Abstract
To explore the potential of the large amount of grape pomace in wineries of China, oils of three Eurasian grape cultivars (Chardonnay, Merlot and Carbernet Sauvignon) and two Chinese traditional grape cultivars (Vitis amurensis and Vitis davidii), were characterised. The results showed seed oil properties differ for various grape varities. Grape seed oils were demonstrated to be good sources of polyunsaturated fatty acid (PUFA) (63.88–77.12 %), sterols (227.99–338.83 mg/100 g oil) and tocotrienols (320.08–679.24 mg/kg oil). Seed oil of V. amurensis exhibited the highest values of polyunsaturated fatty acid, total tocotrienols, total tocols and DPPH· scavenging capacity. Seed oil of Carbernet Sauvignon had the highest contents of squalene, total sterols, total tocopherols and total phenolics. Principal component analysis five grape cultivars differentiated on the basis of bioactive components content and antioxidant properties.
Keywords: Grape seed oil, Vitis davidii, Vitis amurensis, Sterols, Vitamin E, DPPH·
Introduction
Grape is one of the most important fruits grown in China. In 2013, China produced 11,650,024 tons of grapes, being the world’s largest grape producer, which accounts for 15.09 % of the total world production (FAOSTAT 2013). With increasing wineries year by year in China, more and more grape pomace is producing. Grape pomace disposal has become a serious issue, as discharging directly or using as feedstuff is usually adopted, which caused serious environmental pollution and resource waste.
Dry pomace accounts for nearly 25 % (w/w) of grapes, of which about 38 % (w/w) is seed (Rice 1976). The seeds were reported to contain 3.95–20.71 % oil according to different grape varieties (Beveridge et al. 2005; Demirtas et al. 2013; Fernandes et al. 2013). Grape seed oil is rich in unsaturated fatty acids, especially linoleic acid (C18:2) and oleic acid (C18:1), which are beneficial to human cardiovascular health (Wijendran and Hayes 2004; Beveridge et al. 2005; Crews et al. 2006). Besides, grape seed oil contains an appreciable amount of vitamin E (tocopherols and tocotrienols), sterols, phenolics and other bioactive compounds, which all contribute to the antioxidant properties (Bail et al. 2008; Passos et al. 2010; Lutterodt et al. 2011). Vitamin E also possesses neuroprotective and antitumor activities (Khanna et al. 2003; Nesaretnam et al. 2007). Moreover, sterols show important anticancer and cholesterol-lowering effects (Awad and Fink, 2000; Piironen et al. 2000). Therefore, it is a good option to dispose grape pomace by extracting seed oil, which will lower costs on oil production and increase output value of wine industry. The grape seed oil can be applied to food, pharmaceutical and cosmetic industries (Sabir et al. 2012).
To the best of our knowledge, most of the studies have been undertaken using grapes cultivated in Turkey (Demirtas et al. 2013), Portugal (Passos et al. 2010; Fernandes et al. 2013), Canada (Beveridge et al. 2005), France (Crews et al. 2006), Italy (Crews et al. 2006), Spain (Crews et al. 2006; Pardo et al. 2009; Rubio et al. 2009), Austria (Bail et al. 2008) and the United States (Lutterodt et al. 2011). However, there is limited information about grape seed oils extracted from grape cultivars cultivated in China, which is an important producer of grapes according to FAO. Five principle grape varieties cultivated in China were selected, including three Eurasian grape cultivars (Chardonnay, Merlot and Carbernet Sauvignon) and two Chinese traditional cultivars (Vitis amurensis and Vitis davidii). The main objective of this study is to characterise composition and antioxidant properties of oils from seed of five different cultivars grown in China.
Materials and methods
Materials
Five different varieties of grape pomace were collected from wineries in China during the fall of 2013, including one white variety (Chardonnay) and four red varieties (Merlot, Carbernet Sauvignon, V. davidii and V. amurensis). The days between wine extraction and beginning of drying were uniform for all samples. The seeds were separated from pomace and spread on gauze for drying at ambient temperature (20 °C) for 8 days. The seeds were turned over regularly to achieve uniform drying with ultimate moisture content of about 10 %. Then the seeds were vacuum packaged with polyethylene bags and stored at −18 °C until extracting process.
Extraction of grape seed oils
The seeds were ground using a mill and screened through mesh size. After that, the seeds were subjected to supercritical carbon dioxide extraction. CO2 used in the extractions was 99.99 % pure (Beijing Qianxi Gases Company, Beijing, China). The conditions of SFE were optimized by respond surface methodology in previous experiments, the optimum condition was: extraction pressure of 28 MPa, extraction temperature of 45 °C, CO2 flow rate of 25 kg/h, separation pressure of 6 MPa, separation temperature of 50 °C and extraction time of 75 min. The oil yields were calculated through weighing the obtained oil in percent of dry grape seed flour. The oils were stored under nitrogen at −60 °C prior to analysis.
