Abstract
Objectives
Using the human telomerase reverse transcriptase (hTERT) promoter and the modified ferritin heavy chain (Fth) reporter gene, reporter gene expression for MRI was examined in telomerase positive and negative tumour cells and xenografts.
Methods
Activity of the reporter gene expression vector Lenti-hTERT-Fth1-3FLAG-Puro was compared to constitutive CMV-driven expression and to the untransfected parental control in five tumour cell lines: A549, SKOV3, 293T, U2OS and HPDLF. In vitro, transfected cells were evaluated for FLAG-tagged protein expression, iron accumulation and transverse relaxation. In vivo, tumours transduced by lentiviral vector injection were imaged using T2*WI. Changes in tumour signal intensity were validated by histology.
Results
Only telomerase positive tumour cells expressed FLAG-tagged Fth and displayed an increase in R2* above the parental control, with a corresponding change in T2*WI. In addition, only telomerase positive tumours, transduced by injection of the reporter gene expression construct, exhibited a change in signal intensity on T2*WI. Tumour histology verified the expression of FLAG-tagged Fth and iron accumulation in telomerase positive tissue.
Conclusion
Reporter gene expression for MRI, using the Fth reporter and the hTERT promoter, may be a useful strategy for the non-invasive diagnosis of many types of cancer.
Key points
• Modified heavy chain of ferritin can serve as an MR reporter gene
• hTERT promoter can direct the expression of reporter gene in cancer cells
• MR reporter imaging mediated by hTERT promoter can be used for cancer diagnosis
Keywords: Magnetic resonance imaging, Neoplasm, hTERT, Reporter gene expression, Ferritin heavy chain
Introduction
Telomerase is a ribonucleoprotein enzyme that controls the elongation of telomeres in mammalian cells [1]. It is usually repressed in most somatic cells and reactivated in approximately 85 % of human cancer cells, which maintain telomerase activity to sustain proliferation [2]. The selective reactivation of telomerase in cancer cells offers an attractive target for cancer diagnosis. Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase, and a strong correlation is observed between telomerase activity and hTERT mRNA expression in a variety of cancers [3]. Many researchers designed reporter gene imaging formulations in which promoter fragments of hTERT were used to direct the expression of reporter genes [4]. These encoded for proteins that change MRI contrast, bind radiolabelled probes or emit light [5, 6]. By introducing the gene expression system into cells using molecular techniques, the reporter gene of choice was (over)expressed only in telomerase-positive tumour cells as demonstrated in vitro and in vivo by position emission tomography (PET) or charge-coupled device (CCD) camera using reporter genes such as the Na/I symporter (NIS) [7] or the green fluorescence protein (GFP) [8].
MRI has been widely used in the field of modern medical diagnosis and treatment. Compared with optical imaging and nuclear medicine modalities, MRI has the advantages of multi-parameter imaging, higher spatial and temporal resolution, and lack of ionizing radiation. The primary requirement for an MR reporter gene is to provide contrast that can be distinguished from the non-transgenic cells [9]. Ferritin is an iron storage protein, which can accumulate significant amounts of hydrous ferric oxide iron from the intracellular labile iron pool, and then store it as ferrihydrite [10]. The expression of ferritin causes the transverse relaxation rate of transfected cells to increase significantly and generate strong hypointensity on T2 /T2*-weighted images [10].
In the present study, we hypothesized that telomerase-positive tumours and cells would be visualized by MR imaging using the hTERT promoter and the modified ferritin heavy chain (Fth) reporter gene. To test our hypothesis, we generated recombinant lentivirus Lenti-hTERT-Fth1-3FLAG-Puro in which the hTERT promoter was used to direct the expression of the modified Fth reporter gene, and then the recombinant vectors were transduced into cells in vitro and injected into tumour models in nude mice. Our aim was to assess the potential application of MR reporter imaging, mediated by the hTERT promoter, to cancer diagnosis.
