temperature. Anker et al. (2001) reported that WVP of glycerol-plasticized WPI films were relatively constant over aging for 120 days in a climate room (23 °C, 50 % RH) despite significant moisture loss. OP values of the spiced film decreased within the first week of storage and tended to remain unchanged afterwards. For prolonged storage (≥14 days), OP of the film samples kept at 35 and 45 °C (~2.0–2.8 cc mm/kPa day m2) was slightly lower than that of the samples stored at 25 °C (~3.3–4.0 cc mm/kPa day m2). OP of the WPI film without spice powders was 3.47 cc-mm/kPa-day-m2, which was close to that of the spiced film aged at 25 °C after 7 days.
Fig. 3.
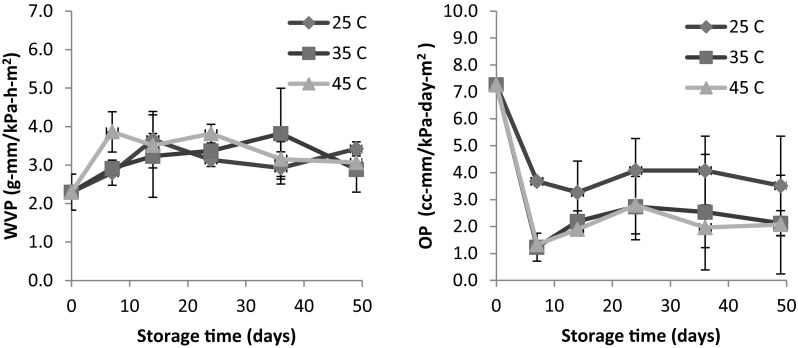
Water vapor permeability and oxygen permeability of the G3 formula spiced films stored at 25–45 °C. Error bar represents a standard deviation
Changes in mechanical properties of the spiced films were evidenced during storage (Fig. 4). Like OP values, TS increased within the first 7 days of storage, regardless of temperature. Thereafter, the value either stayed relatively constant (at 25 °C) or gently increased (at 35 and 45 °C). However, %E gradually decreased after 14 d of storage. Overall results showed that mechanical properties of all film samples were enhanced during extended storage period (>7 days). Improved mechanical properties of WPI films have been reported during aging (Anker et al. 2001; Dangaran and Krochta 2007) and heat curing (Amin and Ustunol 2007; Miller et al. 1997). The enhanced cohesive forces in polymer films induced by gradual molecular rearrangement were hypothesized to be responsible for the stronger and less extensible film. Miller et al. (1997) reported that curing at higher temperature and lower RH accelerated TS increase and %E decrease by eliciting additional covalent cross-linking between protein chains. The higher tensile properties during storage may also be related to the loss of plasticizer and evaporation of water.
Fig. 4.
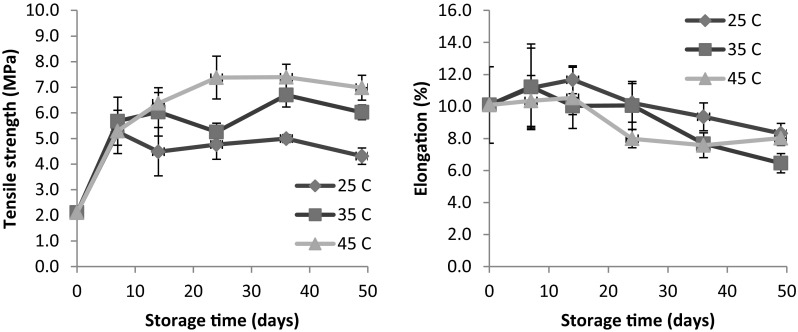
Mechanical properties of the G3 formula spiced films stored at 25–45°C. Error bar represents a standard deviation
Allicin content in film G3 (Table 4) decreased over time (p ≤ 0.05), due to its high volatility (Borlinghaus et al. 2014). Storage temperature did not greatly influence degradation profile of allicin during aging. WPI-based films were reported to be good aroma barrier (Miller et al. 1998) and oil barrier (Janjarasskul and Krochta 2010). However, the transport phenomena were different and faster when volatile compounds were already incorporated in the film matrix. In this case, those compounds were ready to migrate to both sides of the surface, instead of permeating through film matrix from one to another side driven by concentration gradient. Nonetheless, the stability study showed that WPI matrix could effectively retard the loss of allicin. By the end of 47-day-storage, 64–81 % of allicin in WPI film was maintained at 25–45 °C. Rahman et al. (2009) reported up to 29.5 % loss of allicin from slices of garlic cloves during drying at 50 °C for only 7 h. Piperine content decreased over time (p ≤ 0.05), owing to its sensitivity to light, temperature