Reagents and standards
Boron trifluoride-methanol solution (14 %), 5α-cholestane, BSTFA + TMCS (99:1), gallic acid (purity ≥97.5 %), anhydrous pyridine (99.8 %), DPPH·, standards of fatty acid methyl esters, sterols, tocopherols and tocotrienols were purchased from Sigma Aldrich company (St. Louis, MO, USA). Folin–Ciocalteu reagent was purchased from Tianjin Jinke Institute of Fine Chemicals Division (Tianjin, China). All of the reagents used were of analytical grade.
Fatty acids analysis
Fatty acids of different grape seed oils were measured as described by Beveridge et al. (2005) with slight modifications. Grape seed oil (30mg) was reacted with 1 mL methanolic KOH (1 M) for 1 min at ambient temperature followed by reacting with 1 mL 14 % BF3-methanol for 15 min at 95 °C. The mixture was cooled and 0.5 mL of saturated NaCl was added followed by 1 mL n-hexane. The upper layer was used directly for gas chromatography (GC) by a Bruker SCION 456-GC equipped with a FID. A RTX-Wax column (30 m × 0.25 mm with a 0.25 μm film thickness) was used with nitrogen as the carrier gas at a flow rate of 1 mL/min. Samples (1 μL) were injected by a CP-8400 autoinjector at a split ratio of 20:1. The oven temperature program comprised an initial temperature of 140 °C for 2 min, followed by an increase to 190 °C at 10 °C/min and another ramp to 240 °C at 4 °C/min, then held at 240 °C for 5 min. Injector and detector temperatures were 260 °C. Results were reported as peak area percent of total area of all fatty acid peaks. All samples were analysed in duplicate.
Sterols analysis
Sterols of different grape seed oils were detected according to the method described by Shin et al. (2010) with slight modifications. 0.5 g of grape seed oil was weighed into round-bottom flask with 1 mL internal standard (5α-cholestane, 0.2 mg/mL) and then evaporated to dryness. 10 mL of ethanol containing 3 % (w/v) pyrogallol and 1 mL of saturated KOH solution were added. The mixture was vortexed for 1 min and saponified at 80 °C for 30 min purging with N2. The solution was extracted with 20 mL n-hexane for three times and washed with distilled water until the washes with a neutral pH, followed by drying with anhydrous sodium sulfate. The hexane fraction was evaporated to dryness at 40 °C by rotary vacuum evaporation. TMS ether derivatives were prepared by adding 100 μL of anhydrous pyridine and 100 μL of BSTFA + TMCS (99:1) and vortexing. Then they were transferred to vials for GC–MS analysis. The TMS-sterols were determined by an Agilent 7890A GC with an Agilent 5975C inert XL mass selective detector. The conditions were as follows: a DB-5 column (30 m × 0.25 mm with a 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA); 1.0 μL of injection volume with a Gerstel MPS 2 XL multi-purpose sampler; a split ratio of 15:1; helium as the carrier gas at a flow rate of 1 mL/min; an initial oven temperature of 260 °C for 5 min followed by a temperature ramp to 280 °C and held for 30 min; injector temperature were set at 280 °C; a scan range of 50.00–600.00 amu; transfer line temperature was set at 250 °C; an ion source temperature of 230 °C; an electron ionization energy of 70 eV. Different concentrations of the external standards (squalene, campesterol, stigmasterol, β-Sitosterol, sitostanol, △5-avenasterol) and internal standard (5α-cholestane) were derivatized as described above. Sterols of grape seed oil were quantified by constructing standard curves of external standard-internal standard concentration ratio and peak area ratio. All samples were analysed in triplicate.
Vitamin E analysis
Vitamin E composition of different grape seed oils was determined according to a modification of AOCS Official Method Ce 8-89 (1998) using a Shimadzu HPLC system equipped with a RF-10A XL fluorescence detector. 0.15 g of grape seed oil was weighed and diluted with n-hexane to 10 mL. The solution was vortexed and filtered through 0.45 μm pore size syringe filter. Then 10 μL of this solution was injected to HPLC by a SIL-20A autosampler. Separation was achieved by a normal phase Venusil Diol column (4.6 mm × 250 mm, 5 μm) and isocratic mobile phase of n-hexane/2-propanol (99.5:0.5, v/v) at 1.0 mL/min flow rate. Excitation wavelength was set at 290 nm and emission wavelength at 330 nm. Tocopherols and tocotrienols were quantified by external standard method. All samples were run in triplicate.
Extraction of phenolic compounds
3 g of grape seed oil was extracted three times with 3 mL methanol as described by Parry et al. (2006). The combined methanol extract was vacuum evaporated and dissolved in 1 mL of methanol. The solution was kept under nitrogen in the dark prior to further analysis.
