Abstract
This study evaluated the effect of popping and fermentation on the chemical composition of three types of Amaranthus caudatus grains cultivated in Ethiopia. Proximate composition, minerals and mineral absorption inhibitors were analyzed. Popping caused a decrease in protein content by 4 % and an increase in fat, ash, acid detergent fiber (ADF) and neutral detergent fiber (NDF) contents by 12, 10, 15 and 67 %, respectively. While fermentation increased protein, fat and ash content by 3, 22 and 14 %, respectively but did not significantly change ADF and NDF content. Fe, Ca and phytic acid (IP6) decreased during popping but Mg, Zn, galloyl and catechol did not change significantly. On the other hand, fermentation increased Fe and Mg content but decreased IP6, galloyl and catechol content. The decrease in mineral absorption inhibitors especially IP6 during popping and fermentation could contribute to enhance mineral bioavailability. However, due to the presence of high phytate content in raw amaranth, all IP6-to-mineral molar ratios were above the recommended values.
Keywords: Amaranthus caudatus, Popping, Fermentation, Proximate composition, Minerals, Phytic acid, Iron binding polyphenols
Introduction
Amaranth is an ancient plant belonging to the family of Amaranthaceae which is believed to have originated from Central and South America (Gamel et al. 2006). It is a pseudocereal with high grain yield and capable of withstanding extreme climate and soil conditions (Capriles et al. 2008). Amaranth has good nutritional attributes with high level of protein, minerals, and fat as compared to the commonly utilized cereals such as barley, wheat, maize, sorghum (Mustafa et al. 2011; Pedersen et al. 1987). The high level of protein also presents a unique functional attributes such as high foaming and water absorption property of amaranth flour (Shevkani et al. 2014). Moreover, it has excellent amino acid profile with high amount of lysine and sulfur containing amino acids which are lacking in cereals and legumes, respectively (Amare et al. 2015). Amaranth oil is rich in unsaturated fatty acids and also contains a significant amount of squalene which can potentially prevent skin cancer and decrease serum cholesterol level (He et al. 2002).
The seeds of amaranth can be subjected to several treatments such as popping, toasting, cooking, roasting, flaking, extruding or grinding to be consumed as suspensions with water or milk or to be mixed with other cereal flours for bread and pasta making with improved quality (Capriles et al. 2008). However, its nutritive value is limited by the presence of high level of mineral absorption inhibitors especially phytates with a significant variations ranging between 0.52 and 2.24 g/100 g among different species studied so far (Lorenz and Wright 1984; Pedersen et al. 1987). According to Coulibaly et al. (2011) and Feil and Fossati (1997), growing conditions such as season, soil profile, harvesting techniques, stage of maturation, species and genotype are the main possible factors for the existing variations in phytate contents of crops. In line with this, some researchers also documented levels of other potential mineral absorption inhibitors such as tannins (Lorenz and Wright 1984; Mustafa et al. 2011) in different species of amaranth growing in different countries.
In Ethiopia amaranth can grow nearly all over the country yet the crop is underutilized in many parts of the country. In the Southern Nations Nationalities and Peoples (SNNP) region, especially in Bench Majji Zone, a particular ethnic group called Me’enit cultivates the crop and utilize it in different forms. The seed could be popped and milled to prepare thin porridge called “Atmit”, thick porridge called “Genfo” or mixed with cold water to prepare an instant drink called “Besso” or fermented to prepare a drink called “Shamita/Borde”. The raw seeds could also be milled, mixed with other cereal flours like maize and sorghum which then fermented for 3–4 and 48–72 h to prepare “Kita”, unleavened bread and “Injera”, a traditional pancake, respectively.
Although amaranth has a multiple food uses, limited information on its nutritive value and mineral absorption inhibitors of the crop grown under the agro ecological conditions of Ethiopia is available. Therefore, the aim of the present study was to determine the proximate composition, minerals and absorption inhibitors of three different types of Amaranthus caudatus grains cultivated in Ethiopia and evaluate the effect of popping and fermentation.
Materials and methods
Sample collection
Three different types of A. caudatus grains, white, red and brown in color, were purchased from six farmers living in Chat Kebelle, Bench Majji Zone, Southern Nations, Nationalities and Peoples region, Ethiopia in October 2011. Composite samples of each amaranth types prepared from the grains of the six origins were sorted, cleaned and washed to remove immature seeds, sand and soil. The washed seeds were sun dried and stored at 4 °C.
Sample preparation
Preparation of raw amaranth flour
The composite samples for each type of amaranth grain were milled, sieved using 0.425 mm sieve and stored at 4 °C until further analysis.
Popping
Cleaned and sun dried amaranth grains were popped as described in Amare et al. (2015). Briefly, the sun dried seeds were placed in a hot clay pan for 10–15 s until popping. The popped grains were then milled to pass through a 0.425 mm sieve and stored in polyethylene bags at 4 °C for further analysis.
