Abstract
Wine lees, a major waste product of winemaking, is a rich source of polyphenolic compounds. LED-light irradiation at 400-nm elicited microbicidal activity of aqueous extract from wine lees (WLE) against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans, in addition to reactive oxygen species (ROS) formation, including hydroxyl radical (·OH) and hydrogen peroxide (H2O2). Although treatment for 20 min of photoirradiation alone exerted bactericidal activity with a 2- to 3-log reduction, photoirradiated WLE for 20 min achieved a 5-log or greater reduction in viable S. aureus and P. aeruginosa cells. Regarding C. albicans, a 1-log reduction (90 % reduction) of viable cells was achieved by photoirradiated WLE for 40 min, whereas photoirradiation alone did not show any fungicidal effect. ROS analyses revealed that approximately 170 μM ·OH and 600 μM H2O2 were generated in photoirradiated WLE for 20 min. Because the bactericidal activity of photoirradiated WLE was abolished by ·OH scavengers, ROS, especially highly oxidative ·OH, may be responsible for the microbicidal activity of photoirradiated WLE. In addition to its microbicidal activity, WLE may act as an antioxidant as it exerted radical scavenging activity against 2,2-diphenyl-1-picrylhydrazyl, a stable free radical.
Keywords: Wine lees, Microbicidal action, Hydroxyl radical, Hydrogen peroxide
Highlights
Photoirradiated aqueous extract from wine lees (WLE) generates reactive oxygen species.
Hydroxyl radicals generated by photoirradiated WLE could kill microbes effectively.
Photoirradiated WLE could be a novel alternative to H2O2 used in the food industry.
Introduction
Polyphenolic compounds have been noted for their antioxidant activities (Kondo et al. 1999; Liu et al. 2000; Yilmaz and Toledo 2004). In addition to antioxidant activity, their pro-oxidant potential has been applied to various fields such as anticancer treatment. It was reported that an important anticancer mechanism of plant polyphenols is mediated through intracellular copper mobilization and reactive oxygen species (ROS) generation, which is a characteristic feature of pro-oxidant properties of polyphenolic compounds, leading to cancer cell death (Khan et al. 2014). In our previous studies, the pro-oxidant potential of polyphenols was applied to the development of a novel disinfection technique (Nakamura et al. 2012b, 2013, 2015). Exposing an aqueous solution of polyphenols to blue light led to photooxidation of the polyphenolic hydroxyl group, resulting in the generation of hydrogen peroxide (H2O2) produced via electron transfer from photooxidized polyphenols to dissolved oxygen. H2O2, in turn, is homolytically cleaved by blue light, resulting in the generation of hydroxyl radicals (·OH), which are a main contributor of bactericidal activity.
The grape is the largest fruit crop in the world. The annual production worldwide amounts to almost 70 million tons, approximately 80 % of which is used to make wine ( FAO 2014). However, the winemaking process generates large amounts of waste materials or byproducts. The waste from the winemaking process can be divided into three categories: pomace, clarification sediment (such as lees), and yeast sediment. The generated amount of waste depends on the condition of the grapes at the time of harvest, as well as the processing method used. In extreme cases, this can result in waste levels of up to 20 % of the harvested mass (Russ and Meyer-Pittroff 2004). Thus, waste materials or byproducts obtained from the winemaking process could be a valuable resource to be recycled.
One particular byproduct obtained from the winemaking process is wine lees, which may be a good resource to be recycled such as a sustainable source for economic nutrients (Perez-Bibbins et al. 2015). Wine lees are generated during fermentation and the aging process. The solid fraction of lees primary consists of yeast biomass, insoluble carbohydrates (such as cellulosic or hemicellulosic materials), phenolic compounds, lignin, proteins, inorganic salts, organic acid salts (mainly tartrates), and other materials, while the liquid phase is rich in ethanol and organic acids (Perez-Bibbins et al. 2015). As it was reported that wine lees can be applied to the recovery of value-added phytochemicals due to the ability of yeast to form molecular interactions with phenolic compounds (Mena et al. 2014), it is anticipated that the profile of phenolic compounds in lees might differ from that in the residue of crushed grapes.
Recent studies suggested that natural substances, such as naturally occurring phenolic compounds, that possess antibacterial and antioxidative activities are required for food preservatives and sanitizers (Kang et al. 2013; Xu et al. 2014).
H2O2 is an effective microbicide commonly used in the food industry (Demrkol 2009; Yun et al. 2012). We hypothesized that the major ROS generated in photoirradiated aqueous extract of wine lees are H2O2 and ·OH. Thus, photoirradiated wine lees may be a novel alternative to H2O2 in the food industry.