Total phenolic content determination
The total phenolics were determined according to a modified Folin–Ciocalteu antioxidant capacity assay (Berker et al. 2013). 200 μL of methanol extract was added to 300 μL of Folin–Ciocalteu reagent (diluted at a volume ratio of 1:2 with isobutanol), followed by 3.5 mL of 0.1 M aqueous NaOH, then diluted to 10 mL with distilled water. After 20 min of reaction at ambient temperature, the absorbance was read at 665 nm. The total phenolics were quantified according to the standard curve of gallic acid and expressed as mg gallic acid equivalents (GAE) per kg grape seed oil. All samples were carried out in triplicate.
DPPH· scavenging capacity
The radical scavenging capacity of samples was assessed against the stable radical DPPH· by a kinetic approach (Terpinc and Abramovič 2010; Chen et al. 2014). 200 μL of methanol extract was added to 3.8 mL 0.1 mmol/L DPPH· ethanol solution and shaken vigorously. The absorbance was measured at 517 nm at regular time intervals for 30 min. A control reaction was prepared by 200 μL of methanol extract adding to 3.8 mL ethanol, and a blank reaction with 200 μL of methanol adding to 3.8 mL 0.1 mmol/L DPPH· ethanol solution. The radical scavenging ability was calculated using the following equations:
where As(t=x) is the absorbance of sample at t = x s; Ac and Ab are the absorbances of control and blank at 30 min, respectively. All samples were run in triplicate.
Statistical analysis
Data were reported as the mean ± standard deviation (SD). ANOVA and Duncan’s Multiple Range Tests were carried out using the software SAS 8.0 (SAS Institute, Cary, NC, USA) at a significance level of P < 0.05.
Results and discussion
Oil yields
The oil yields of different grape seeds are shown in Table 1. The SFE extraction yields of various grape seed cultivars ranged from 13.71 to 15.92 % with significant differences (P < 0.05), which were higher than those (5.85-13.6 %) reported by Beveridge et al. (2005). Merlot variety showed the highest oil yield while Chardonnay (white grape variety) showed the lowest oil yield.
Table 1.
Oil yields and fatty acid composition of different grape seed oils
| Chardonnay | Merlot | Carbernet Sauvignon | Vitis amurensis | Vitis davidii | |
|---|---|---|---|---|---|
| Oil yields (g/100 g dry seed flour) | 13.71 ± 0.04e | 15.92 ± 0.05a | 14.45 ± 0.03d | 15.73 ± 0.03b | 15.08 ± 0.04c |
| Fatty acid (% of total FAME) | |||||
| C14:0 | 0.04 ± 0.00c | 0.04 ± 0.00c | 0.05 ± 0.00b | 0.02 ± 0.00d | 0.08 ± 0.00a |
| C16:0 | 6.67 ± 0.02d | 7.04 ± 0.02c | 7.54 ± 0.05b | 6.56 ± 0.02e | 8.55 ± 0.01a |
| C16:1 | 0.15 ± 0.00b | 0.13 ± 0.00c | 0.15 ± 0.00b | 0.13 ± 0.00c | 0.26 ± 0.00a |
| C17:0 | 0.05 ± 0.00b | 0.06 ± 0.00b | 0.06 ± 0.00b | 0.08 ± 0.00a | 0.08 ± 0.00a |
| C18:0 | 3.46 ± 0.00c | 3.59 ± 0.03b | 3.39 ± 0.03c | 2.06 ± 0.01d | 4.59 ± 0.02a |
| C18:1 | 18.34 ± 0.01b | 15.01 ± 0.04c | 14.43 ± 0.04d | 13.63 ± 0.00e | 22.03 ± 0.02a |
| C18:2 | 70.47 ± 0.01c | 73.26 ± 0.08b | 73.39 ± 0.08b | 76.77 ± 0.05a | 63.52 ± 0.04d |
| C18:3 | 0.35 ± 0.00c | 0.39 ± 0.00b | 0.46 ± 0.02a | 0.35 ± 0.00c | 0.35 ± 0.00c |
| C19:0 | 0.03 ± 0.01a | 0.02 ± 0.00a | 0.02 ± 0.00a | 0.02 ± 0.00a | 0.04 ± 0.02a |
| C20:0 | 0.15 ± 0.00c | 0.17 ± 0.01b | 0.17 ± 0.01b | 0.11 ± 0.00d | 0.21 ± 0.00a |
| C20:1 | 0.21 ± 0.00bc | 0.23 ± 0.00a | 0.22 ± 0.01ab | 0.20 ± 0.00c | 0.24 ± 0.01a |
| C22:0 | 0.06 ± 0.00ab | 0.05 ± 0.00b | 0.08 ± 0.01a | 0.06 ± 0.01ab | 0.04 ± 0.00b |
| C24:0 | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.03 ± 0.01a | 0.01 ± 0.00b | 0.01 ± 0.00b |
| SFA | 10.47 ± 0.01d | 10.98 ± 0.04c | 11.34 ± 0.07b | 8.92 ± 0.05e | 13.60 ± 0.01a |
| MUFA | 18.71 ± 0.00b | 15.36 ± 0.04c | 14.81 ± 0.03d | 13.96 ± 0.00e | 22.53 ± 0.03a |
| PUFA | 70.82 ± 0.00c | 73.66 ± 0.08b | 73.85 ± 0.09b | 77.12 ± 0.05a | 63.88 ± 0.04d |
* Different letters in the same row indicate significant difference (P < 0.05)
Fatty acids