Fermentation
Natural fermentation was carried out according to the method described by Ibrahim et al. (2005) with modification. Briefly, 250 g of amaranth flour was mixed with 500 mL distilled water in a 600 mL beaker and then left to ferment for 48 h at room temperature (22 ± 2 °C). Thereafter, the sample was mixed with a glass rod and transferred to three aluminum dishes (30 cm diameter each) and dried in a hot oven (Heraeus UT 5042, Germany) at 50 °C for 20 h. The dried sample was then ground to pass a 0.425 mm screen and stored in polyethylene bags at 4 °C for further analysis.
Proximate composition analysis
Crude protein content was determined according AOAC standard method 979.09 (AOAC 2000), where nitrogen content was determined by the Kjeldahl method and a conversion factor of 5.85 was considered as suggested by Berghofer and Schoenlechner (2002). The amount of crude fat was determined by the Soxhlet extraction according to AOAC standard method 920.39 (AOAC 2000). The ash content was determined after the removal of organic matter by dry ashing according to AOAC standard method 923.03 (AOAC 2000).
Neutral detergent fiber and Acid detergent fiber
Neutral detergent fibre (NDF) content, which corresponds approximately to cellulose, hemicellulose and lignin content, and acid detergent fibre (ADF) content, which corresponds approximately to cellulose and lignin content, were determined according to the gravimetric method of Van Soest (1963) using a Fibertec 1020 (Foss, Hillerod, Denmark).
Determination of available carbohydrates
Available carbohydrate was determined by difference and the result was expressed as g/100 g DM.
Mineral analysis
The analysis of minerals was made by flame atomic absorption spectrophotometry (AAnalyst 800, Perkin Elmer, France) following the method described in Hama et al. (2011) with modification. Briefly, about 0.4 g of sample flour was mixed with a mixture of 7 mL of concentrated nitric acid and 3 mL hydrogen peroxide and left to equilibrate for 30 min followed by digestion using microwave digester (Ethos-1, Milestone, Italy).
Mineral absorption inhibitors
Determination of phytic acid
Myo-inositol hexaphosphate (IP6) content was determined by high-performance anion-exchange chromatography (Dionex, Sunnyvale, USA), after acid extraction, according to the method described in Hama et al. (2011). Briefly, 100 mg of the dried sample was extracted by using 5 mL of 0.5 M HCl in a boiling water bath for 6 min. The mixture was cooled in an ice bath and centrifuged at 4500 g for 20 min at 4 °C. The supernatant was filtered using 0.2 µm membrane filter before drying in a Speedvac (JOUAN, Saint Herblain, France). After drying, the sample was reconstituted with 2 mL of MilliQ water and injected into a high-performance anion-exchange chromatography using an AS-11 precolumn and column kit (Dionex, Sunnyvale, USA) for analysis with conductimetric detector. The HPLC conditions were: mobile phase 200 mM NaOH and HPLC grade water with gradient elution (15–40 % of 200 mM NaOH for 8 min, 40 % 200 mM NaOH for 1 min and 15 % 200 mM NaOH for 10 min), flow rate: 1 mL/min, column temperature: 30 °C, injection volume: 50 µL. Standard phytic acid solutions were also prepared from sodium phytate (Sigma) to develop a calibration curve. Results were expressed in gram per 100 g DM.
Determination of iron binding polyphenols
Iron-binding polyphenols (galloyl and catechol groups) were analyzed using the method of Brune et al. (1991). Briefly, to 50 mg of sample, 5 mL of 50 % dimethylformamide (Carlo Erba) in 0.1 N acetate buffer (pH 4.4) was added and the mixture was incubated for 16 h at 28 °C in a shaking water bath at 160 rpm. The solution was filtered using Whatman GF/A filter paper. From the supernatant, 0.5 mL was taken and mixed with 2 mL FAS (ferric ammonium sulfate) reagent containing 50 % urea (Merck) in 0.1 M acetate buffer (pH 4.4), 1 % gum arabic (sigma) and 5 % ferric ammonium sulfate (Riedel–de-Haen) in 1 M HCl. The mixture was incubated for 15 min at 30 °C and the absorbance of the colored complexes was measured at 680 and 578 nm, corresponding to the absorption maxima of Fe-galloyl and Fe-catechol complexes, respectively against a reagent blank consisting of 0.5 mL 50 % DMF and 2 mL of FAS-reagent. A food blank was also made by mixing 0.5 mL of 50 % DMF and 2 mL of sample extract and the absorbance was subtracted. The concentration of galloyl groups (expressed as tannic acid equivalents) and catechol groups (expressed as catechin equivalents) were calculated from standard curves for catechin (sigma) and tannic acid (Merck) at the two wavelengths. All analysis were done in triplicate.