Materials and methods
Reagents
Reagents were purchased from the following sources: 5,5-dimethyl-1-pyrroline N-oxide (DMPO) from Labotec (Tokyo, Japan); catalase from bovine liver, H2O2, dimethyl sulfoxide (DMSO), thiourea, and l-ascorbic acid from Wako Pure Chemical Industries (Osaka, Japan); 4-hydroxy-2,2,6,6-tetramethylpiperidine N-oxyl (TEMPOL) from Sigma-Aldrich (St. Louis, MO, USA); and 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Tokyo Chemical Industry (Tokyo, Japan). All other reagents used were of analytical grade.
Preparation of aqueous extract of wine lees
Strained wine lees were obtained from a white wine grape variety (Niagara) harvested in Hokkaido, Japan after fermentation for 1–2 weeks and freeze-dried. Pure water (at a ratio of 3 ml pure water per 1 g powder) was added to the dried lees powder, and the resultant mixture was agitated at 150 rpm overnight at room temperature. The upper layer was taken and centrifuged at 1000×g for 20 min, and the supernatant was collected. Following membrane filtration (pore size, 0.22 μm), the supernatant was subjected to total polyphenol determination using the Folin–Denis method in which gallic acid was used as a standard (Schanderl 1970). The aqueous extract solution (hereafter termed WLE) was adjusted to contain 0.2 mg total polyphenol/ml with pure water and stored at −20 °C until further analysis. In the DPPH scavenging assay, WLE was further freeze-dried and tested. One gram of freeze-dried WLE was obtained from 58.7 ml of WLE.
Light source
An experimental device equipped with a light emitting diode (LED) with a wavelength of 400 nm (NHH105UV, Lustrous Technology, Shiji, Taiwan) was used. The output power of the LED measured using a power meter (FieldMate, Coherent, Santa Clara, CA, USA) was set at 400 mW per LED corresponding to an irradiance of 130 mW/cm2 at a distance of 15 mm from the LED. A four-sided, clear methacrylate plastic cuvette containing the sample was placed in the experimental device. LED-light irradiation was performed on both sides of the plastic cuvette (total irradiance: 260 mW/cm2).
Microbicidal assay
Staphylococcus aureus JCM 2413, Pseudomonas aeruginosa JCM 6119, and Candida albicans JCM 153 purchased from the Japan Collection of Microorganisms, RIKEN BioResource Center (Wako, Japan) were used. Each suspension of S. aureus and P. aeruginosa was prepared in sterile physiological saline from a culture grown on brain heart infusion (BHI) agar (Becton–Dickinson Labware, Franklin Lakes, NJ, USA) aerobically at 37 °C overnight. A suspension of C. albicans was prepared in sterile physiological saline from a culture grown on Sabouraud dextrose agar (SDA) at 37 °C overnight. In a plastic cuvette, 450 µl of WLE or pure water was mixed with 50 µl of the bacterial or fungal suspension to reach a final concentration of approximately 107 colony forming units (CFU)/ml for the two bacterial strains and 107 cells/ml for C. albicans. Then, the samples were exposed to LED light for 10, 20, or 40 min. After irradiation, 50 µl of the sample was mixed with an equal volume of sterile catalase solution (5000 U/ml phosphate buffer [pH 7.4]) to terminate the bactericidal effect of H2O2 generated by photooxidation of polyphenols in WLE. A tenfold serial dilution of the mixture was prepared using sterile physiological saline, and 10 µl of the diluted solution were seeded onto a BHI agar plate for bacteria or a SDA plate for C. albicans. The agar plates were cultured as described above for 2 days, and the CFU/ml or cells/ml was determined. In addition, as controls, samples were kept for 10, 20, or 40 min in a light-shielding box, instead of being exposed to LED light, and subjected to the same procedures. The initial bacterial count (inoculum size) was evaluated using the viable counting method, and the initial count of C. albicans was microscopically determined.
Because one of the potential pivotal constituents of photoirradiated WLE is H2O2, the bactericidal and fungicidal effects of 3 % H2O2, which is within the range of commonly-used concentrations for food sanitary research (Demrkol 2009; Mcwatters et al. 2002; Ukuku and Fett 2004), were also examined. In a plastic cuvette, 450 µl of H2O2 or pure water was mixed with 50 µl of S. aureus or C. albicans suspension to reach final concentrations of 3 % (w/v) for H2O2, approximately 107 CFU/ml for S. aureus and 107 cells/ml for C. albicans. Control samples kept for 10, 20, or 40 min in a light-shielding box were also subjected to the bactericidal and fungicidal assays, as described above. All tests were performed in triplicate.