Fatty acid composition of grape seed oils is shown in Table 1. Thirteen kinds of fatty acids were detected in grape seed oil samples. Significant differences (P < 0.05) were observed among various grape varieties. Results revealed that grape seed oil was mainly composed of unsaturated fatty acids which account for 86.41–91.08 % of total fatty acids. The most abundant fatty acid was linoleic acid (C18:2) ranging from 63.52 to 76.77 %, followed by oleic acid (C18:1) (13.63–22.03 %), palmitic acid (C16:0) (6.56–8.55 %) and stearic acid (C18:0) (2.06–4.59 %). These values were in accordance with those reported in literature (Beveridge et al. 2005; Crews et al. 2006; Pardo et al. 2009; Rubio et al. 2009; Lutterodt et al. 2011; Fernandes et al. 2013). Interestingly, the highest linoleic acid content was found in V. amurensis (76.77 %), followed by Carbernet Sauvignon, Merlot, Chardonnay, and the lowest in V. davidii (63.52 %), while oleic acid contents was in reverse order with the highest for V. davidii (22.03 %) and the lowest for V. amurensis (13.63 %). This result verified the observation of Pardo et al. (2009) found in different Spain grape seed oils and Sabir et al. (2012) found in different Turkey grape seed oils. V. davidii exhibited obviously higher monounsaturated fatty acid (MUFA) content and lower polyunsaturated fatty acid (PUFA) content than other varieties. Differences in grape seed oil composition may be caused by different cultivars of grape seed. Sabir et al. (2012) drew the same conclusion that differences of Turkey grape seed oils may be the result of different cultivation conditions as well as cultivar aptitude. Beveridge et al. (2005) also observed variation due to different grape varieties grown in Canada.
Sterols and squalene
Squalene and sterols are the major unsaponifiable matters in grape seed oils. Five kinds of sterols, including campesterol, stigmasterol, β-sitosterol, sitostanol and △5-avenasterol, were identified in grape seed oils and quantified by corresponding sterol standards (Table 2). The plant sterols were demonstrated to be able to lower blood cholesterol by inhibiting cholesterol absorption (Abuajah et al. 2015). The total sterols content ranged from 227.99 to 338.83 mg/100 g oil, which consisted with the values reported by Rubio et al. (2009) (368.6 mg/100 g), Pardo et al. (2009) (241.7–311.0 mg/100 g) and Demirtas et al. (2013) (267.2–300.0 mg/100 g). Carbernet Sauvignon showed obviously higher sterol contents (338.83 mg/100 g) than other cultivars (P < 0.05), while the other cultivars have no significant difference (P > 0.05) in total sterol contents. The sterol composition of grape seed oil was in agreement with that found by other researchers with β-sitosterol as the most abundant sterol, followed by stigmasterol, campesterol, sitostanol and △5-avenasterol. Carbernet Sauvignon showed the highest individual sterols among all varieties except for △5-avenasterol, and V. amurensis exhibited the highest △5-avenasterol value.
Table 2.
Squalene and sterols of seed oils extracted from different grape varieties
| Sterols (mg/100 g oil) | Chardonnay | Merlot | Carbernet Sauvignon | Vitis amurensis | Vitis davidii |
|---|---|---|---|---|---|
| Squalene | 10.20 ± 0.68e | 10.96 ± 0.04d | 16.29 ± 0.28a | 14.17 ± 0.13c | 15.22 ± 0.08b |
| Campesterol | 21.23 ± 0.22d | 24.83 ± 1.09c | 30.40 ± 1.25a | 26.75 ± 0.77b | 22.67 ± 0.34d |
| Stigmasterol | 41.48 ± 1.16b | 40.67 ± 0.73b | 47.92 ± 1.23a | 39.41 ± 1.91b | 30.14 ± 0.36c |
| β-Sitosterol | 150.87 ± 6.90b | 150.95 ± 5.23b | 230.64 ± 9.11a | 146.77 ± 9.99b | 155.31 ± 2.45b |
| Sitostanol | 9.69 ± 0.19c | 11.90 ± 0.38b | 19.53 ± 0.99a | 11.64 ± 0.72b | 12.37 ± 0.44b |
| △5-Avenasterol | 4.73 ± 0.24d | 5.46 ± 0.17d | 10.33 ± 0.60b | 20.59 ± 1.22a | 7.73 ± 0.52c |
| Total sterols | 227.99 ± 8.17b | 233.80 ± 6.74b | 338.83 ± 12.94a | 245.15 ± 14.42b | 228.22 ± 3.35b |
* Different letters in the same row indicate significant difference (P < 0.05)
Squalene was also determined when detecting sterols by GC–MS. He et al. (2003) reported that the squalene in vegetable oils decreased risk for various cancers and reducing serum cholesterol levels. Squalene contents ranges from 10.20 to 16.29 mg/100 g oil in different grape seed oils, which were consistent with the squalene content in sunflowerseed oil (9.2 mg/100 g) and soybean oil (12.5 mg/100 g) (Nergiz and Çelikkale 2011). Significant differences (P < 0.05) were observed among different cultivars. Carbernet Sauvignon seed oil exhibited the highest squalene content, and Chardonnay exhibited the lowest squalene content than all other red varieties.