Estimation of mineral bioavailability
Molar ratios
Molar ratios of phytic acid to minerals are the most frequently used method to estimate mineral bioavailability. The desirable molar ratios suggested for optimal absorption for adult diets are less than 1.0 or preferably less than 0.4 for phytic acid:iron (Hurrell 2004), less than 0.17 for phytic acid:calcium (Umeta et al. 2005), and less than 15 for phytic acid:zinc (Gibson 2006).
Quality assurance and quality control
In order to validate the accuracy of the analytical methods for protein, fat, ash and minerals three certified reference materials from the Institute for Reference Materials and Measurements (EU-IRMM), namely BCR-381 (rye flour), BCR-191 (brown bread) and BCR-679 (white cabbage) were used. The measurements were conducted based on the above procedure and the data was compared with the certified reference values (Table 1).
Table 1.
Quality assurance results for nutrient analysis of amaranth grain
| Parameter | Units | Method of analysis | Reference material | Certified value | Analyzed values | Recovery (%) |
|---|---|---|---|---|---|---|
| Proximate | ||||||
| Protein | g/100 g | Kjeldahl | BCR 381 (rye flour) | 1.56 ± 0.01 | 1.48 ± 0.06 | 95 |
| Fat | Soxhlet extraction | 1.36 ± 0.16 | 1.25 ± 0.05 | 92 | ||
| Ash | Ignition on muffle furnace | 1.08 ± 0.11 | 1.09 ± 0.04 | 101 | ||
| Mineral | ||||||
| Iron | mg/100 g | Microwave digestion and flame AAS determination | BCR 191 (brown bread) | 4.07 ± 0.23 | 3.90 ± 0.38 | 96 |
| Zinc | 1.95 ± 0.05 | 1.99 ± 0.04 | 102 | |||
| Calcium | 41.00 | 39.70 ± 0.82 | 97 | |||
| Iron | BCR 679 (white cabbage) | 7.97 ± 0.27 | 7.55 ± 0.18 | 95 | ||
| Zinc | 5.5 ± 0.25 | 5.72 ± 0.01 | 104 | |||
| Calcium | 776.8 ± 65.5 | 747.8 ± 1.2 | 96 | |||
Statistical analysis
Data were analyzed using two way analysis of variance (ANOVA) to determine significant differences among the processing methods using Statgraphics plus 5.1 (Statpoint, Warrenton, USA) software. Duncan multiple range test was used to compare the means.
Results and discussion
Proximate composition of raw amaranth grains
Table 2 shows the proximate composition of three types of A. caudatus grain. The protein and fat content of raw amaranth falls in the range of 14.0–15.5 and 7.5–7.7 g/100 g DM, respectively. The results were in agreement with previous reports by Kaur et al. (2010) and He et al. (2002). Fat content in amaranth grain was higher as compared to most conventional cereals such as wheat, maize and teff (Forsido et al. 2013). The available carbohydrate content of brown amaranth was lower than that of white and red colored amaranth grains (Table 2). This is associated with the high level of dietary fiber in brown amaranth as all the three types of amaranth contain comparable quantities of other nutrients.
Table 2.
Proximate composition of three types of raw A. caudatus grains
| Grain type | DM (g/100 g DM) | Protein (g/100 g DM) | Fat (g/100 g DM) | Available carbohydrate (g/100 g DM) | Ash (g/100 g DM) | ADF (g/100 g DM) | NDF (g/100 g DM) |
|---|---|---|---|---|---|---|---|
| White amaranth | 89.23 ± 0.10 | 13.88 ± 0.16 | 7.64 ± 0.05 | 68.41 ± 0.35 | 2.99 ± 0.08 | 5.49 ± 0.14 | 7.08 ± 0.36 |
| Red amaranth | 89.14 ± 0.09 | 15.15 ± 0.08 | 7.54 ± 0.06 | 67.15 ± 0.35 | 2.57 ± 0.02 | 5.99 ± 0.16 | 7.59 ± 0.41 |
| Brown amaranth | 88.88 ± 0.57 | 15.53 ± 0.24 | 7.72 ± 0.03 | 59.50 ± 1.98 | 3.10 ± 0.16 | 14.15 ± 1.89 | 14.79 ± 0.14 |
Values are mean of triplicates ±SD
DM dry matter, ADF acid detergent fiber, NDF neutral detergent fiber
The amount of ash in the three types of amaranth whole grains was in the range between 2.6 and 3.1 g/100 g DM, the maximum being for brown colored amaranth and the minimum for red colored amaranth. This was consistent with reportes by Kaur et al. (2010). The content of acid detergent fiber (ADF), which corresponds to cellulose and lignin content, was the highest for brown amaranth (14.2 g/100 DM) followed by red (6.0 g/100 g DM) and white (5.5 g/100 g DM) (Table 2). Neutral detergent fiber (NDF) content was the highest in brown amaranth than other two types (Table 2). Pedersen et al. (1987) also demonstrated that colored amaranth have high amount of dietary fiber. The results of NDF in raw amaranth grains were also in agreement with the report by Mustafa et al. (2011) and higher than the amount of insoluble dietary fiber present in Amaranthus hypochondriacus reported by Czerwinski et al. (2004). The difference between ADF and NDF was comparable in white and red amaranth but lower in brown amaranth. This indicates that brown amaranth had lower amount of hemicellulose, which is included during NDF determination, than the other types.
