Abstract
Sea fennel, a rediscovered star of the coastal cuisine, has been investigated for its phytochemical profile and biological potential. Sea fennel flowers, stems and leaves were analyzed for essential oils (EOs) isolated by hydrodistillation, as well as non-volatiles obtained by ethanolic extraction. Limonene were found to be a dominant compound in EOs and ethanolic extracts; ranging from 57.5–74.2 % and 0.7–8.1 mg/g dry plant material, respectively. In addition total phenolic content was determined for ethanolic extracts. All samples and their main phytochemicals were tested for various methods. EO and extract obtained from flowers were tested for vasodilatory activity on rat aortic rings. Antioxidant activity of EOs was extremely low in comparison to extracts, on the contrary to cholinesterase inhibition where EOs showed better activity than extracts. Flower extract and chlorogenic acid showed stronger vasodilators in comparison to EO and limonene. The obtained results point out the potential impact of the dominant compounds from EO and extract on the biological properties of the sea fennel.
Keywords: Chlorogenic acid, Limonene, Antioxidation, Cholinesterase inhibition, Vasodilatation
Introduction
Crithmum maritimum L. is a facultative halophyte from the family of Apiaceae. Commonly known as sea fennel or rock samphire, this wild, naturally salt-tolerant plant is usually found on maritime rocks, piers and sandy beaches along the Mediterranean, Black sea and European Atlantic coasts (Meot-Duros and Magné 2009).
Sea fennel has a number of uses in culinary, medicine and cosmetics. This highly aromatic herb has been used in many European countries as edible vegetable or spice. Beside cooked, it is often used for food flavouring, also young leaves are usually prepared for salads or pickled in vinegar and used as condiments. This herb has been forgotten for a long time, but in the last few years it has been rediscovered and became the star of the coastal cuisine (Özcan et al. 2001; Maleš et al. 2003; Renna and Gonnella 2012). Sea fennel was also used in folk medicine as an appetizer, tonic, carminative, diuretic, or for treating obesity due to its richness of biologically active compounds such as vitamin C, ω-3 and ω-6 fatty acids, iodine, carotenoids, minerals, organic acids and phenolics (Frankie 1982; Guil-Guerrero and Rodriguez-Garcia 1999; Atia et al. 2011; Siracusa et al. 2011). In the past, sea fennel consummation protected sailors from scurvy. Its essential oils (EOs) are also widely used in cosmetics (Atia et al. 2011; Jallali et al. 2014). Although in the case of sea fennel usually leaves are used, other plant parts which remain unused could also be useful for culinary or medicinal purposes. Due to the possible different phytochemical composition and their yield in different plant parts (Pateira et al. 1999; Houta et al. 2011), it is important to investigate them separately in order to get the insight into their biological potential. Also, the majority of studies on sea fennel investigated the qualitative and quantitative compositions of its EOs: from Turkey (Senatore et al. 2000; Özcan et al. 2001), Portugal (Pateira et al. 1999), Italy (Ruberto et al. 2000), and Croatia (Maleš et al. 2003; Kulišić-Bilušić et al. 2010), with aim to support the hypothesis that several different chemotypes of sea fennel grow in the Mediterranean region. They reported significant fluctuations in EOs composition containing monoterpenes as the main compounds, while studies on sea fennel chemistry, especially on its polar components and their biological activities are scarce (Meot-Duros and Magné 2009).
Due to the oxidative stress a large percentage of population is affected by chronic diseases, such as neurodegenerative disease, cardiovascular diseases, obesity, and cancer but many studies show that natural compounds have potential in reducing risk of such diseases. As one antioxidant method cannot fully describe the antioxidant activity of the samples, for the full evaluation multiple method approach is necessary (Generalić Mekinić et al. 2014). For that reason, three antioxidant methods were used, that are based on the fundamentally different reaction mechanisms: Ferric Reducing/Antioxidant Power (FRAP) (Electron Transfer, ET), 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging (Hydrogen Atom Transfer, HAT), and a relatively new and not so common Briggs–Rauscher (BR) reaction (combined mechanisms). One of the most common forms of neurodegenerative disorders, affecting many elderly people, is Alzheimer disease and inhibition of acetylcholinesterase (AChE)/butyrylcholinesterase (BuChE) serves as a strategy for its treatment (Singh et al. 2013). Also, different experimental studies indicated that a great variety of phytochemicals, especially those from different herbs, have the potential to protect the vascular system and improve the function of blood vessels. In our previous researches (Mudnić et al. 2010), in addition to and independently from their antioxidant effects, several plant extracts and pure substances enhance the production of vasodilating agents (nitric oxide, endothelium-derived hyperpolarizing factor) and inhibit the production of vasoconstrictor factors in endothelial cells.
Thus, the aim of this study was to explore the phytochemical profile of sea fennel (EOs and ethanolic extracts) from the different plant parts in order to explore their antioxidant, AChE/BuChE inhibitory and vasodilatory potential.