Vitamin E
Vitamin E composition of oil from different grape varieties was showed in Table 3. Four isomers of tocopherol and four isomers of tocotrienol were detected in different grape seed oils. The total vitamin E content ranged from 397.75 to 755.79 mg/kg in which tocotrienols accounted for 68.4–89.9 %. The most abundant tocol form was α-tocotrienol in the range of 177.77–521.11 mg/kg, followed by γ-tocotrienol (128.87–183.70 mg/kg) and α-tocopherol (50.80–131.34 mg/kg). The α-tocotrienol values were comparable with those reported by Beveridge et al. (2005) (102–228 mg/kg), Fernandes et al. (2013) (69–319 mg/kg) and Demirtas et al. (2013) (137–264 mg/kg), except for that observed for V. amurensis. The γ-tocotrienol values were similar to those reported for Demirtas et al. (2013) (75–322 mg/kg), but they were found in lower concentration compared to those reported by Beveridge et al. (2005) (217–383 mg/kg) and Fernandes et al. (2013) (499–1575 mg/kg). This may be ascribed to the differences in grape seed variety and cultivation conditions. The highest total tocols and total tocotrienols content were found in V. amurensis, while the highest total tocopherols content was observed for white Chardonnay exhibited the lowest total tocols and total tocotrienols contents than all other red varieties.
Table 3.
Vitamin E composition of seed oils from different grape varieties
| Tocols (mg/kg oil) | Chardonnay | Merlot | Carbernet Sauvignon | Vitis amurensis | Vitis davidii |
|---|---|---|---|---|---|
| α-Tocopherol | 59.19 ± 1.32d | 90.00 ± 1.28b | 131.34 ± 1.26a | 66.95 ± 0.22c | 50.80 ± 0.54e |
| α-Tocotrienol | 177.77 ± 3.92e | 239.95 ± 3.82c | 201.59 ± 1.90d | 521.11 ± 4.35a | 277.69 ± 4.93b |
| β-Tocopherol | 0.86 ± 0.05d | 1.75 ± 0.06c | 2.53 ± 0.10a | 2.09 ± 0.08b | 0.25 ± 0.02e |
| β-Tocotrienol | 2.41 ± 0.09c | 2.73 ± 0.08b | 2.73 ± 0.01b | 7.66 ± 0.23a | 1.92 ± 0.09d |
| γ-Tocopherol | 17.37 ± 0.09c | 22.19 ± 0.04b | 40.51 ± 0.73a | 7.27 ± 0.09e | 10.82 ± 0.21d |
| γ-Tocotrienol | 128.87 ± 2.77d | 156.29 ± 4.98c | 164.71 ± 2.19b | 132.15 ± 4.46d | 183.70 ± 2.78a |
| δ-Tocopherol | 0.26 ± 0.01c | 0.80 ± 0.04b | 1.48 ± 0.08a | 0.24 ± 0.02c | 0.13 ± 0.01d |
| δ-Tocotrienol | 11.02 ± 0.19c | 12.39 ± 0.22b | 12.07 ± 0.09b | 18.31 ± 1.02a | 7.55 ± 0.32d |
| total-Tocopherols | 77.67 ± 1.23c | 114.74 ± 1.28b | 175.87 ± 0.68a | 76.55 ± 0.33c | 62.00 ± 0.65d |
| total-Tocotrienols | 320.08 ± 2.07e | 411.36 ± 9.03c | 381.10 ± 3.61d | 679.24 ± 8.74a | 470.87 ± 5.70b |
| total-Tocols | 397.75 ± 2.71d | 526.10 ± 9.63c | 556.97 ± 4.28b | 755.79 ± 8.55a | 532.87 ± 6.33c |
* Different letters in the same row indicate significant difference (P < 0.05)
Total phenolics
Total phenolics of oils from seeds of different grape varieties is reported in Table 4. Total phenolic contents ranged from 46.60 mg GAE/kg to 98.19 mg GAE/kg (Table 4). The results were in accordance with the values of 59.0–115.5 mg GAE/kg reported by Bail et al. (2008), and higher than the values of 21.9–47.0 mg GAE/kg reported by Demirtas et al. (2013) and 10.68–34.43 mg GAE/kg reported by Pardo et al. (2009). The highest content of total phenolics (98.19 mg GAE/kg) was found for Carbernet Sauvignon. Chardonnay (white grape variety) represented the lowest total phenolic content (46.60 mg GAE/kg) comparing with other red grape varieties. This may be due to the main phenolics in presence of anthocyanins which appear to be red.