Effect of popping and fermentation on proximate composition
The effect of popping and fermentation on the content of protein, fat, available carbohydrate (CHO), ash and dietary fiber (ADF and NDF) of three types of amaranth is shown in Table 3. Popping resulted in a significant decrease (p < 0.05) in protein content from 14.9 to 14.3 g/100 g DM. The decrease in protein content might be attributed to the partial oxidation of heat labile amino acids. On the other hand, fermentation brought a significant increment in protein content. This is attributed to the loss of carbohydrates during fermentation (Svanberg and Lorri 1997). As the fermentation was spontaneous, i.e. without adding yeast or bacteria, no huge difference in protein was expected (Kazanas and Fields 1981). Fat content was significantly increased (p < 0.05) from 7.6 to 8.5 and 9.2 g/100 g DM during popping and fermentation, respectively. The increase in fat content during fermentation could be due to the activation of yeast strains which could produce fat (Khetarpaul and Chauhan 1989). While the improvement observed after popping could be due to the partial removal of pericarp that was low in fat.
Table 3.
Effect of processing on composition of the three types of A. caudatus grains
| Treatment | Protein content (g/100 g DM) | Fat content (g/100 g DM) | Available carbohydrate (g/100 g DM) | Ash content (g/100 g DM) | ADF content (g/100 g DM) | NDF content (g/100 g DM) |
|---|---|---|---|---|---|---|
| Raw | 14.9 ± 0.8b | 7.6 ± 0.1c | 65.0 ± 4.3a | 2.9 ± 0.3b | 8.8 ± 4.5b | 9.6 ± 3.6b |
| Popped | 14.3 ± 0.5c | 8.5 ± 0.6b | 57.9 ± 5.5c | 3.2 ± 0.4a | 10.1 ± 5.6a | 16.2 ± 4.7a |
| Fermented | 15.3 ± 0.9a | 9.2 ± 0.2a | 63.0 ± 4.8b | 3.3 ± 0.6a | 8.6 ± 4.6b | 9.3 ± 3.5b |
Values are means of nine measurements ±SD and means followed by different letters in the same column are significantly different at p < 0.05
DM dry matter, ADF acid detergent fiber, NDF neutral detergent fiber
The ash content significantly (p < 0.05) increased during popping and fermentation by 10 and 14 %, respectively. This may be associated with loss of dry matter allowing the minerals to concentrate (Svanberg and Lorri 1997). ADF and NDF content increased significantly after popping from 8.8 to 10.1 and 9.6 to 16.2 g/100 g DM (p < 0.05), respectively. Ramulu and Rao (1997) also observed increased levels of insoluble dietary fiber during heat treatment of cereals. But the effect of fermentation on both ADF and NDF was not noticeable.
Mineral contents of raw amaranth grains
Total iron, calcium and magnesium content was the highest in brown colored amaranth (Table 4). Zinc content in the three types of amaranth was lower than that reported by Mustafa et al. (2011) and Nascimento et al. (2014). Fe content in raw grains showed variation ranging between 12.29 and 21.22 mg/100 g DM and the result was in agreement with the report by Mustafa et al. (2011) but higher than the report by Nascimento et al. (2014). Ca content also exhibited huge variation among the three types of amaranth (102–215 mg/100 g DM in raw samples). Brown amaranth showed the highest mineral contents, particularly Fe and Ca which were nearly twice of that measured in white and red amaranth. The result was not far from the reports by Pedersen et al. (1987) and Mustafa et al. (2011). Mg content ranged from 292 to 341 mg/100 g DM for raw grains. This was comparable with that reported by Mustafa et al. (2011) but higher than that reported by Nascimento et al. (2014).
Table 4.