Materials and methods
All used reagents and solvents were of analytical grade. Chlorogenic acid, acetylcholinesterase (AChE, from Electrophorus electricus), butyrylcholinesterase (BuChE, from equine serum), acetylthiocholine iodide (ATChI), butyrylthiocholine iodide (BuTChI), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), noradrenaline and acetylcholine chloride were purchased from Sigma-Aldrich GmbH (Steinheim, Germany).
Plant material collection, essential oil extraction and extract preparation
The aerial parts of wild-grown sea fennel were harvested in Central Dalmatia (Split, Croatia) during flowering stage (August 2013). The plant material (flowers, stems and leaves) was air-dried for 10 days at room temperature in a shaded and aerated place. For the extract preparation dried plant material was pulverised (1 min in high speed grinder) into powder.
The volatiles were isolated from dried flowers, stem, and leaves (100 g) by hydrodistillation in Clevenger type apparatus according to the procedure described by Kulišić-Bilušić et al. (2010).
Extracts were prepared by mixing 10 g of the powdered material with 100 mL of 80 % aqueous ethanol (v/v). In order to obtain better extraction yield, ultrasonic assisted extraction (35 kHz) was used (1 h, 50 °C, under reflux). After the extraction, the suspensions were filtered. All extracts were prepared in triplicate (i.e. for each plant part three equal amounts of plant material were extracted separately), and obtained extracts from the same plant part were combined into the final extract that was used in further experiments. The extracts and essential oils (EOs) were stored at −20 °C until analyses.
Gas chromatography and mass spectrometry (GC–MS)
Gas chromatography analyses were performed on gas chromatograph (model 3900; Varian Inc., Lake Forest, CA, USA) equipped with mass spectrometer (model 2100T) and non-polar capillary column VF-5MS (30 m × 0.25 mm i.d., coating thickness 0.25 μm) (Varian Inc.). Chromatographic conditions were as follows: helium was carrier gas at 1 mL/min, injector temperature was 250 °C. The column temperature was programmed at 60 °C isothermal for 3 min, and then increased to 246 °C at a rate of 3 °C/min and held isothermal for 25 min. The injected volume was 1 μL and the split ratio was 1:50. MS conditions were: ionization voltage 70 eV; ion source temperature 200 °C; mass scan range: 40–350 mass units. The analyses were carried out in duplicate. The individual peaks were identified by comparison of their retention indices (relative to C8–C20 n-alkanes for VF-5MS) to those of authentic samples and literature, as well as by comparing their mass spectra with the Wiley 7 MS library (Wiley, New York, NY, USA) and NIST02 (Gaithersburg, MD, USA) mass spectral database. The percentage composition of the samples was computed from the GC peak areas using the normalization method (without correction factors). The component percentages were calculated as mean values from duplicate GC–MS analyses.
Total phenolic content
The total phenolic content in sea fennel ethanolic extracts was estimated using the Folin–Ciocalteu method (Katalinić et al. 2013). Spectrophotometric measurements were performed on a SPECORD 200 Plus, Edition 2010 (Analytik Jena AG, Jena, Germany). The measurements were performed in triplicate for each sample and the results are expressed as mg of gallic acid equivalents per g of dry plant material (mg GAE/g of d.p.m.).
High-performance liquid chromatography (HPLC–DAD)
HPLC used was Perkin Elmer Series 200 with UV/VIS detector (Perkin-Elmer Inc., Shelton, CT, USA). Chlorogenic acid was separated on an UltraAqueous C18 column (250 × 4.6 mm, 5 mm, maintained at 30 °C, Restek, Bellefonte, PA, USA). The flow rate was 0.8 mL/min. Chlorogenic acid peak was identified by comparing the retention time and absorption spectra with those obtained using the standard under the same conditions, at 280 nm, and it was quantified using external standard calibration curve. Each sample was injected twice in the chromatographic system. A gradient consisting of solvent A (water/phosphoric acid, 99.8:0.2, v/v) and solvent B (methanol/acetonitrile, 50:50, v/v) was applied as follows: from 96 % A at 0.5 min to 50 % A at 40 min, to 40 % A at 45 min, to 0 % A at 60 min, to 0 % A at 68 min, to 96 % A at 70 min and maintaining 96 % A for 10 min (80 min).
Antioxidant activity
The reducing potential of sea fennel EOs and EtOH extracts was measured as ferric reducing/antioxidant power (FRAP) as described by Benzie and Strain (1996). In this assay, antioxidants are evaluated as reductants of Fe3+ to Fe2+, which is chelated by 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) to form a Fe2+–TPTZ complex absorbing at 593 nm. The results are expressed in µmol Fe2+ per litre of extract (µmol Fe2+/L). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging ability of the samples was measured according to the procedure reported by Katalinić et al. (2013). The results for radical scavenging activities were expressed as inhibition percentage of DPPH radical (% Inhibition).
The ability of samples to stop the oscillations in Briggs–Rauscher (BR) assay was performed as described previously (Generalić Mekinić et al. 2014). In this assay, when antioxidants are added to an active oscillating BR reaction mixture there is an immediate quenching of the oscillations. The inhibition time linearly depends on the type and concentration of the added antioxidant. Oscillations in the BR mixtures were followed spectrophotometrically at 620 nm and the results are expressed as the inhibition time (in min).