Materials and methods
Construction and production of lentiviral vectors
The ferritin heavy chain (Fth1) gene was synthesized by Shanghai Generay Biotech Co., Ltd according to the Fth1 gene sequence in GenBank (NM_010239) with the exclusion of regulatory iron response elements, and then tagged with three consecutive FLAG sequence (3FLAG). The construct was subcloned into the NheI and BamHI restriction sites in the lentiviral vector pCDH-CMV-MCS-Puro to form the pCDH-CMV-Fth1-3FLAG-Puro vector. The pCDH-CMV- Fth1-3FLAG-Puro vector was linearized by restriction digest with SpeI and XbaI, and purified to remove the CMV promoter fragment. Polymerase chain reaction (PCR) was used to amplify the hTERT promoter from plasmid pLenti-hTERT-EGFP, a gift from Mr Li (Department of Pathophysiology, Third Military Medical University, Chongqing, China), using forward primer 5′-TTTTATCGATACTAGTCACAGACGCCCAGGACCGCGCTTC-3′ and reverse primer 5′-ATTCGCTAGCTCTAGACCACGTGCGCCCACGTGCGCCCAC-3′ containing SpeI and XbaI restriction sites, respectively. After restrictive digestion with SpeI and XbaI, the hTERT promoter fragment was successfully inserted into the linearized plasmid to form the pCDH-hTERT-Fth1-3FLAG-Puro vector.
Lentiviral vectors were produced by cotransfecting 293T cells with three plasmids (the reconstructed plasmids pCDH-hTERT-Fth1-3FLAG-Puro or pCDH-CMV-Fth1-3FLAG-Puro, the packaging plasmids pCD/NL-BH*DDD and pLTR-G) at a ratio of 3:2:1. Forty-eight hours after transfection, the viruses were collected and concentrated. The constructed lentiviral vectors were named Lenti-hTERT-Fth1-3FLAG-Puro and Lenti-CMV-Fth1-3FLAG-Puro.
Culture of cells
Five human cell lines were chosen for this study. Lung carcinoma A549, ovarian carcinoma SKOV3, embryonic kidney 293T, osteosarcoma U2OS and primary periodontal ligament fibroblast (HPDLF) cells were all obtained from the central laboratory of Xin Qiao Hospital (Chongqing, China). A549, SKOV3, 293T and HPDLF cells were maintained in DMEM medium (Gibco), and U2OS cells were cultured in McCoy’s 5A medium (Gibco). All media were supplemented with 10 % fetal bovine serum (Gibco) and 100 U/ml of penicillin–streptomycin (Gibco). Immunofluorescence staining and western blotting were performed as described below to determine the presence of hTERT protein in all cell lines.
Lentivirus transfection
Cells were incubated with Lenti-hTERT-Fth1-3FLAG-Puro and Lenti-CMV-Fth1-3FLAG-Puro in the presence of polybrene (Sigma, 5–8 μg/ml) for 24 h [11]. The expression of FLAG-tagged Fth in all cells was analysed by immunofluorescence staining and an enzyme-linked immunosorbent assay (ELISA) as described below. Iron accumulation was determined by Prussian blue iron staining and by using a total iron assay kit as described below.
Western blot analysis
Cells (2 × 106) were lysed on ice in 200 μl RIPA buffer with protease inhibitor (Sigma). The concentration of the collected cellular protein was evaluated by the bicinchoninic acid (BCA) assay according to the manufacturer’s instructions (Pierce Biotechnology) [12], and ca. 15 μg of the protein samples was loaded onto 10 % gels for SDS–PAGE. The proteins were then transferred onto PVDF membranes for blotting. The membranes were blocked in 5 % bovine serum albumin for 2 h and then incubated overnight at 4 °C with rabbit anti-TERT monoclonal antibody (1:1000, Abcam) or mouse anti-β-actin monoclonal antibody (1:1000, Abcam). After thoroughly washing with Tris-buffered saline with 0.1 % Tween 20 (0.1 % TBST), the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (1:1000, Santa Cruz) for 1 h. The protein bands were visualized by enhanced chemiluminescent (ECL) agents (Pierce Biotechnology) following the manufacturer’s protocol.