Mineral content of three types of raw A. caudatus grains
| Grain type | Fe (mg/100 g DM) | Zn (mg/100 g DM) | Ca (mg/100 g DM) | Mg (mg/100 g DM) |
|---|---|---|---|---|
| White amaranth | 13.06 ± 1.79 | 3.40 ± 0.42 | 122 ± 2 | 292 ± 33 |
| Red amaranth | 12.29 ± 0.51 | 2.73 ± 0.08 | 102 ± 5 | 301 ± 10 |
| Brown amaranth | 21.15 ± 1.4 | 3.43 ± 0.21 | 215 ± 2 | 341 ± 8 |
Values are mean of triplicates ±SD
Effect of popping and fermentation on mineral contents
Popping significantly decreased (p < 0.05) iron and calcium content (31 and 8 %, respectively). This decrease in Fe content could be attributed to the loss of pericarp during popping as more than 66 % of total minerals were found in the bran and germ fractions in amaranth (Berghofer and Schoenlechner 2002). On the other hand, no significant effect (p < 0.05) was observed on Zn and Mg content due to popping (Table 5). It was concluded that the outer layer of amaranth grain contained high amount of Fe and Ca. On contrary, fermentation brought significant increase (p < 0.05) in Fe and Mg but no significant change was observed in Zn and Ca (Table 5).
Table 5.
Effect of processing on mineral content of three types of A. caudatus grains
| Treatment | Fe (mg/100 g DM) | Zn (mg/100 g DM) | Ca (mg/100 g DM) | Mg (mg/100 g DM) |
|---|---|---|---|---|
| Raw | 15.50 ± 4.4b | 3.19 ± 0.4a | 147 ± 52a | 311 ± 28b |
| Popped | 10.67 ± 0.78c | 3.24 ± 0.4a | 135 ± 46b | 327 ± 32ab |
| Fermented | 17.65 ± 4.9a | 3.34 ± 0.5a | 146 ± 51a | 330 ± 20a |
Values are mean of nine measurements ±SD and means followed by different letters in the same column are significantly different at p < 0.05
Mineral absorption inhibitors
Table 6 presents myo-inositol hexakisphosphate (IP6) content in raw amaranth grains. The amount was higher than that present in other cereals such as wheat, maize, buckwheat and rye (Egli et al. 2003). Such a high content of phytate probably strongly impairs the mineral bioavailability of amaranth based products and limits utilization of the crop to a wider consumer groups. In addition to phytate, polyphenols were also responsible for lowering mineral bioavailability (Brune et al. 1991; Khokhar and Owusu Apenten 2003). Therefore, polyphenols particularly iron binding polyphenols (galloyls and catechols) were measured in all raw amaranth grains and the result is shown in Table 6. Galloyl and catechol content was the highest for raw brown colored amaranth compared to raw red and raw white colored amaranth. Pigmented grains were known to have high amount of phenolic compounds (Manach et al. 2004) and this was also confirmed by the results of total polyphenol in the three differently colored amaranth in this study (data not shown).
Table 6.
Mineral absorption inhibitors in raw A. caudatus grains
| Grain type | IP6 (g/100 g DM) | Galloyl (mg TAE/100 g DM) | Catechol (mg CE/100 g DM) |
|---|---|---|---|
| White amaranth | 2.20 ± 0.15 | 93 ± 11 | ND |
| Red amaranth | 1.85 ± 0.25 | 100 ± 15 | 24 ± 16 |
| Brown amaranth | 2.09 ± 0.15 | 143 ± 10 | 54 ± 21 |
Values are mean of triplicates ±SD
ND not detected, IP6 myo-inositol hexakisphosphate
DM dry matter, TAE tannic acid equivalent, CE catechin equivalent
Substantial reduction in IP6 occurred during popping and fermentation by 39 and 77 %, respectively. This may be attributed to partial removal of pericarp during popping as phytates were known to be concentrated in the bran of most cereals (Hama et al. 2011). The impact of heat treatment (roasting and autoclaving) in reducing phytic acid content in chickpea cultivars has also been reported by Hussain et al. (1989). Repo-Carrasco-Valencia et al. (2009) also reported that extrusion cooking decreased the content of phytic acid in amaranth although not significant. The decrease during fermentation could be due to the activation of endogenous phytase or due to the action of exogenous phytase from fermentation microorganisms (yeast and lactic acid bacteria) when favorable pH was created (Baye et al. 2014).
Popping didn’t exhibit a significant effect on the content of both galloyl and catechol. On the other hand, fermentation significantly decreased galloyl and catechol content (Table 7). This was in agreement with the reports of Mouquet-Rivier et al. (2008). An increased activity of polyphenol oxidase might be the contributing factor to the polymerization of polyphenols making them less extractable.
Table 7.