Acetylcholinesterase/butyrylcholinesterase inhibitory activity
AChE/BuChE inhibitory activity measurements were carried out by using slightly modified Ellman method as described before for AChE inhibitory activity (Generalić Mekinić et al. 2013). The measurements were performed on a Tecan MicroPlate Reader, model Sunrise (Tecan Group Ltd., Männedorf, Switzerland). A typical run consisted of 180 μL of phosphate buffer (0.1 M, pH 8), 10 μL of DTNB (at a final concentration of 0.3 mM prepared in 0.1 M phosphate buffer pH 7 with 0.12 M sodium bicarbonate added for stability), 10 μL of sample solution (dissolved in EtOH), and 10 μL of AChE/BuChE solution (at a final concentration 0.03 U/mL). Reactants were mixed in a cuvette and reaction was initialized by adding 10 μL of acetylthiocholine iodide/butyrylthiocholine iodide (ATChI/BuTChI), at a final concentration of 0.5 mM. As a negative control EtOH was used instead of sample solution. Also non-enzymatic hydrolysis was monitored by measurement of two blank runs for each run. In short, in first blank mixture AChE/BuChE was replaced with equivalent buffer amount and in second blank mixture ATChI/BuTChI was replaced by equivalent buffer amount. All measurements were done spectrophotometrically at 409 nm and room temperature for 6 min period. The results are expressed as percentage inhibition of enzyme activity.
Vasodilatory activity
Vasodilatory activity was analyzed by measuring isometric force of the isolated vascular rings (force transducers FORT 10, World Precision Instruments, Berlin, Germany), linked to an amplifier QUAD Bridge and a computerized acquisition system MacLab/8e (ADInstruments, Castle Hill, Australia). The biological signals were processed by CHART 4.2.4. software (ADInstruments). The vasodilatory activity was determined in the isolated rat aortic rings (n = 20 male Sprague–Dawley rats) as previously described (Musić et al. 2005). The precontracted rings were randomly exposed to cumulative concentrations of EO, limonene and chlorogenic acid (1 mg/mL stock solution) as well as extract having 0.5 ‰ to 6 ‰ final dilutions in organ baths (n = 10 for all samples tested). The relaxation was expressed as the percentage decrease of the noradrenaline-induced vasoconstriction. The 80 % aqueous ethanol (v/v) which was used as solvent in preparation of sea fennel flowers extract had no vasodilatory effect.
Statistical analysis
Statistical analysis was performed using GraphPad InStat3 (GraphPad Software, San Diego, USA). For statistical analysis of vasodilatory activity, 1 and 2-way analysis of variance, followed by Bonferroni post hoc tests was used. Data are expressed as mean ± standard error (SEM). P < 0.05 was considered statistically significant. The tested samples differed significantly in total phenolic content so we used dilution, instead of concentration, to express the effective concentration, EC50, which was calculated using non-linear regression analysis.
Results and discussion
Chemical composition
Different parts of sea fennel (flowers, leaves and stems) were used for isolation of essential oils (EOs) and preparation of EtOH extracts. EOs, were analyzed by GC–MS, the phenolic content of extracts by Folin–Ciocalteu method, while the concentration of chlorogenic acid was evaluated using HPLC. The results are shown in Table 1.
Table 1.
Phytochemical composition of sea fennel
| No. | Component | RI | Flowers | Stems | Leaves |
|---|---|---|---|---|---|
| Essential oilsa | |||||
| Monoterpene hydrocarbons | |||||
| 1. | α-Pinene | 938 | 4.9 | 0.1 | 0.1 |
| 2. | Sabinene | 975 | 12.0 | 8.1 | 13.4 |
| 3. | β-Pinene | 990 | 0.1 | tr | tr |
| 4. | Limonene | 1039 | 62.2 | 74.2 | 57.5 |
| 5. | γ-Terpinene | 1065 | 13.8 | 4.6 | 12.0 |
| 6. | Terpinolene | 1089 | 0.3 | 1.1 | 1.5 |
| 7. | Alloocimene | 1136 | 0.9 | 1.8 | 3.5 |
| Oxygenated monoterpenes | |||||
| 8. | Terpinen-4-ol | 1192 | 2.0 | 5.9 | 6.9 |
| 9. | α-Terpineol | 1211 | 0.2 | 0.3 | 0.4 |
| 10. | Thymol methyl ether | 1238 | 0.2 | 0.4 | 0.3 |
| Total identified (%) | 96.6 | 96.5 | 95.6 | ||
| Yield (%) | 2.44 | 0.19 | 0.55 | ||
| EtOH extracts | |||||
| Total phenols (mg GAE/g of d.p.m.)b | 32.6 ± 1.0 | 7.6 ± 0.2 | 35.1 ± 0.2 | ||
| Chlorogenic acid (mg/g of d.p.m.)c | 7.7 ± 1.3 | 0.7 ± 0.0 | 8.1 ± 0.1 | ||
aAnalysed by GC–MS; RI = retention indices on VF-5MS column; tr—traces (<0.1 %)
bAnalysed spectrophotometrically by Folin–Ciocalteu method
cAnalysed by HPLC–DAD; d.p.m.—dry plant material