and oxygen. This active constituent of black pepper oleoresin can undergo hydrolysis to piperidine and piperinic acid and also photolysis to iso-chavicine, resulting in the loss of typical pepper flavor and medicinal properties (Shaikh et al. 2006). Storage at higher temperature tended to induce greater rate and extent of piperine degradation. Approximately 20–37 % of piperine was retained in the film matrix at 47 days of storage at 25–45 °C. The decreasing of both allicin and piperine content gradually plateaued over time, similar to the profile of OP. As previously shown in Fig. 3, the WVPs stabilized and OPs decreased significantly after 1 week of storage. This finding indicated that the improved oxygen barrier of the film matrix during prolonged storage could decrease the degradation rate of allicin and piperine content.
Table 4.
Allicin and piperine content in the G3 formulaa film stored under different conditionsb,c
| Storage time | Allicin content (μg/g film) | Piperine content (μg/g film) | ||||
|---|---|---|---|---|---|---|
| (days) | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C |
| 0 | 0.75d ± 0.07 | 0.75c ± 0.07 | 0.75c ± 0.07 | 32.87d ± 4.58 | 32.87c ± 4.58 | 32.87d ± 4.58 |
| 7 | 0.61c ± 0.05 | 0.63c ± 0.12 | 0.79c ± 0.10 | 21.51bc ± 1.23 | 21.89b ± 3.16 | 14.29bc ± 1.70 |
| 14 | 0.56bc ± 0.10 | 0.44b ± 0.00 | 0.46a ± 0.08 | 25.87c ± 3.88 | 17.16ab ± 3.09 | 15.61c ± 1.57 |
| 26 | 0.58bc ± 0.05 | 0.23a ± 0.03 | 0.42a ± 0.07 | 19.16b ± 0.85 | 15.08a ± 2.00 | 10.70b ± 0.92 |
| 35 | 0.29a ± 0.04 | 0.41b ± 0.06 | 0.36a ± 0.02 | 16.68ab ± 1.18 | 15.76a ± 1.00 | 11.42b ± 1.00 |
| 47 | 0.48b ± 0.02 | 0.48b ± 0.08 | 0.61b ± 0.10 | 12.12a ± 1.24 | 11.93a ± 1.46 | 6.72a ± 0.36 |
aG3 formula contained 3 g of garlic powder, 0.6 g of pepper powder, 0.4 g of salt per 100 g of casting solution
bValues are the averages ± standard deviations
cDifferent superscripts (a, b, c, …) indicate significant differences within the same column (p ≤ 0.05)
nsNo significant differences within the same column (p > 0.05)
Conclusions
Incorporation of spice powders into WPI films influenced film properties. Spiced films had inferior mechanical properties and WVP compared to the control films. This could be related to their heterogeneous structure; soluble and insoluble spice biopolymers and small air bubbles that could hinder protein–protein interactions. However, a model spiced film showed reasonable storage stability at 25–45 °C. Mechanical properties were gradually improved and barrier properties were relatively unchanged during prolonged storage (14–49 days). Up to 81 % allicin and 37 % piperine was retained in the spiced film at the end of storage test. Therefore, addition of spice powders into edible film matrices could be a promising approach to enhance functionality and application of edible films in the food industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was funded by the Ratchadapisek Sompoch Endowment Fund (2014), Chulalongkorn University (CU-57-009-FW) and the senior project research fund, Faculty of Science, Chulalongkorn University. Authors are grateful to Prof. Dr. Juan L. Silva for proofreading and English language editing.