To determine if the bactericidal effect of photoirradiated WLE could be attributable to ·OH, DMSO or thiourea, which are well-known ·OH scavengers (Dorfman and Adams 1973; Halliwell and Gutteridge 2007), were added to the reaction mixture. The reaction mixture consisting of 425 μl of WLE, 50 µl of S. aureus suspension and 25 μl of DMSO or thiourea was prepared to reach final concentrations of approximately 107 CFU/ml S. aureus and 700 mM for DMSO or 150 mM for thiourea. Then, the samples were irradiated with LED light for 20 min. The CFU/ml was determined after each treatment as described above. All tests were performed in triplicate.
Electron spin resonance (ESR) analysis of ·OH and colorimetric determination of H2O2
Qualitative and quantitative analyses of ·OH generated by photoirradiation of WLE were performed using an ESR spin trapping technique as reported previously (Nakamura et al. 2010a). An aliquot (483 μl) of undiluted or 2–8-fold diluted WLE was mixed with 17 μl of DMPO in a plastic cuvette to reach a final concentration of 300 mM DMPO. Then, the sample was irradiated with LED light for 0, 10, 20, and 60 s. After irradiation, the sample was transferred to a quartz cell for ESR spectrometry, and the ESR spectrum was recorded on an X-band ESR spectrometer (JES-FA-100, JEOL, Tokyo, Japan). The measurement conditions for ESR were as follows: field sweep, 331.89–341.89 mT; field modulation frequency, 100 kHz; field modulation width, 0.1 mT; amplitude, 200; sweep time, 2 min; time constant, 0.03 s; microwave frequency, 9.420 GHz; and microwave power, 4 mW. TEMPOL (2 µM) was used as a standard to calculate the concentration of spin-trapped radicals, and the ESR spectrum of manganese held in the ESR cavity was used as an internal standard.
Because linearity of the increase in DMPO-OH, a spin adduct of DMPO and ·OH, was confirmed in all undiluted and diluted WLE within 10 s of irradiation, the two following experiments were conducted. First, to examine whether ·OH was continuously generated during LED-light irradiation for 20 min, undiluted and eightfold diluted WLE were irradiated with LED light for 20 min, and then DMPO was added to each photoirradiated sample to reach a final concentration of 300 mM. Immediately after addition of DMPO, the sample was further irradiated with LED light for 10 s. Then, ESR analysis was performed as described above. Second, to estimate the amount of ·OH generated in undiluted photoirradiated WLE for 20 min, an aliquot (483 μl) of undiluted WLE in a plastic cuvette was irradiated with LED light for 0, 0.5, 1, 2, 3, 4, 5, 10, and 20 min in the absence of DMPO. Then, 17 μl of DMPO was added to each cuvette to reach a final concentration of 300 mM, and the cuvette was again irradiated with LED light for 10 s followed by ESR determination of DMPO-OH, as described above. Because the slope of each linear line for the DMPO-OH yield from 0 to 10 s indicates the velocity of ·OH generation (μM/s), the area under the curve, which is a function of the velocity of ·OH generation and LED-irradiation time without DMPO, was calculated to estimate the amount of ·OH generated for 20 min of LED-light irradiation.
For H2O2 determination, 500 μl of undiluted WLE in a plastic cuvette was irradiated with LED light for 0, 5, 10, and 20 min. Immediately after irradiation, the H2O2 concentration was determined using a colorimetric method based on the peroxide-mediated oxidation of Fe2+ followed by the reaction of Fe3+ with xylenol orange (Jiang et al. 1990). All tests were performed in triplicate.
Scavenging effect on the stable radical DPPH
Freeze-dried WLE and l-ascorbic acid were dissolved in pure water followed by filtration (pore size, 0.22 μm). An aliquot (80 μl) of each aqueous solution was mixed with 16 μl of 100 mM Tris–HCl buffer (pH 7.5), 64 μl of 100 % ethanol, and 40 μl of 1 mM DPPH dissolved in 100 % ethanol in a well of a 96-well microplate. The plate was then left in a light-shielding box for 20 min. Absorbance at 520 nm was read by a microplate reader (FilterMax F5, Molecular Devices, Sunnyvale, CA, USA). The rate of DPPH scavenging was calculated according to the following equation:
([A520 of the solvent control–A520 of the specimen]/A520 of the solvent control) × 100, where A520 is absorbance at 520 nm. All tests were performed in duplicate.
Statistical analyses
Statistical differences in the viable counts obtained in the microbicidal assay were assessed by the Tukey–Kramer HSD multi-comparison test. Statistical analysis of results obtained from the microbicidal assay was performed following logarithmic conversion. When colonies were not detected, the value of the detection limit (102 CFU/ml) was used for the statistical analysis. Regarding the yield of H2O2, since H2O2 was not detected in the pure water group, statistical significance for the remaining four groups was assessed by the Tukey–Kramer HSD multi-comparison test. P < 0.05 was considered to be significant.