The yield of EO in flowers was 2.44 %, while the significantly lower amounts were isolated from leaves and stems, 0.55 and 0.19 %, respectively. This was in accordance with the results obtained by Pateira et al. (1999). Similar like in the previous studies, monoterpenes constituted the major fraction of the EOs (Senatore et al. 2000; Özcan et al. 2001; Kulišić-Bilušić et al. 2010; Jallali et al. 2014). Ten compounds, representing ca. 96 % of the EOs, were identified and in all samples limonene, followed by γ-terpinene and sabinene, were the major compounds. Other researchers also confirmed the limonene as major compound in sea fennel EOs (Pateira et al. 1999; Ruberto et al. 2000; Senatore et al. 2000; Özcan et al. 2001; Maleš et al. 2003; Kulišić-Bilušić et al. 2010). The highest percentage of limonene was found in stems (74.2 %) while its content in flowers and leaves was 62.2 and 57.5 %, respectively. According to this, Croatian and Sicilian sea fennel due to distinctly high content of limonene could be a special chemotype regarding those from Turkey, Portugal and Southern Italy (Pateira et al. 1999; Senatore et al. 2000; Özcan et al. 2001; Jallali et al. 2014). EOs from flowers and leaves were rich in γ-terpinene while the amount of this compound in stems was threefold lower.
While there are many reports on sea fennel EOs, the information on polar component, like phenolics and chlorogenic acid were scarce. The samples differed in the content of total phenolics (Table 1), extracts of leaves and flowers contained relatively high concentrations, 35.1 and 32.6 mg GAE/g of d.p.m., respectively, while in stems more than fourfold lower amount was detected. Meot-Duros and Magné (2009) and Jallali et al. (2014) reported on relatively high concentrations of phenolic compounds in sea fennel. These authors highlighted that the content of phenolics in sea fennel was affected by different biotic and/or abiotic factors that can influence the levels and the biosynthesis of these plant metabolites. In their study chlorogenic acid (5-caffeoylquinic acid) was the dominant phenolic compound, while Siracusa et al. (2011) even reported that chlorogenic acid, its isomers, and higher derivatives were identified as almost the sole class of phenolics in sea fennel infusions. Chlorogenic acid is a phenolic compound widespread in the plant kingdom with a broad spectrum of biological activities (Upadhyay and Mohan Rao 2013). Although it is not officially specified as a chemotaxonomic marker of the Apiaceae plant family (like rosmarinic acid in Lamiaceae plants), its presence, in relatively high amounts, in plants belonging to this botanical family was widely described in scientific literature (Pandey et al. 2012; Upadhyay and Mohan Rao 2013; etc.). In this study we explored the distribution of chlorogenic acid in flowers, leaves and stems. Its concentration ranged from 0.7 to 8.1 mg/g of d.p.m. with the highest amounts found in leaves and flowers. The presented results were in accordance with those previously described by Meot-Duros and Magné (2009) who reported that sea fennel was one of the most chlorogenic acid-rich Apiaceae plants. Similar observations could be made regarding the results on phenolics and chlorogenic acid content in other Apiaceae species (Pandey et al. 2012; Maulidiani et al. 2014).
Biological activities
Antioxidant activity
The reducing activity of the samples, as well as limonene and chlorogenic acid was tested by Ferric Reducing/Antioxidant Power (FRAP) assay, and the free radical scavenging activity was evaluated by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and Briggs–Rauscher (BR) method (Table 2). In order to draw conclusions about the activities of the dominant compounds on antioxidant capacity of EOs and extracts, limonene and chlorogenic acid at concentration of 1 mg/mL were also tested.
Table 2.
Antioxidant activity of sea fennel extracts and essential oils and its referent compounds
| Sample | FRAP (µmol Fe2+/L) | DPPH (inhibition %) | BR (min) | |
|---|---|---|---|---|
| Essential oils | Flowers | 22.0 ± 2.3 | 2.8 ± 0.0 | 0.3 ± 0.1 |
| Stems | 42.0 ± 2.3 | 2.8 ± 0.2 | 0.8 ± 0.0 | |
| Leaves | 8.4 ± 2.4 | 2.6 ± 0.2 | 0.6 ± 0.0 | |
| Ethanolic extracts | Flowers | 16065.6 ± 95.3 | 61.0 ± 3.8 | 49.2 ± 0.1 |
| Stems | 3009.4 ± 39.8 | 13.0 ± 0.7 | 1.7 ± 0.3 | |
| Leaves | 17335.0 ± 276.0 | 61.8 ± 3.8 | 68.0 ± 1.0 | |
| Referent compounds | Chlorogenic acid | 370.2 ± 6.9 | 91.6 ± 0.7 | 114.3 ± 2.5 |
| Limonene | 42.9 ± 2.8 | 2.7 ± 0.2 | 0.3 ± 0.0 |
EOs, limonene and chlorogenic are tested at concentration of 1 mg/mL (the final concentration in reaction system is 33 µg/mL), while the tested EtOH extracts (phenolic concentrations for flowers, stems and leaves were 32.6, 7.6 and 35.1 mg of gallic acid equivalents (GAE)/g of dry plant material, respectively) were tenfold diluted prior analysis. Values are expressed as mean ± standard deviation
The antioxidant activity of EOs was very low (Table 2), due to absence of phenolics. Previous studies used different methods for antioxidant evaluation of sea fennel EOs and showed that monoterpenic hydrocarbons were weak radical scavengers but have exhibited inhibitory activity on lipid oxidation in food and biological systems (Ruberto et al. 2000; Kulišić-Bilušić et al. 2010; Jallali et al. 2014).