References
- Amin S, Ustunol Z. Solubility and mechanical properties of heat-cured whey protein-based edible films compared with that of collagen and natural castings. Int J Dairy Technol. 2007;60:149–153. doi: 10.1111/j.1471-0307.2007.00305.x. [DOI] [Google Scholar]
- Anker M, Standing M, Hermansson A-M. Aging of whey protein films and the effect on mechanical and barrier properties. J Agric Food Chem. 2001;49:989–995. doi: 10.1021/jf000730q. [DOI] [PubMed] [Google Scholar]
- ASTM . Annual book of American standard testing methods. Philadelphia: American Society for Testing and Material; 1989. Standard test methods for water-vapor transmission of materials (E96-E80) pp. 730–739. [Google Scholar]
- ASTM . Annual book of American standard testing methods. Philadelphia: American Society for Testing and Material; 1997. Standard test methods for tensile properties of thin plastic sheeting (D882-95) pp. 162–170. [Google Scholar]
- ASTM . Annual book of American society for testing methods. Philadelphia: American Society for Testing and Material; 2002. Standard test methods for oxygen gas transmission rate through plastic film and sheeting using a coulometric sensor (D3985-95) pp. 472–477. [Google Scholar]
- Borlinghaus J, Albrecht F, Gruhlke MCH, Nwachukwu ID, Slusarenko AJ. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CM, McClements DJ. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci Technol. 1998;9:143–151. doi: 10.1016/S0924-2244(98)00031-4. [DOI] [Google Scholar]
- Campos CA, Gerschenson LN, Flores SK. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011;4:849–875. doi: 10.1007/s11947-010-0434-1. [DOI] [Google Scholar]
- Dangaran KL, Krochta JM. Preventing the loss of tensile, barrier and appearance properties caused by plasticizer crystallization in whey protein films. Int J Food Sci Technol. 2007;42:1094–1100. doi: 10.1111/j.1365-2621.2006.01355.x. [DOI] [Google Scholar]
- Fagen HJ, Kolen EP, Hussong RV. Spice analysis, spectrophotometric method for determining piperine in oleoresins of black pepper. J Agric Food Chem. 1955;3:860–862. doi: 10.1021/jf60056a009. [DOI] [Google Scholar]
- Garcia FT, Sobral PJA. Effect of the thermal treatment of the filmogenic solution on the mechanical properties, color and opacity of films based on muscle proteins of two varieties of Tilapia. J Food Sci Technol. 2005;38:289–296. [Google Scholar]
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stand A Phys Chem. 1977;81A:89–96. doi: 10.6028/jres.081A.011. [DOI] [Google Scholar]
- Han JH, Krochta JM. Physical properties of whey protein coating solutions and films containing antioxidants. J Food Sci. 2007;72:E308–E314. doi: 10.1111/j.1750-3841.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Izquierdo VM (2007) Thermal transitions, extrusion, and heat-sealing of whey protein edible films [dissertation]. University of California
- Hernandez-Izquierdo VM, Reid DS, McHugh TH, Berrios JDJ, Krochta JM. Thermal transitions and extrusion of glycerol-plasticized whey protein mixtures. J Food Sci. 2008;73:E169–E175. doi: 10.1111/j.1750-3841.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Muñoz P, López-Rubio A, del-Valle V, Almenar E, Gavara R. Mechanical and water barrier properties of glutenin films influenced by storage time. J Agric Food Chem. 2004;52:79–83. doi: 10.1021/jf034763s. [DOI] [PubMed] [Google Scholar]
- Janjarasskul T, Krochta JM. Edible packaging materials. Annu Rev Food Sci Technol. 2010;1:415–488. doi: 10.1146/annurev.food.080708.100836. [DOI] [PubMed] [Google Scholar]
- Janjarasskul T, Tananuwong K, Krochta JM. Whey protein film with oxygen scavenging function by incorporation of ascorbic acid. J Food Sci. 2011;76:E561–E568. doi: 10.1111/j.1750-3841.2011.02409.x. [DOI] [PubMed] [Google Scholar]