Results and discussion
Microbicidal assay
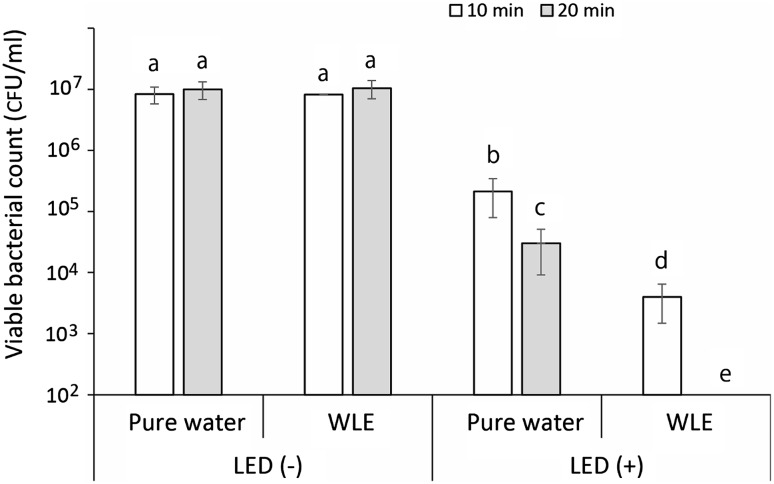
The result of the bactericidal assay against S. aureus is summarized in Fig. 1. Under the condition without LED-light irradiation, WLE kept in a light-shielding box for 10 and 20 min showed almost no bactericidal activity in comparison with that of the corresponding pure water groups. LED-light irradiation alone showed slight bactericidal activity. LED-light irradiation of pure water for 10 and 20 min showed an approximate 1.5- and 2.5-log reduction of viable bacterial counts, respectively, compared with the corresponding pure water groups without LED-light irradiation. Furthermore, LED-light irradiation of WLE for 10 min effectively killed the bacteria with an approximate 3-log reduction, and LED-light irradiation for 20 min achieved a 5-log reduction. The results of the bactericidal assay against P. aeruginosa and the fungicidal assay against C. albicans are summarized in Fig. 2. Similar to S. aureus, although LED-light irradiation of P. aeruginosa in pure water for 20 min showed an approximate 3-log reduction of viable bacterial counts, LED-light irradiation of the bacteria in WLE for 20 min effectively killed the bacteria with a >5-log reduction. Unlike the two bacterial species tested, LED-light irradiation of C. albicans in pure water for 40 min showed almost no fungicidal effect. When C. albicans in WLE were irradiated with LED light for 40 min, the fungi were killed with an approximately 1-log reduction (90 % reduction).
Fig. 1.
Number of viable Staphylococcus aureus cells in suspension after each treatment (suspended in pure water or WLE with or without LED-light irradiation for 10 or 20 min). Each value indicates the mean of triplicate determinations with the standard deviation. Significant differences (P < 0.01) within each group are denoted by lowercase letters (i.e., bars with the different letter are significantly different)
Fig. 2.
Number of viable Pseudomonas aeruginosa and Candida albicans cells suspended in pure water or WLE with or without LED-light irradiation. P. aeruginosa and C. albicans were irradiated with LED light for 20 and 40 min, respectively. Each value indicates the mean of triplicate determinations with the standard deviation. Significant differences (P < 0.01) within each group are denoted by lowercase letters (i.e., bars with the different letter are significantly different). ND not detected
The effect of 3 % H2O2 as a reference disinfectant revealed that treatment of S. aureus showed a time-dependent bactericidal activity, and 20 min-treatment achieved a 5-log reduction of viable cells (Fig. 3). In contrast, treatment of C. albicans with 3 % H2O2 showed almost no fungicidal effect, and even 40-min treatment resulted in only a slight reduction of viable cells.
Fig. 3.
Number of viable Staphylococcus aureus and Candida albicans cells suspended in 3 % H2O2. S. aureus was exposed to 3 % H2O2 for 10 and 20 min, and C. albicans for 20 and 40 min. Each value indicates the mean of triplicate determinations with the standard deviation. Significant differences (P < 0.01 for S. aureus and P < 0.05 for C. albicans) within each group are denoted by lowercase letters (i.e., bars with the different letter are significantly different). ND not detected
These results clearly demonstrate that LED-light irradiation of WLE at 400 nm had the ability to elicit bactericidal activity comparable to that of 3 % H2O2, which is within the range of commonly-used concentrations for food sanitary research. Regarding the fungicidal activity, photoirradiated WLE against C. albicans appears to be more potent than 3 % H2O2. One of the reasons that C. albicans is resistant to 3 % H2O2 could be the catalase activity of C. albicans cells. It was reported that the catalase activity of C. albicans cells was comparable to that of aerobes (Nakamura et al. 2010b), which would result in resistance to oxidative stress (Nakamura et al. 2012a).