Effect of processing on mean mineral absorption inhibitors of three types of A. caudatus grain
| Treatment | IP6 (g/100 g DM) | Galloyl (mg TAE/100 g DM) | Catechol (mg CE/100 g DM) |
|---|---|---|---|
| Raw | 2.05 ± 0.23c | 112.00 ± 25.66b | 26.11 ± 1.31b |
| Popped | 1.25 ± 0.26b | 104.56 ± 32.94b | 24.78 ± 2.01b |
| Fermented | 0.47 ± 0.31a | 4.78 ± 22.06a | 0.00 ± 0.00a |
Values are mean of nine measurements ±SD and means followed by different letters in the same column are significantly different at p < 0.05
IP6 myo-inositol hexakisphosphate
DM dry matter, TAE tannic acid equivalent, CE catechin equivalent
Estimation of mineral bioavailability using molar ratio
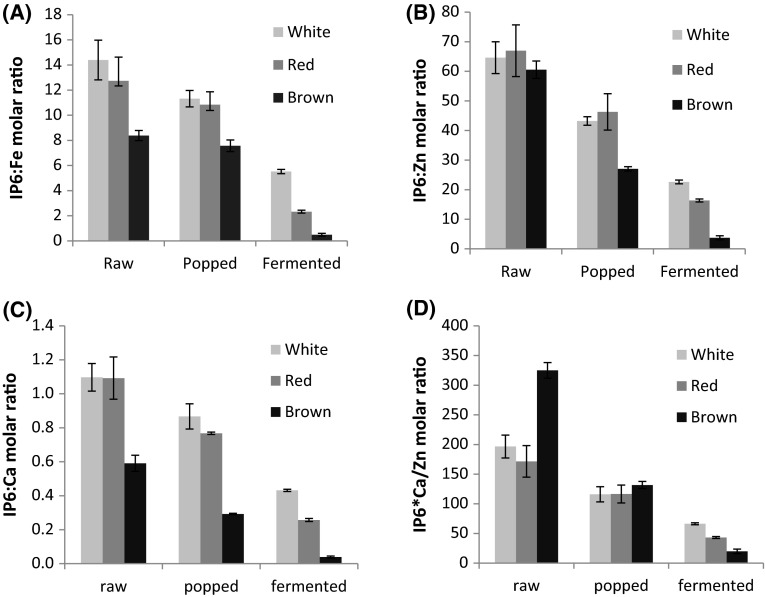
The molar ratios of IP6:Fe for the three types of amaranth under different processing is shown in Fig. 1a. The result showed that IP6:Fe molar ratio was the highest for white amaranth followed by red and brown amaranth. The result also showed that popping and fermentation brought a marked decrease in IP6:Fe molar ratio. However, only fermentation in brown amaranth enabled to achieve the recommendation (IP6:Fe < 1). The fermented samples of the two other types should be consumed with mineral absorption enhancers in which case higher IP6:Fe molar ratio could be entertained to achieve better mineral bioavailability (Hurrell 2004).
Fig. 1.
Effect of processing on molar ratios of IP6:Fe (a), IP6:Zn (b), IP6:Ca (c) and IP6 × Ca:Zn (d)
The effect of popping and fermentation significantly reduced the molar ratios of IP6:Zn in all the three amaranth (Fig. 1b). However, fermented brown amaranth met the cut off level (IP6:Zn < 15). It has been observed that high levels of Ca exacerbate the inhibitory effect of phytic acid on Zn absorption in humans by forming a Ca–Zn–phytic acid complex in the intestine, a more insoluble complex than phytate complexes formed in either ion alone (Fordyce et al. 1987). Thus the use of IP6 × Ca/Zn has been suggested as a better indicator of Zn bioavailability and if the value is above 200, the deleterious effect of Ca on Zn absorption is expected (Hemalatha et al. 2007). As seen in Fig. 1d, both popping and fermentation decreased the value of IP6 × Ca/Zn significantly and all, except the raw brown amaranth, had molar ratio above 200 due the relatively high amount of calcium in brown amaranth (Table 4). Similar effect was also observed on molar ratios of IP6:Ca where 21–51 and 61–93 % reduction was achieved during popping and fermentation, respectively. However, only fermented brown amaranth scored below the critical level (IP6:Ca < 0.17) (Fig. 1c).
In general, both processing methods (popping and fermentation) were effective in reducing the level of phytate to a greater extent. The effect of fermentation in reducing the content of phytic acid was far better than popping. However, due to the particularly high initial phytate content of amaranth, almost all IP6-to-mineral molar ratios were above the recommended values except for fermented brown amaranth. It is therefore necessary to design further strategies to maximize phytate degradation and improve mineral bioavailability.
Conclusion
All the three types of amaranth cultivated in Ethiopia have been found to be rich source of protein and fat compared to most commonly consumed cereals in developing countries. These are also a good source of iron, zinc and calcium making them a potential crop for complementing both cereals and legumes. However, all the three types contained high amount of mineral absorption inhibitors mainly phytate that could significantly be reduced after popping or fermentation. Despite the attempt done to decrease the level of mineral absorption inhibitors using popping and fermentation, the level was high enough to bind minerals and consequently decrease bioavailability. It is therefore necessary to investigate further optional processing methods to fully exploit the crop to its nutritional potential. Furthermore, investigating the in vitro and/or in vivo bioavailability of key nutrients (iron, zinc and calcium) in those grain amaranth types and their interaction with anti-nutritional factors such as phytate, polyphenols and oxalate are also required.