- Kechichian V, Ditchfield C, Veiga-Santos P, Tadini CC. Natural antimicrobial ingredients incorporated in biodegradable films based on cassava starch. LWT Food Sci Technol. 2010;43:1088–1094. doi: 10.1016/j.lwt.2010.02.014. [DOI] [Google Scholar]
- Labuza TP, Tannenbaum SR, Karel M. Water content and stability of low-moisture and intermediate-moisture foods. Food Technol. 1970;24:543–550. [Google Scholar]
- Leuangsukrerk M, Phupoksakul T, Tananuwong K, Borompichaichartkul C, Janjarasskul T. Properties of konjac glucomannan–whey protein isolate blend films. LWT Food Sci Technol. 2014;59:94–100. doi: 10.1016/j.lwt.2014.05.029. [DOI] [Google Scholar]
- McClements DJ, Keogh MK. Physical properties of cold-setting gels formed from heat-denatured whey protein isolate. J Sci Food Agric. 1995;69:7–14. doi: 10.1002/jsfa.2740690103. [DOI] [Google Scholar]
- McHugh TH, Krochta JM. Sorbitol- vs glycerol-plasticized whey protein edible films: integrated oxygen permeability and tensile property evaluation. J Agric Food Chem. 1994;42:841–845. doi: 10.1021/jf00040a001. [DOI] [Google Scholar]
- Miller KS, Chiang MT, Krochta JM. Heat curing of whey protein films. J Food Sci. 1997;62:1189–1193. doi: 10.1111/j.1365-2621.1997.tb12241.x. [DOI] [Google Scholar]
- Miller KS, Upadhyaya SK, Krochta JM. Permeability of d-Limonene in whey protein films. J Food Sci. 1998;63:244–247. doi: 10.1111/j.1365-2621.1998.tb15718.x. [DOI] [Google Scholar]
- Miron T, Shin I, Feigenblat G, Weiner L, Mirelman D, Wilchek M, Rabinkov A. A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: reaction of 4-mercaptopyridine with thiosulfinates. Anal Biochem. 2002;307:76–83. doi: 10.1016/S0003-2697(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Murillo-Martínez MM, Tello-Solís SR, García-Sánchez MA, Ponce-Alquicira E. Antimicrobial activity and hydrophobicity of edible whey protein isolate films formulated with nisin and/or glucose oxidase. J Food Sci. 2013;78:M560–M566. doi: 10.1111/1750-3841.12078. [DOI] [PubMed] [Google Scholar]
- Nisha P, Singhal RS, Pandit AB. The degradation kinetics of flavor in black pepper (Piper nigrum L.) J Food Eng. 2009;92:44–49. doi: 10.1016/j.jfoodeng.2008.10.018. [DOI] [Google Scholar]
- Pradeep KU, Geervani P, Eggum BO. Common Indian spices: nutrient composition, consumption and contribution to dietary value. Plant Food Hum Nutr. 1993;44:137–148. doi: 10.1007/BF01088378. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Al-Shamsi QH, Bengtsson GB, Sablani SS, Al-Alawi A. Drying kinetics and allicin potential in garlic slices during different methods of drying. Dry Technol. 2009;27:467–477. doi: 10.1080/07373930802683781. [DOI] [Google Scholar]
- Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M. Use of essential oils in bioactive edible coatings: a review. Food Eng Rev. 2011;3:1–16. doi: 10.1007/s12393-010-9031-3. [DOI] [Google Scholar]
- Shaikh J, Bhosale R, Singhal R. Microencapsulation of black pepper oleoresin. Food Chem. 2006;94:105–110. doi: 10.1016/j.foodchem.2004.10.056. [DOI] [Google Scholar]
- Sothornvit R, Krochta JM. Plasticizers in edible films and coatings. In: Han JH, editor. Innovations in food packaging. London: Academic Press; 2005. pp. 403–433. [Google Scholar]
- Trezza T, Krochta JM. Color stability of edible coatings during prolonged storage. J Food Sci. 2000;65:1166–1169. doi: 10.1111/j.1365-2621.2000.tb10259.x. [DOI] [Google Scholar]
- USDA (2015) National nutrient database for standard reference release 27. http://ndb.nal.usda.gov/ndb/search. Accessed 22 Apr 2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.