Table 4.
Total phenolics, R(DPPH) values, coefficients and analysis of variance of the fit function that was obtained by non-linear regression analysis in DPPH radical scavenging assay
| Total phenolics (mg GAE/kg) | R2 | Adj. R2 | y0 | A | B | P value | R(DPPH) (s−1) | |
|---|---|---|---|---|---|---|---|---|
| Chardonnay | 46.60 ± 3.24d | 0.9998 | 0.9997 | 15.4703 | 84.5297 | 132.8727 | <0.0001 | −0.5901 |
| Merlot | 80.68 ± 3.97b | 0.9993 | 0.9992 | 11.4689 | 88.5311 | 99.5213 | <0.0001 | −0.8045 |
| Carbernet Sauvignon | 98.19 ± 0.02a | 0.9983 | 0.9981 | 14.9319 | 85.0681 | 88.7868 | <0.0001 | −0.8561 |
| Vitis amurensis | 86.69 ± 6.53b | 0.9998 | 0.9998 | 5.5587 | 94.4413 | 52.0916 | <0.0001 | −1.4963 |
| Vitis davidii | 66.88 ± 2.05c | 0.9987 | 0.9986 | 10.6741 | 89.3259 | 67.9326 | <0.0001 | −1.1349 |
* Different letters in the same column indicate significant difference (P < 0.05)
DPPH· scavenging capacity
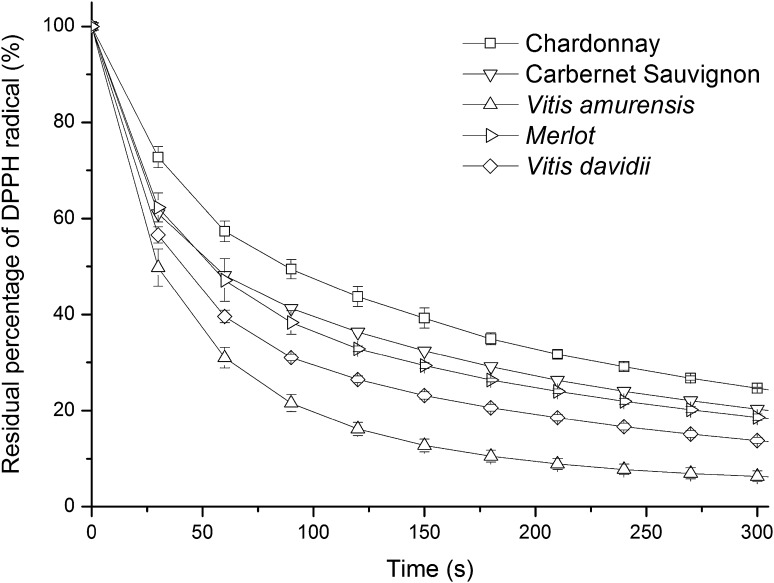
A kinetic approach was used to quantitatively evaluate the DPPH· scavenging capacity of grape seed oils. The kinetic curves of DPPH· residual percentages against time were plotted (Fig. 1). According to the data analysis, the mathematical model that most satisfactorily described the time dependence of DPPH· residual percentage was exponential function:
where y represents the DPPH· residual percentage and x denotes the reaction time. The corresponding coefficients (y 0, A and B) were presented in Table 4. The high values of R2 and Adj. R2 indicated perfect fits of exponential function which reached very significant levels (P < 0.01).
Fig. 1.
The percentage of DPPH radical remaining in the system on time of incubation with a concentration of 150 mg/mL of grape seed oils
Initial rate of this process (R(DPPH)) at x = 10 s was used to estimate the efficiency of DPPH· scavenging and calculated as the first derivative of the exponential function (Terpinc and Abramovič 2010):
The values of R(DPPH) are shown in Table 4. A lower R(DPPH) value indicates a higher DPPH· scavenging capacity (Chen et al. 2014). The Chinese traditional red grape variety of V. amurensis showed the highest DPPH· scavenging capacity, while white grape variety of Chardonnay exhibited the lowest among all varieties. In addition, Chinese traditional grape varieties (V. amurensis and V. davidii) exhibited a higher DPPH· scavenging capacity than the Eurasian grape varieties cultivated in China (Merlot and Carbernet Sauvignon). There were no good correlations between values of R(DPPH) and total phenolics, which indicated that the antioxidant activities of grape seed oil were not just the function of phenolics but might be a combined effect of phenolics, vitamin E and sterols.