In the experiment in which the effect of ·OH scavengers was examined, LED-light irradiation of WLE for 20 min resulted in a >5-log reduction of viable S. aureus cells. This bactericidal activity of photoirradiated WLE was completely abrogated in the presence of 700 mM DMSO and 150 mM thiourea, and the viable bacterial counts in both cases were similar or even superior to that in the photoirradiated pure water group (Fig. 4). Thus, the results strongly suggest that the major contributor to the bactericidal effect of photoirradiated WLE is ·OH.
Fig. 4.
Influence of ·OH scavengers on the bactericidal effect of photoirradiated WLE. LED-light irradiation was performed for 20 min. Each value indicates the mean of triplicate determinations with the standard deviation. Significant differences (P < 0.01) within each group are denoted by lowercase letters (i.e., bars with the different letter are significantly different)
ESR analysis of ·OH and colorimetric determination of H2O2
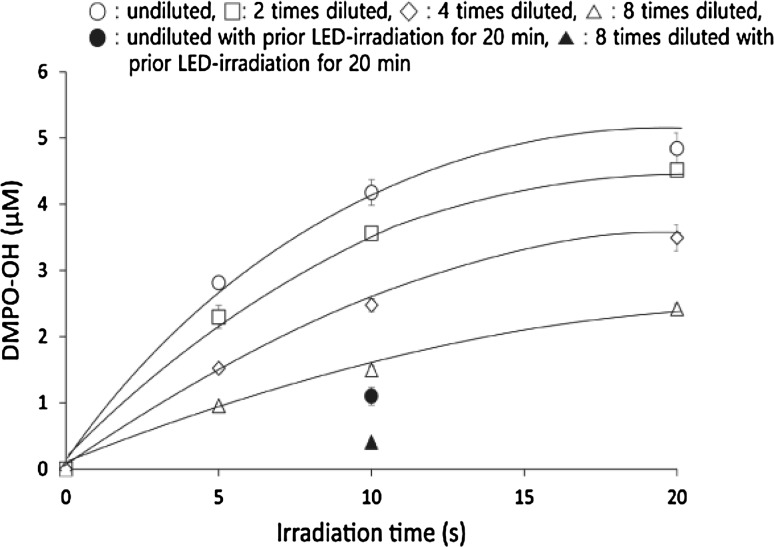
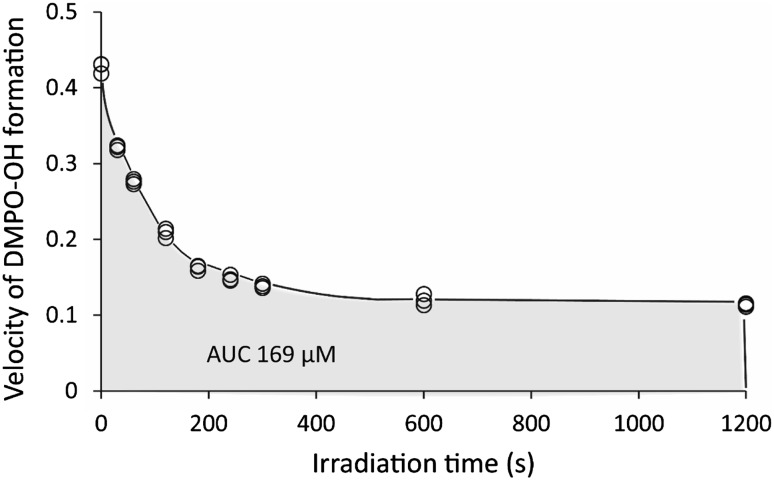
When WLE was irradiated with LED light in the presence of 300 mM DMPO, the ESR signal of DMPO-OH was detected. The presence of the spin adduct was confirmed by hyperfine coupling constants of aN = aH = 1.49 mT for DMPO-OH (Buettner 1987). Figure 5 summarizes the yields of DMPO-OH after LED-light irradiation of undiluted and diluted WLE. The yield increased in an irradiation time- and WLE concentration-dependent manner. Within 10 s of irradiation, linearity of the increase in DMPO-OH was confirmed in all undiluted and diluted WLE (Fig. 5). To examine whether ·OH was continuously generated during LED-light irradiation, undiluted and eightfold diluted WLE irradiated with LED light for 20 min in the absence of DMPO was furthered irradiated with LED light for 10 s in the presence of 300 mM DMPO. This resulted in an approximate 75 % reduction of the DMPO-OH yield in both cases as compared to the yield without prior LED-light irradiation for 20 min (Fig. 5). Furthermore, to estimate the amount of ·OH generated in undiluted photoirradiated WLE for 20 min, WLE was irradiated with LED light for 0, 0.5, 1, 2, 3, 4, 5, 10, and 20 min in the absence of DMPO. Then, ESR determination of DMPO-OH generated in 10 s of additional LED-light irradiation with DMPO was conducted. The curve of the function of velocity of ·OH and LED-light irradiation time indicated that the estimated total amount of ·OH generated in undiluted photoirradiated WLE for 20 min was 169 μM (Fig. 6).