Immunofluorescence staining
Cells were cultured in confocal petri dishes (NEST Biotechnology) and rinsed with phosphate buffered saline (PBS) pH 7.4 before examination. Following fixation in 4 % paraformaldehyde (PFA) for 10 min, the cells were blocked with 5 % normal goat serum in PBS at 37 °C for 30 min. To examine hTERT protein expression, the cells were incubated with rabbit anti-hTERT monoclonal antibody (1:500, Abcam) overnight at 4 °C and then visualized with Alexa Flour 488-conjugated goat anti-rabbit antibody (1:1000, Beyotime). To examine FLAG protein expression, the cells were incubated with mouse anti-FLAG monoclonal antibody (1:1000, Sigma) overnight at 4 °C and then visualized with Alexa Flour 555-conjugated goat anti-mouse antibody (1:1000, Beyotime). The cell nuclei were counterstained with DAPI (1:1000, Beyotime).
Enzyme-linked immunosorbent assay
Extracts of 106 cells were collected, and the BCA assay was used to determine the total protein concentration of each sample. The total ferritin heavy chain (Fth) content in each cell sample was analysed using an ELISA kit (Uscn Life Science Inc, Wuhan, China) according to the manufacturer’s instructions. The concentration of Fth in the samples was determined by comparing the O.D. of the samples to the standard curve. The results are expressed as nanograms of Fth per milligram of total protein. Values are the means ± SD of three independent experiments.
Assessment of iron accumulation
Cells were cultured in medium supplemented with ferric ammonium citrate (FAC, 1 mM, Sigma) for 48 h and then washed thoroughly with PBS to remove any free iron. For Prussian blue staining, cells were fixed in 4 % PFA for 10 min and then incubated in Prussian blue working solution, which was prepared by mixing equal parts of 1 % potassium ferric ferrocyanide solution and 5 % hydrochloric acid solution for 30 min [12]. Cells were counterstained with nuclear fast red (Beyotime) for 5 min. The iron content of all cells was further determined by using an iron assay kit according to the manufacturer’s instructions (Sigma). Briefly, 2 × 106 cells were homogenized in cold iron assay buffer, and the cell extracts were collected to determine their iron contents. Absorbance was immediately measured at 593 nm. The iron content for each sample was determined by dividing the iron content by the number of cells (pg/cell). Three sets of samples were measured.
Tumour models
Twelve BALB/c nude mice (male, 4 weeks old, and 16–18 g) of specific pathogen-free grade were purchased from the Animal Department, Third Military Medical University. We performed experiments in accordance with the local ethics committee of the Third Military Medical University. The mice were treated with an intraperitoneal injection of 400 μl iron dextran (50 mg/2 ml, Zhejiang Ruian Pharmaceutical Factory, China) every 3 days for a total of six injections. After the fourth iron injection, the bilateral hind flanks of the mice were subcutaneously inoculated with A549 or U2OS cells (106 cells/position) (n = 6 for each tumour sample). When tumours reached approximately 5–10 mm in diameter, each tumour model was randomly divided into two groups (n = 3). In one group, 100 μl Lenti-hTERT-Fth1-3FLAG-Puro viral particles (5 × 108 IU/ml) were intratumorally injected into the left-side tumour and nothing into the right-side tumour. In the other group, 100 μl Lenti-CMV-Fth1-3FLAG-Puro viral particles (5 × 108 IU/ml) were injected into the left-side tumour and 100 μl PBS was injected into the right-side tumour.