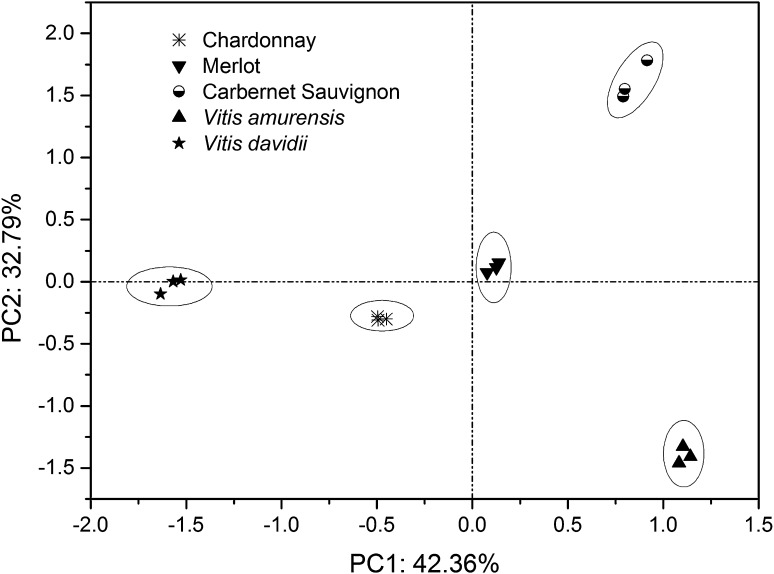
Principal component analysis (PCA)
PCA was applied to find principle components from the multivariate data of all properties detected above. As shown in Fig. 2, the first two principle components could separate the five grape cultivars effectively, which accounted for 75.15 % (PC1 = 42.36 % and PC2 = 32.79 %, respectively) of the total variation. PC1 was mainly correlated with linoleic acid (0.962), polyunsaturated fatty acid (0.964), β-tocopherol (0.952) and δ-tocotrienol (0.863), whereas inversely correlated with palmitoleic acid (−0.840), stearic acid (−0.867), oleic acid (−0.976), saturated fatty acid (−0.803) and monounsaturated fatty acid (−0.976). PC2 was highly contributed by nonadecanoic acid (0.900), teracosanoic acid (0.922), β-sitosterol (0.814), sitostanol (0.814), γ-tocopherol (0.926), δ-tocopherol (0.841) and total tocopherol (0.826). Carbernet Sauvignon showed both higher PC1 and PC2 scores than other cultivars as it had higher linoleic acid, nonadecanoic acid, teracosanoic acid, polyunsaturated fatty acid, β-sitosterol, sitostanol, β-tocopherol, γ-tocopherol, δ-tocopherol, δ-tocotrienol and total tocopherol. V. amurensis exhibited the highest PC1 score, which indicated the higher contents of linoleic acid, polyunsaturated fatty acid, β-tocopherol and δ-tocotrienol than other cultivars. And V. davidii showed the highest PC2 score with higher contents of nonadecanoic acid, teracosanoic acid, β-sitosterol, sitostanol, γ-tocopherol, δ-tocopherol and total tocopherol.
Fig. 2.
PCA plot of different grape seed oils
Conclusion
The present study demonstrated that grape seed oils from different grape varieties presented different compositions. Chinese traditional cultivars (V. amurensis and V. davidii) exhibited relatively higher tocotrienol and DPPH· scavenging capacity than Eurasian grape varieties (Chardonnay, Merlot and Carbernet Sauvignon) cultivated in China. Although Carbernet Sauvignon had significantly higher sterols and phenolics contents than all other varieties, a weaker DPPH· scavenging capacity was found compared with V. amurensis and V. davidii, which indicated a more important role of tocotrienol on the antioxidant ability of grape seed oil. Chardonnay (White varieties) seed oil showed the lowest sterols, vitamin E and phenolic content, therefore the lowest antioxidant ability. The results revealed that grape pomace of V. amurensis, V. davidii and Carbernet Sauvignon were good sources of high quality grape seed oil with superior nutritional value, such as relatively higher content of squalene, sterols, vitamin E, total phenolics and better DPPH· scavenging capacity. Due to the availability of high amounts of grape pomace in China, perspective reuse of the waste for seed oil extraction appears to be highly profitable.
Acknowledgments
This research was supported by a grant of Ministry of Agriculture of the People’s Republic of China, Special Fund for Agro-scientific Research in the Public Interest (Project No. 201303076). We appreciated the generous fund support for this work.
Compliance with ethical standards
Conflict of interest
None.