Fig. 5.
·OH yields (open circles, open squares, open diamond, open triangle) generated by LED-light irradiation of undiluted and 2- to 8-fold diluted WLE for 0, 10, and 20 s, and ·OH yields (filled circle, filled triangle) generated by irradiation (10 s) of undiluted and eightfold diluted WLE subjected to prior LED-light irradiation for 20 min without DMPO. Each value indicates the mean of triplicate determinations with the standard deviation
Fig. 6.
Relationship between the velocity of DMPO-OH generation and LED-light irradiation time. The area under the curve indicates the estimated amount of ·OH generated during LED-light irradiation for 20 min. Open circles indicate individual data
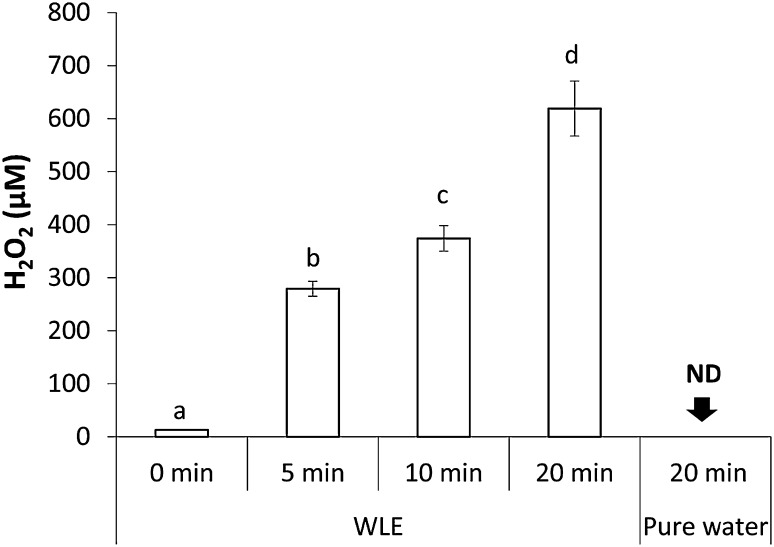
WLE with LED light generated H2O2 in an irradiation time-dependent manner (Fig. 7). In contrast, only a small amount of H2O2 was found in WLE without irradiation, and H2O2 was not detected in pure water irradiated with LED light for 20 min. The average yields of H2O2 generated in WLE with LED-light irradiation for 5, 10, and 20 min were approximately 280, 370, and 620 µM, respectively.
Fig. 7.
H2O2 yields generated by LED-light irradiation of WLE for 0, 5, 10, and 20 min. Each value indicates the mean of triplicate determinations with the standard deviation. Significant differences between the two groups are shown as P < 0.01. ND not detected
According to the ESR analysis, ·OH was generated in an irradiation time- and WLE concentration-dependent manner, at least up to 20 s of irradiation. However, once photoirradiation was increased to 20 min without DMPO, the yield of DMPO-OH generated for 10 s of additional photoirradiation decreased to approximately one fourth of that obtained by 10 s of photoirradiation without prior irradiation, indicating that the velocity of ·OH generation decreased gradually with irradiation time (0–20 min). Nonetheless, the total amount of ·OH generated in 20 min was estimated to be approximately 169 μM. Our previous result using photolysis of H2O2 as an ·OH generation system suggested that 200–300 µM ·OH yielded within 3 min would be needed to produce a >5-log reduction in S. aureus (Ikai et al. 2010). Thus, the amount of ·OH obtained in the present study would be sufficient to kill the bacteria within 20 min. As H2O2 was also detected in photoirradiated WLE in an irradiation time-dependent manner, ·OH is likely generated via photolysis of H2O2.