Acknowledgments
Authors are grateful to Addis Ababa University, SIDA SAREC Swedish Government and Institute de recherche pour le development (IRD), Montpellier, France for supporting the study.
References
- Amare E, Mouquet-Rivier C, Servent A, Morel G, Adish A, Haki GD. Protein quality of amaranth grains cultivated in Ethiopia as affected by popping and fermentation. Food Nutr Sci. 2015;6:38–48. doi: 10.4236/fns.2015.61005. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Baye K, Mouquet-Rivier C, Icard-Vernière C, Picq C, Guyot J-P. Changes in mineral absorption inhibitors consequent to fermentation of Ethiopian injera: implications for predicted iron bioavailability and bioaccessibility. Int J Food Sci Technol. 2014;49(1):174–180. doi: 10.1111/ijfs.12295. [DOI] [Google Scholar]
- Berghofer E, Schoenlechner R. Grain amaranth. In: Belton P, Taylor J, editors. Pseudocereals and less common cereals. Berlin: Springer; 2002. pp. 219–260. [Google Scholar]
- Brune M, Hallberg L, Skanberg A. Determination of iron binding phenolic groups in foods. J Food Sci. 1991;56(1):128–131. doi: 10.1111/j.1365-2621.1991.tb07992.x. [DOI] [Google Scholar]
- Capriles VD, Coelho KD, Guerra-Matias AC, Arêas JAG. Effects of processing methods on amaranth starch digestibility and predicted glycemic index. J Food Sci. 2008;73(7):H160–H164. doi: 10.1111/j.1750-3841.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Coulibaly A, Kouakou B, Chen J. Phytic acid in cereal grains: structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am J Plant Nutr Fertil Technol. 2011;1:1–22. doi: 10.3923/ajpnft.2011.1.22. [DOI] [Google Scholar]
- Czerwinski J, Bartnikowska E, Leontowicz H, Lange E, Leontowicz M, Katrich E, Trakhtenberg S, Gorinstein S. Oat (Avena sativa L.) and amaranth (Amaranthus hypochondriacus) meals positively affect plasma lipid profile in rats fed cholesterol-containing diets. J Nutr Biochem. 2004;15(10):622–629. doi: 10.1016/j.jnutbio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Egli I, Davidsson L, Juillerat M-A, Barclay D, Hurrell R. Phytic acid degradation in complementary foods using phytase naturally occurring in whole grain cereals. J Food Sci. 2003;68(5):1855–1859. doi: 10.1111/j.1365-2621.2003.tb12342.x. [DOI] [Google Scholar]
- Feil B, Fossati D. Phytic acid in triticale grains as affected by cultivar and environment. Crop Sci. 1997;37(3):916–921. doi: 10.2135/cropsci1997.0011183X003700030036x. [DOI] [Google Scholar]
- Fordyce EJ, Forbes RM, Robbins KR, Erdman JW. Phytate·calcium/zinc molar ratios: are they predictive of zinc bioavailability? J Food Sci. 1987;52:440–444. doi: 10.1111/j.1365-2621.1987.tb06634.x. [DOI] [Google Scholar]
- Forsido SF, Rupasinghe HV, Astatkie T. Antioxidant capacity, total phenolics and nutritional content in selected ethiopian staple food ingredients. Int J Food Sci Nutr. 2013;64(8):1–6. doi: 10.3109/09637486.2013.806448. [DOI] [PubMed] [Google Scholar]
- Gamel TH, Linssen JP, Mesallam AS, Damir AA, Shekib LA. Effect of seed treatments on the chemical composition of two amaranth species: oil, sugars, fibres, minerals and vitamins. J Sci Food Agr. 2006;86(1):82–89. doi: 10.1002/jsfa.2318. [DOI] [Google Scholar]
- Gibson RS. Zinc: the missing link in combating micronutrient malnutrition in developing countries. Proc Nutr Soc. 2006;65:51–60. doi: 10.1079/PNS2005474. [DOI] [PubMed] [Google Scholar]
- Hama F, Icard-Vernière C, Guyot J-P, Picq C, Diawara B, Mouquet-Rivier C. Changes in micro- and macronutrient composition of pearl millet and white sorghum during in field versus laboratory decortication. J Cereal Sci. 2011;54(3):425–433. doi: 10.1016/j.jcs.2011.08.007. [DOI] [Google Scholar]
- He H-P, Cai Y, Sun M, Corke H. Extraction and purification of squalene from Amaranthus grain. J Agr Food Chem. 2002;50(2):368–372. doi: 10.1021/jf010918p. [DOI] [PubMed] [Google Scholar]