The results of antioxidant activity obtained for ethanolic extracts are also presented in Table 2. It can be seen that the antioxidant capacity of extracts was significantly higher in comparison to the EOs. The best results using all three methods were obtained for leaves extract (FRAP value 17,335 μmol Fe2+/L; DPPH inhibition % of 61.8; BR inhibition 68 min), while the lowest activity was detected for stems. This is in accordance with the results of Houta et al. (2011) who reported on DPPH activity of sea fennel metanolic extracts.
In this study, beside well-known DPPH and FRAP assays, a relatively new BR method was also used. BR inhibition times ranged from 1.7 min for stems up to 68.0 min for leaves extract. The results for antiradical activity of extracts from flowers and leaves (obtained using both methods) reflect the high antioxidant potential of these plant organs. Siracusa et al. (2011) reported a very strong free radical scavenging activity of sea fennel infusions which was comparable with commercial antioxidants like BHT and BHA. The results obtained for pure chlorogenic acid which exhibited extremely high inhibition of DPPH radicals (91.6 %) and long inhibition of BR reactions (114.3 min) (Table 2). Previous studies confirmed the role of phenolic compounds as major antioxidants in plants, especially within halophytic species (Jallali et al. 2014). Chlorogenic acid is one of the most effective antioxidants from the group of phenolic acids. The vicinal hydroxyl groups on the aromatic ring of chlorogenic acid are believed to be responsible for good hydrogen donating capacity, reducing and radical scavenging activity (Sato et al. 2011; Saqib et al. 2016). Although, extracts of sea fennel flowers and leaves with the highest content of total phenolics and chlorogenic acid provided highest antioxidant activity and previous studies reported possible consequences of these compounds on plant extracts bioactivity (Meot-Duros and Magné 2009; Jallali et al. 2014); their good antioxidant activity could attributed to the presence of other antioxidative phytochemicals present in sea fennel with such as vitamin C, carotenoids, organic acids, etc.
Cholinesterases inhibitory activity
Enzyme acetylcholinesterase (AChE) represents an important target in the first stage of Alzheimer’s disease (AD) while butyrylcholinesterase (BuChE) enzyme is more important in the later stages of AD. The most promising approaches for treating Alzheimer’s disease are usually based on AChE and BuChE inhibitors (Wszelaki et al. 2010), and several medicinal plants have been reported to contain or possesses AChE and BuChE inhibitory activity (Mukherjee et al. 2007; Wszelaki et al. 2010; Generalić Mekinić et al. 2013).
In present study, sea fennel EOs and extracts were screened for their cholinesterase inhibitory activity (Table 3). Generally, all investigated samples exerted a better inhibition on AChE rather than on BuChE, while EOs were more effective in comparison with the extracts. The strongest AChE inhibitory activity, higher than 50 %, was found in EOs from stems while the lowest activity was observed for EO from leaves with the highest content of oxygenated monoterpenes. The hydrophobic active site of AChE is reported to be susceptible to hydrophobic interactions. According to the findings presented in Mukherjee et al. (2007) monoterpenes possess carbon skeleton which may contribute to their anti-cholinesterase activity. Limonene, a cyclic hydrocarbon, alone provided good inhibitory activity, especially on AChE (68.8 %), while moderate and threefold lower activity was detected on BuChE (21.2 %).
Table 3.
Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitory activity of sea fennel essential oils, extracts and its referent compounds
| Sample | AChE inhibition (%) | BuChE inhibition (%) | |
|---|---|---|---|
| Essential oils | Flowers | 44.4 ± 2.9 | 16.8 ± 0.6 |
| Stems | 65.2 ± 0.2 | 24.8 ± 1.0 | |
| Leaves | 38.3 ± 4.7 | 24.5 ± 0.9 | |
| Ethanolic extracts | Flowers | 24.0 ± 0.6 | 15.4 ± 1.1 |
| Stems | n.a. | 14.0 ± 0.4 | |
| Leaves | 27.2 ± 0.9 | 19.6 ± 0.8 | |
| Reference compounds | Chlorogenic acid | 48.1 ± 1.5 | 29.8 ± 0.7 |
| Limonene | 68.8 ± 0.6 | 21.2 ± 1.6 |
EOs and limonene were tested at concentration of 1 mg/mL (the final concentration in reaction system is 33 µg/mL), while chlorogenic acid was tested at concentration of 5 mg/mL (the final concentration in the reaction mixture is 227 μg/mL). n.a.—no activity. Values are expressed as mean ± standard deviation
The most active ethanolic extract was from sea fennel leaves (AChE inhibition was 27.2 %), which can be attributed to the high concentration of the chlorogenic acid, but the presence of other biologically active compounds should not be neglected. Pure chlorogenic acid, at a concentration of 1 mg/mL, showed inhibition on AChE of 48.1 % while it was found to exhibit weaker inhibitory effect against BuChE. Leaves extract, having the highest share of phenolics, showed the best BuChE inhibition (19.6 %). Contrary, having the lowest phenolic content, stems extract showed the weakest inhibitory activity (below 15 %).
The similarity in the molecular weight and hydrophobic moieties in the chemical structure of phenolics with the well-known anti-cholinesterase inhibitors (tacrine, donepezil, galantamine, etc.) may explain the observed anti-cholinesterase inhibitory activity.
Vasodilatory activity
Aqueous or alcoholic (ethanol or methanol) plant extracts are the most commonly used for testing vasodilatory activity of plants, while organic solvents are rarely used. However, number of studies, concerning vasodilatory effects of various essential oils and their constituents, is increasing (Magalhães et al. 2008; Pereira et al. 2013).
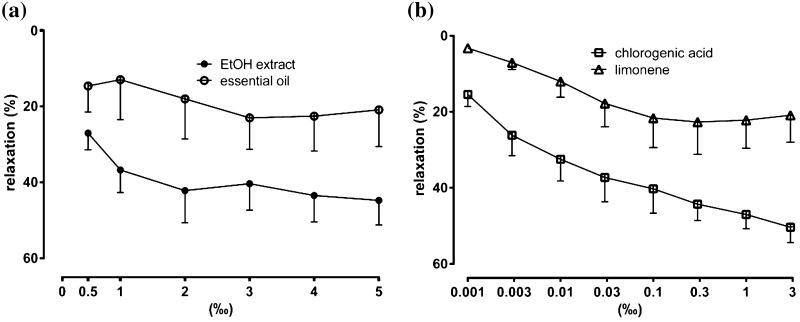
Vasoactive effects were screened in the noradrenaline-precontracted rat aortic rings (Fig. 1). Basal tension of the rat aortic rings (n = 40) following exposure to noradrenaline was 16.6 ± 0.8 mN. All examined samples and compounds showed similar vasodilator potency (EC50 of 1.1 ‰ and 2.0 ‰ for EtOH extract and EO, respectively; 0.004 ‰ and 0.007 ‰ for chlorogenic acid and limonene, respectively). The maximal vasodilatory effect (Emax) for the EO was 23.0 ± 8.3 %, while for EtOH extract was 44.7 ± 6.5 % (Fig. 1a). Pure limonene and chlorogenic acid showed dose-dependent and direct vasodilatory activity until reaching Emax (22.7 ± 8.6 % and 50.4 ± 4.1 %, respectively) (Fig. 1b).
Fig. 1.
Vasodilatory activity of: a sea fennel flower essential oil and ethanolic extract; b chlorogenic acid and limonene. Vasodilatory activity is expressed as the percentage decrease of the noradrenaline-precontracted rat aortic rings. Results are shown as mean ± standard error (SEM) (n = 10)
The vasodilatory effect of EO was relatively weak and similar to the activity of its main constituent, limonene. Till date vasodilatory activity of limonene was not evaluated. However, other monoterpenes compounds like terpinen-4-ol, carvacrol and thymol were shown to have vasodilatory activity but in much higher concentrations (up to 6000 µM) in comparison to limonene, which was 70 microM and appeared to be several fold lower from other monoterpenes (70 µM) (Peixoto-Neves et al. 2010; Maia-Joca et al. 2014). Selected concentration range was used to roughly “cover” concentrations that are feasible with the in vivo conditions (Miller et al. 2013).
When comparing the vasodilatory effectiveness of the EtOH extract and chlorogenic acid we can see that they are almost equally strong vasodilators. Since EtOH extract is rich with chlorogenic acid, its strong vasodilatory activity can be, at least partially, attributed to this phenolic compound, but the overall effect of the EtOH extract could be affected by other present phenolics. It was previously shown that a synergistic effect of a blend of different phenolics is responsible for maximal induction of endothelial NO synthase in human endothelial cells (Wallerath et al. 2005). Also, some non-phenolic compounds could affect vasodilatory activity. The inactivation of nitric oxide by oxygen free radicals, which contributes to endothelial dysfunction in essential hypertension, is decreased by vitamin C. However, vasodilatory activity of numerous plant extracts is strongly correlated with their content of total phenolics, whereas there is no such correlation with the content of individual phenolic compound. In the previous study, among 9 tested phenolic acids, gallic acid, an excellent antioxidant, was shown to have no vasodilatory effects, whereas caffeic acid was found to be the “strongest” vasodilator (Mudnić et al. 2010). In this study, chlorogenic acid, an ester of caffeic and quinic acid, was shown to have two fold higher Emax than caffeic acid. Vasodilatory effect of chlorogenic acid in vivo was also shown in animals (Taguchi et al. 2014) and its hypotensive effects in humans (Mubarak et al. 2012).