Scavenging effect on the stable radical DPPH
Freeze-dried WLE and l-ascorbic acid scavenged DPPH in a concentration-dependent manner (Fig. 8), showing that WLE possesses antioxidant potential. However, the effect of freeze-dried WLE was much less potent than that of l-ascorbic acid. The effective concentrations showing 50 % scavenging of freeze-dried WLE and l-ascorbic acid were 7.85 and 0.02 mg/mL, respectively, indicating that the activity of 1 g freeze-dried WLE corresponds to that of 0.0025 g l-ascorbic acid. In other words, 1 L of WLE would possess antioxidant potential equivalent to approximately 40 mg l-ascorbic acid. Previous studies showed that 0.05 % (500 mg/L) ascorbic acid was effective not only to prevent the degradation of phenolic compounds in fresh lettuce (Altunkaya and Gökmen 2009), but also lipid oxidation of ground beef (Ismail et al. 2009). Therefore, WLE may be applicable as a food preservative and sanitizer in terms of antioxidative potential if concentrated WLE is used (e.g., tenfold concentrated).
Fig. 8.
Scavenging activity of freeze-dried WLE upon DPPH treatment. Each value indicates the mean with individual data (filled circle). EC50 indicates the effective concentration showing 50 % scavenging activity
Regarding the potential application of photoirradiated WLE, one of the specific examples is as a sanitizer for fresh fruits and vegetables. Although the consumption of fresh fruits and vegetables is essential to deliver health benefits (Wang et al. 2014), fruits and vegetables must be sanitized to avoid disease outbreaks associated with microbial contamination (Callejon et al. 2015; Kozak et al. 2013; Lynch et al. 2009; Mellmann et al. 2011). The major limitation of the present study was that the effect of photoirradiated WLE was examined only in in vitro conditions. Thus, further study to simulate real-world situations is needed.
Conclusion
Photoirradiated WLE showed comparable microbicidal activity to the bactericidal and fungicidal effects of 3 % H2O2, which was within the range of commonly used concentrations for food sanitary research. Therefore, photoirradiated WLE could be a novel alternative to H2O2 for use in the food industry.
Acknowledgments
This research was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C), 26460116, 2014.
References
- Altunkaya A, Gökmen V. Effect of various anti-browning agents on phenolic compounds profile of fresh lettuce (L. sativa) Food Chem. 2009;117:122–126. doi: 10.1016/j.foodchem.2009.03.085. [DOI] [Google Scholar]
- Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3:259–303. doi: 10.1016/S0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- Callejon RM, Rodriguez-Naranjo MI, Ubeda C, Hornedo-Ortega R, Garcia-Parrilla MC, Troncoso AM. Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathog Dis. 2015;12:32–38. doi: 10.1089/fpd.2014.1821. [DOI] [PubMed] [Google Scholar]
- Demrkol O. Effects of hydrogen peroxide treatment on thiol contents in fresh-cut asparagus (Asparagus officinalis) spears. Int J Food Sci Nutr. 2009;60:80–88. doi: 10.1080/09637480701602969. [DOI] [PubMed] [Google Scholar]
- Dorfman LM, Adams GE. Reactivity of the hydroxyl radical in aqueous solutions. Springfield: National Bureau of Standards (NSRDS-NBS no. 46); 1973. pp. 1–59. [Google Scholar]
- FAO (2014) FAO STAT. http://faostat.fao.org. Accessed February 2014
- Halliwell B, Gutteridge JM (2007) 2.5.1 Hydroxyl radical. In: Free radicals in biology and medicine, 4th edn. Oxford University Press: Oxford, pp 42–45
- Ikai H, Nakamura K, Shirato M, Kanno T, Iwasawa A, Sasaki K, Niwano Y, Kohno M. Photolysis of hydrogen peroxide, an effective disinfection system via hydroxyl radical formation. Antimicrob Agents Chemother. 2010;54:5086–5091. doi: 10.1128/AAC.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail HA, Lee EJ, Ko KY, Paik HD, Ahn DU. Effect of antioxidant application methods on the color, lipid oxidation, and volatiles of irradiated ground beef. J Food Sci. 2009;74:C25–C32. doi: 10.1111/j.1750-3841.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Woollard AC, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-N. [DOI] [PubMed] [Google Scholar]