References
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medical properties of food: a review. J Food Sci Technol. 2015;52(5):2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC Official Method Ce 8-89 (1998) Determination of tocopherols and tocotrienols in vegetable oils and fats by HPLC. In: Official methods and recommended practices of the American Oil Chemists’ Society, 5th edn. AOCS, Champaign
- Awad AB, Fink CS. Phytosterols as anticancer dietary components: evidence and mechanism of action. J Nutr. 2000;130(9):2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- Bail S, Stuebiger G, Krist S, Unterweger H, Buchbauer G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008;108(3):1122–1132. doi: 10.1016/j.foodchem.2007.11.063. [DOI] [PubMed] [Google Scholar]
- Berker KI, Ozdemir Olgun FA, Ozyurt D, Demirata B, Apak R. Modified Folin–Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J Agric Food Chem. 2013;61(20):4783–4791. doi: 10.1021/jf400249k. [DOI] [PubMed] [Google Scholar]
- Beveridge THJ, Girard B, Kopp T, Drover JCG. Yield and composition of grape seed oils extracted by supercritical carbon dioxide and petroleum ether: varietal effects. J Agric Food Chem. 2005;53(5):1799–1804. doi: 10.1021/jf040295q. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gan Z, Zhao J, Wang Y, Zhang S, Li J, Ni Y. In vitro comparison of antioxidant capacity of cumin (Cuminum cyminum L.) oils and their main components. LWT-Food. Sci Technol. 2014;55(2):632–637. [Google Scholar]
- Crews C, Hough P, Godward J, Brereton P, Lees M, Guiet S, Winkelmann W. Quantitation of the main constituents of some authentic grape-seed oils of different origin. J Agric Food Chem. 2006;54(17):6261–6265. doi: 10.1021/jf060338y. [DOI] [PubMed] [Google Scholar]
- Demirtas I, Pelvan E, Özdemir İS, Alasalvar CandErtas E. Lipid characteristics and phenolics of native grape seed oils grown in Turkey. Eur J Lipid Sci Technol. 2013;115(6):641–647. doi: 10.1002/ejlt.201200159. [DOI] [Google Scholar]
- FAOSTAT (2013) Grapes production of the World and China in 2013. http://faostat3.fao.org/browse/Q/QC/E. Accessed 30 Dec 2015
- Fernandes L, Casal S, Cruz R, Pereira JA, Ramalhosa E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res Int. 2013;50(1):161–166. doi: 10.1016/j.foodres.2012.09.039. [DOI] [Google Scholar]
- He HP, Cai JG, Corke H. Studies of unsaponifiables in several vegetable oils. Lipids. 2003;9:658–663. [Google Scholar]
- Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, Sen CK. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278(44):43508–43515. doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterodt H, Slavin M, Whent M, Turner E, Yu L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011;128(2):391–399. doi: 10.1016/j.foodchem.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Nergiz C, Çelikkale D. The effect of consecutive steps of refining on squalene content of vegetable oils. J Food Sci Technol. 2011;48(3):382–385. doi: 10.1007/s13197-010-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesaretnam K, Yew WW, Wahid MB. Tocotrienols and cancer: beyond antioxidant activity. Eur J Lipid Sci Technol. 2007;109(4):445–452. doi: 10.1002/ejlt.200600212. [DOI] [Google Scholar]
- Pardo JE, Fernández E, Rubio M, Alvarruiz A, Alonso GL. Characterization of grape seed oil from different grape varieties (Vitis vinifera) Eur J Lipid Sci Technol. 2009;111(2):188–193. doi: 10.1002/ejlt.200800052. [DOI] [Google Scholar]
- Parry J, Haob Z, Luthera M, Sua L, Zhoua K, Yu L. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J Am Oil Chem Soc. 2006;83(10):847–854. doi: 10.1007/s11746-006-5036-8. [DOI] [Google Scholar]
- Passos CP, Silva RM, Da Silva FA, Coimbra MA, Silva CM. Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. Chem Eng J. 2010;160(2):634–640. doi: 10.1016/j.cej.2010.03.087. [DOI] [Google Scholar]
- Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric. 2000;80(7):939–966. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.0.CO;2-C. [DOI] [Google Scholar]
- Rice AC. Solid waste generation and by-product recovery potential from winery residues. Am J Enol Vitic. 1976;27:21–26. [Google Scholar]
- Rubio M, Alvarez-Orti M, Alvarruiz A, Fernandez E, Pardo JE. Characterization of oil obtained from grape seeds collected during berry development. J Agric Food Chem. 2009;57(7):2812–2815. doi: 10.1021/jf803627t. [DOI] [PubMed] [Google Scholar]
- Sabir A, Unver A, Kara Z. The fatty acid and tocopherol constituents of the seed oil extracted from 21 grape varieties (Vitis spp.) J Sci Food Agric. 2012;92(9):1982–1987. doi: 10.1002/jsfa.5571. [DOI] [PubMed] [Google Scholar]
- Shin EC, Pegg RB, Phillips RD, Eitenmiller RR. Commercial peanut (Arachis hypogaea L.) cultivars in the United States: phytosterol composition. J Agric Food Chem. 2010;58(16):9137–9146. doi: 10.1021/jf102150n. [DOI] [PubMed] [Google Scholar]
- Terpinc P, Abramovič H. A kinetic approach for evaluation of the antioxidant activity of selected phenolic acids. Food Chem. 2010;121(2):366–371. doi: 10.1016/j.foodchem.2009.12.037. [DOI] [Google Scholar]
- Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Ann Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]