Conclusions
The EOs and extracts from different parts of Croatian sea fennel, with high content of phytochemicals, limonene and chlorogenic acid, were found to have different impact on investigated biological properties. EOs were found to be very effective against cholinesterase enzymes. On the other hand, sea fennel flower extract showed to have good vasodilatory activity. These properties can imply the potential for use of sea fennel, especially flowers and stems which utilization is not so traditional, in food, pharmaceutical and other industries.
Acknowledgments
This work has been fully supported by Croatian Science Foundation under the projects IP-2014-09-6897 and IP-2013-11-8652.
References
- Atia A, Barhoumi Z, Mokded R, Abdelly C, Smaoui A. Enviromental eco-physiology and economical potential of the halophtye Crithmum maritimum L. (Apiaceae) J Med Plants Res. 2011;5:3564–3571. [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Frankie W. Vitamin C in sea fennel (Crithmum maritimum), an edible wild plant. Econ Bot. 1982;36:163–165. doi: 10.1007/BF02858711. [DOI] [Google Scholar]
- Generalić Mekinić I, Burčul F, Blažević I, Skroza D, Kerum D, Katalinić V. Antioxidative/acetylcholinesterase inhibitory activity of some Asteraceae plants. Nat Prod Commun. 2013;8:471–474. [PubMed] [Google Scholar]
- Generalić Mekinić I, Skroza D, Ljubenkov I, Šimat V, Smole Možina S, Katalinić V. In vitro antioxidant and antibacterial activity of Lamiaceae phenolic extracts: a correlation study. Food Technol Biotechnol. 2014;52:119–127. [Google Scholar]
- Guil-Guerrero JL, Rodriguez-Garcia I. Lipid classes, fatty acids and carotenes of the leaves of six edible wild plants. Eur Food Res Technol. 1999;209:313–316. doi: 10.1007/s002170050501. [DOI] [Google Scholar]
- Houta O, Akrout A, Neffati M, Amri H. Phenolic contents, antioxidant and antimicrobial potentials of Crithmum maritimum cultivated in Tunisia Arid zones. J Biol Act Prod Nat. 2011;1:138–143. [Google Scholar]
- Jallali I, Zaouali Y, Missaoui I, Smeoui A, Abdelly C, Ksouri R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 2014;145:1031–1038. doi: 10.1016/j.foodchem.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Katalinić V, Smole Možina S, Generalić I, Skroza D, Ljubenkov I, Klančnik A. Phenolic profile, antioxidant capacity and antimicrobial activity of crude leaf extracts of six Vitis vinifera L. varieties. Int J Food Prop. 2013;16:45–60. doi: 10.1080/10942912.2010.526274. [DOI] [Google Scholar]
- Kulišić-Bilušić T, Blažević I, Dejanović B, Miloš M, Pifat G. Evaluation of the antioxidant activity of essential oils from caper (Capparis spinosa) and sea fennel (Crithmum maritimum) by different methods. J Food Biochem. 2010;34:286–302. doi: 10.1111/j.1745-4514.2009.00330.x. [DOI] [Google Scholar]
- Magalhães PJ, Lahlou S, Jucá DM, Coelho-de-Souza LN, da Frota PT, da Costa AM, Leal-Cardoso JH. Vasorelaxation induced by the essential oil of Croton nepetaefolius and its constituents in rat aorta are partially mediated by the endothelium. Fundam Clin Pharmacol. 2008;22:169–177. doi: 10.1111/j.1472-8206.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- Maia-Joca RP, Joca HC, Ribeiro FJ, do Nascimento RV, Silva-Alves KS, Cruz JS, Coelho-de-Souza AN, Leal-Cardoso JH. Investigation of terpinen-4-ol effects on vascular smooth muscle relaxation. Life Sci. 2014;115:52–58. doi: 10.1016/j.lfs.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Maleš Ž, Žuntar I, Nigović B, Plazibat M, Bilušić Vundač V. Quantitative analysis of the polyphenols of the aerial parts of rock samphire—Crithmum maritimum L. Acta Pharm. 2003;53:139–144. [PubMed] [Google Scholar]
- Maulidiani Abas F, Khatib A, Shaari K, Lajis NH. Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Ind Crops Prod. 2014;55:238–247. doi: 10.1016/j.indcrop.2014.02.013. [DOI] [Google Scholar]
- Meot-Duros L, Magné C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol Biochem. 2009;47:37–41. doi: 10.1016/j.plaphy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Miller JA, Lang JE, Ley M, Nagle R, Hsu CH, Thompson PA, Cordova C, Waer A, Chow HH. Human breast tissue disposition and bioactivity of limonene in women with early stage breast cancer. Cancer Prev Res. 2013;6:577–584. doi: 10.1158/1940-6207.CAPR-12-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarak A, Bondonno CP, Liu AH, Considine MJ, Rich L, Mas E, Croft KD, Hodgson JM. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: a randomized trial. J Agric Food Chem. 2012;60:9130–9136. doi: 10.1021/jf303440j. [DOI] [PubMed] [Google Scholar]