- Kang SN, Goo YM, Yang MR, Ibrahim RI, Cho JH, Kim IS, Lee OH. Antioxidant and antimicrobial activities of ethanol extract from the stem and leaf of Impatiens balsamina L. (Balsaminaceae) at different harvest times. Molecules. 2013;18:6356–6365. doi: 10.3390/molecules18066356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HY, Zubair H, Faisal M, Ullah MF, Farhan M, Sarkar FH, Ahmad A, Hadi SM. Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: a mechanism for cancer chemopreventive action. Mol Nutr Food Res. 2014;58:437–446. doi: 10.1002/mnfr.201300417. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M. Scavenging mechanisms of (−)-epigallocatechin gallate and (−)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radic Biol Med. 1999;27:855–863. doi: 10.1016/S0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- Kozak GK, MacDonald D, Landry L, Farber JM. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J Food Prot. 2013;76:173–183. doi: 10.4315/0362-028X.JFP-12-126. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ma LP, Zhou B, Yang L, Liu ZL. Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein. Chem Phys Lipids. 2000;106:53–63. doi: 10.1016/S0009-3084(00)00133-X. [DOI] [PubMed] [Google Scholar]
- Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect. 2009;137:307–315. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]
- McWatters LH, Chinnan MS, Walker SL, Doyle MP, Lin CM. Consumer acceptance of fresh-cut iceberg lettuce treated with 2% hydrogen peroxide and mild heat. J Food Prot. 2002;65:1221–1226. doi: 10.4315/0362-028x-65.8.1221. [DOI] [PubMed] [Google Scholar]
- Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, McLaughlin SF, Henkhaus JK, Leopold B, Bielaszewska M, Prager R, Brzoska PM, Moore RL, Guenther S, Rothberg JM, Karch H. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104: H4 outbreak by rapid next generation sequencing technology. PLoS ONE. 2011;6:e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena P, Ascacio-Valdes JA, Girones-Vilaplana A, Del Rio D, Moreno DA, Garcia-Viguera C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly)phenolic compounds. Food Chem. 2014;145:327–334. doi: 10.1016/j.foodchem.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kanno T, Ikai H, Sato E, Mokudai T, Niwano Y, Ozawa T, Kohno M. Reevaluation of quantitative ESR spin trapping analysis of hydroxyl radical by applying sonolysis of water as a model system. Bull Chem Soc Jpn. 2010;83:1037–1046. doi: 10.1246/bcsj.20100078. [DOI] [Google Scholar]
- Nakamura K, Kanno T, Mokudai T, Iwasawa A, Niwano Y, Kohno M. A novel analytical method to evaluate directly catalase activity of microorganisms and mammalian cells by ESR oximetry. Free Radic Res. 2010;44:1036–1043. doi: 10.3109/10715762.2010.495750. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kanno T, Mokudai T, Iwasawa A, Niwano Y, Kohno M. Microbial resistance in relation to catalase activity to oxidative stress induced by photolysis of hydrogen peroxide. Microbiol Immunol. 2012;56:48–55. doi: 10.1111/j.1348-0421.2011.00400.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamada Y, Ikai H, Kanno T, Sasaki K, Niwano Y. Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J Agric Food Chem. 2012;60:10048–10054. doi: 10.1021/jf303177p. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Shirato M, Ikai H, Kanno T, Sasaki K, Kohno M, Niwano Y. Photo-irradiation of proanthocyanidin as a new disinfection technique via reactive oxygen species formation. PLoS ONE. 2013;8:e60053. doi: 10.1371/journal.pone.0060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Ishiyama K, Sheng H, Ikai H, Kanno T, Niwano Y. Bactericidal activity and mechanism of photoirradiated polyphenols against gram-positive and -negative bacteria. J Agric Food Chem. 2015 doi: 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- Perez-Bibbins B, Torrado-Agrasar A, Salgado JM, Oliveira RP, Dominguez JM. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: an overview. Waste Manag. 2015;40:72–81. doi: 10.1016/j.wasman.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Russ W, Meyer-Pittroff R. Utilizing waste products from the food production and processing industries. Crit Rev Food Sci Nutr. 2004;44:57–62. doi: 10.1080/10408690490263783. [DOI] [PubMed] [Google Scholar]
- Schanderl SH. Tannins and related phenolics. In: Joslyn MA, editor. Methods in food analysis. New York: Academic; 1970. pp. 701–724. [Google Scholar]
- Ukuku DO, Fett WF. Method of applying sanitizers and sample preparation affects recovery of native microflora and Salmonella on whole cantaloupe surfaces. J Food Prot. 2004;67:999–1004. doi: 10.4315/0362-028x-67.5.999. [DOI] [PubMed] [Google Scholar]
- Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Yagiz Y, Hsu WY, Simonne A, Lu J, Marshall MR. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J Agric Food Chem. 2014;62:6640–6649. doi: 10.1021/jf501073q. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- Yun HS, Kim Y, Oh S, Jeon WM, Frank JF, Kim SH. Susceptibility of Listeria monocytogenes biofilms and planktonic cultures to hydrogen peroxide in food processing environments. Biosci Biotechnol Biochem. 2012;76:2008–2013. doi: 10.1271/bbb.120238. [DOI] [PubMed] [Google Scholar]