- Hemalatha S, Platel K, Srinivasan K. Zinc and iron contents and their bioaccessibility in cereals and pulses consumed in India. Food Chem. 2007;102:1328–1336. doi: 10.1016/j.foodchem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74(6):445–452. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- Hussain B, Khan S, Ismail M, Sattar A. Effect of roasting and autoclaving on phytic acid content of chickpea. Food/Nahrung. 1989;33(4):345–348. doi: 10.1002/food.19890330416. [DOI] [PubMed] [Google Scholar]
- Ibrahim FS, Babiker EE, Yousif NE, El Tinay AH. Effect of fermentation on biochemical and sensory characteristics of sorghum flour supplemented with whey protein. Food Chem. 2005;92(2):285–292. doi: 10.1016/j.foodchem.2004.07.024. [DOI] [Google Scholar]
- Kaur S, Singh N, Rana JC. Amaranthus hypochondriacus and Amaranthus caudatus germplasm: characteristics of plants, grain and flours. Food Chem. 2010;123(4):1227–1234. doi: 10.1016/j.foodchem.2010.05.091. [DOI] [Google Scholar]
- Kazanas N, Fields M. Nutritional improvement of sorghum by fermentation. J Food Sci. 1981;46(3):819–821. doi: 10.1111/j.1365-2621.1981.tb15356.x. [DOI] [Google Scholar]
- Khetarpaul N, Chauhan BM. Effect of fermentation on protein, fat, minerals and thiamine content of pearl millet. Plant Foods Hum Nutr. 1989;39(2):169–177. doi: 10.1007/BF01091897. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Owusu Apenten RK. Iron binding characteristics of phenolic compounds: some tentative structure activity relations. Food Chem. 2003;81(1):133–140. doi: 10.1016/S0308-8146(02)00394-1. [DOI] [Google Scholar]
- Lorenz K, Wright B. Phytate and tannin content of amaranth. Food Chem. 1984;14(1):27–34. doi: 10.1016/0308-8146(84)90015-3. [DOI] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Mouquet-Rivier C, Icard-Vernière C, Guyot J-P, Tou EH, Rochette I, Trèche S. Consumption pattern, biochemical composition and nutritional value of fermented pearl millet gruels in Burkina Faso. Int J Food Sci Nutr. 2008;59(7):716–729. doi: 10.1080/09637480802206389. [DOI] [PubMed] [Google Scholar]
- Mustafa AF, Seguin P, Gelinas B. Chemical composition, dietary fibre, tannins and minerals of grain amaranth genotypes. Int J Food Sci Nutr. 2011;62(7):750–754. doi: 10.3109/09637486.2011.575770. [DOI] [PubMed] [Google Scholar]
- Nascimento AC, Mota C, Coelho I, et al. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: proximates, minerals and trace elements. Food Chem. 2014;148:420–426. doi: 10.1016/j.foodchem.2013.09.155. [DOI] [PubMed] [Google Scholar]
- Pedersen B, Kalinowski LS, Eggum BO. The nutritive value of amaranth grain (Amaranthus caudatus) Plant Foods Hum Nutr. 1987;36(4):309–324. doi: 10.1007/BF01892352. [DOI] [PubMed] [Google Scholar]
- Ramulu P, Rao PU. Effect of proccesing on dietary fiber content of cereals and pulses. Plant Foods Hum Nutr. 1997;50(3):249–257. doi: 10.1007/BF02436061. [DOI] [PubMed] [Google Scholar]
- Repo-Carrasco-Valencia R, Peña J, Kallio H, Salminen S. Dietary fiber and other functional components in two varieties of crude and extruded kiwicha (Amaranthus caudatus) J Cereal Sci. 2009;49(2):219–224. doi: 10.1016/j.jcs.2008.10.003. [DOI] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Physicochemical, pasting, and functional properties of amaranth seed flours: effects of lipids removal. J Food Sci. 2014;79(7):C1271–C1277. doi: 10.1111/1750-3841.12493. [DOI] [PubMed] [Google Scholar]
- Svanberg U, Lorri W. Fermentation and nutrient availability. Food Control. 1997;8(5):319–327. doi: 10.1016/S0956-7135(97)00018-2. [DOI] [Google Scholar]
- Umeta M, West CE, Fufa H. Content of zinc, iron, calcium and their absorption inhibitors in foods commonly consumed in Ethiopia. J Food Compos Anal. 2005;18:803–817. doi: 10.1016/j.jfca.2004.09.008. [DOI] [Google Scholar]
- Van Soest PS. Use of detergents in the analysis of fibrous feeds II-a rapid method for the determination of fibre and lignin. J Assoc Off Agric Chem. 1963;46:829–835. [Google Scholar]