- Mudnić I, Modun D, Rastija V, Vuković J, Brizić I, Katalinić V, Kozina B, Medić-Šarić M, Boban M. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010;119:1205–1210. doi: 10.1016/j.foodchem.2009.08.038. [DOI] [Google Scholar]
- Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Musić I, Modun D, Katalinić V, Salamunić I, Kozina B, Boban M. Effects of four weeks moderate drinking of red wine and ethanol on the rat isolated heart and aortic rings reactivity during ischemia and hypoxia. Period Biol. 2005;107:165–173. [Google Scholar]
- Özcan M, Akgül A, Başcr KHC, Özck T, Tabanca N. Essential oil composition of sea fennel (Crithmum maritimum) form Turkey. Nahrung/Food. 2001;45:353–356. doi: 10.1002/1521-3803(20011001)45:5<353::AID-FOOD353>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pandey MM, Vijayakumar M, Rastogi S, Rawat AKS. Phenolic content and antioxidant properties of selected Indian spies of Apiaceae. J Herbs Spices Med Plants. 2012;18:246–256. doi: 10.1080/10496475.2012.680548. [DOI] [Google Scholar]
- Pateira L, Nogueira T, Antunes A, Venâncio F, Tavares R, Capelo J. Two chemotypes of Crithmum maritimum L. from Portugal. Flavour Fragr J. 1999;14:333–343. doi: 10.1002/(SICI)1099-1026(199909/10)14:5<333::AID-FFJ839>3.0.CO;2-V. [DOI] [Google Scholar]
- Peixoto-Neves D, Silva-Alves KS, Gomes MD, Lima FC, Lahlou S, Magalhães PJ, Ceccatto VM, Coelho-de-Souza AN, Leal-Cardoso JH. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam Clin Pharmacol. 2010;24:341–350. doi: 10.1111/j.1472-8206.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- Pereira SL, Marques AM, Sudo RT, Kaplan MA, Zapata-Sudo G. Vasodilator activity of the essential oil from aerial parts of Pectis brevipedunculata and its main constituent citral in rat aorta. Molecules. 2013;18:3072–3085. doi: 10.3390/molecules18033072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M, Gonnella M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int J Gastron Food Sci. 2012;1:111–115. doi: 10.1016/j.ijgfs.2013.06.004. [DOI] [Google Scholar]
- Ruberto G, Tiziana Baratta M, Deans SG, Damien Dorman HJ. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oil. Planta Med. 2000;66:687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- Saqib M, Iqbal S, Mahmood A, Akram R. Theoretical investigation for exploring the antioxidant potential of chlorogenic acid: a density functional theory study. Int J Food Prop. 2016;19:745–751. doi: 10.1080/10942912.2015.1042588. [DOI] [Google Scholar]
- Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;403:136–1388. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Senatore F, Napolitano F, Özcan M. Composition and antibacterial activity of the essential oil from Crithmum maritimum L. (Apiaceae) growing wild in Turkey. Flavour Fragr J. 2000;15:186–189. doi: 10.1002/1099-1026(200005/06)15:3<186::AID-FFJ889>3.0.CO;2-I. [DOI] [Google Scholar]
- Singh B, Beg S, Lohan S, Kapil R. Crossing blood-brain barriers using drug delivery: a successful venture using lipidic nanostructured systems. Pharm Rev. 2013;21:41–47. [Google Scholar]
- Siracusa L, Kulisic-Bilusic T, Politeo O, Krause I, Dejanovic B, Ruberto G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J Agric Food Chem. 2011;59:12453–12459. doi: 10.1021/jf203096q. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Hida M, Matsumoto T, Ikeuchi-Takahashi Y, Onishi H, Kobayashi T. Effect of short-term polyphenol treatment on endothelial dysfunction and thromboxane A2 levels in Streptozotocin-induced diabetic mice. Biol Pharm Bull. 2014;37:1056–1061. doi: 10.1248/bpb.b14-00157. [DOI] [PubMed] [Google Scholar]
- Upadhyay R, Mohan Rao L. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit Rev Food Sci Nutr. 2013;53:968–984. doi: 10.1080/10408398.2011.576319. [DOI] [PubMed] [Google Scholar]
- Wallerath T, Li H, Godtel-Ambrust U, Schwarz PM, Forstermann U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide. 2005;12:97–104. doi: 10.1016/j.niox.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Wszelaki N, Kuciun A, Kiss AK. Screening of traditional European herbal medicines for acetylcholinesterase and butyrylcholinesterase inhibitory activity. Acta Pharm. 2010;60:119–128. doi: 10.2478/v10007-010-0006-y. [DOI] [PubMed] [Google Scholar]