Magnetic resonance imaging
Cells were grown in medium supplemented with FAC (1 mM) for 48 h before MR imaging. After thorough washing with PBS to remove extracellular iron, dissociating with trypsin, and fixing in 4 % PFA for 10 min, the cells were suspended in 1 ml PBS and transferred into 2-ml microcentrifuge tubes (5 × 107 cells/tube), which were precoated with 500 μl 1 % agarose. After the cells had settled for 20–30 min and formed a loose pellet on the top of the agarose, MRI was performed with a 3.0-T MR unit with a knee coil (GE Signa HDx). T2*-weighted images were acquired using a GRE (gradient recalled echo) sequence with the following parameters: TR (repetition time) = 400 ms, TE (echo time) = 12 ms, flip angle = 30° and slice thickness = 1.0 mm. In a second GRE acquisition, R2* measurements were derived from eight images using the following parameters: TR = 1000 ms, TE = 2.5–27.0 ms (8 echoes) and slice thickness = 2.2 mm.
Tumours were imaged 15 days after virus injection using a 1.5-T MR scanner (GE Signa HDx). Mice were anaesthetized with 2 % sodium pentobarbital and placed in a wrist coil. T2-weighted images of tumours were obtained using an FSE (fast spin echo) sequence (TR = 2000 ms, TE = 47.5 ms, FOV (field of view) = 6 × 6 cm and slice thickness = 2.0 mm). T2*-weighted images were obtained using a GRE sequence (TR = 540 ms, TE = 10 ms, FOV = 6 × 6 cm and slice thickness = 2.0 mm).
Immunohistochemistry and histology of tumour tissues
After MR scanning, mice were decapitated, and tumours were removed and fixed with 4 % PFA. Each tumour was embedded in OCT (optical cutting temperature) and sectioned using a cryostat microtome. Prepared frozen sections (4 μm in thickness) were used for immunofluorescence staining and Prussian blue iron staining to determine FLAG-tagged Fth expression and iron accumulation in tumour tissues, respectively, as described above.
Statistical analysis
Data are presented as the mean ± SD. Significance tests were performed using one-way ANOVA (Graphpad Prism version 5.0). Differences were considered significant at p values of less than 0.05.
Results
Expression of hTERT proteins in cell lines
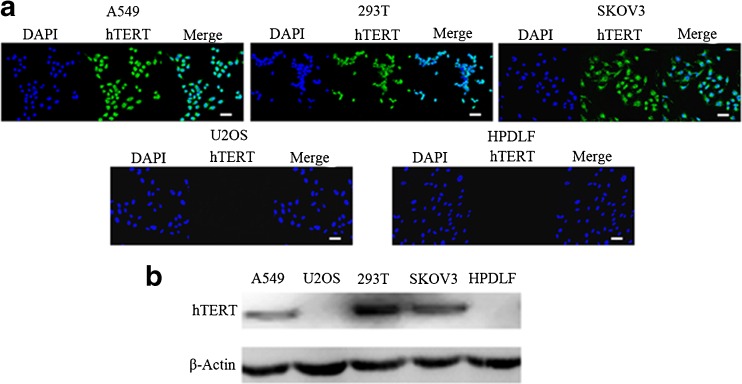
By performing immunofluorescence staining and western blot analysis, we confirmed the expression of hTERT proteins in A549, SKOV3 and 293T cell lines; whereas, no hTERT protein was detected in U2OS or HPDLF cell lines (Fig. 1). These results are consistent with previously published work [13–16]. As the expression of hTERT protein is good indicator of telomerase activity [2], A549, SKOV3 and 293T cell lines were considered telomerase-positive, while U2OS and HPDLF cell lines were considered telomerase-negative in this study.
Fig. 1.
Expression of hTERT protein in cell lines. a Immunofluorescence staining in cells with anti-hTERT antibody. In immunofluorescence images, hTERT was visible in A549, SKOV3 and 293T cells but not in U2OS or HPDLF cells. Green fluorescence represents hTERT proteins; blue fluorescence represents cell nuclei. Scale bar 50 μm. b Western blots for hTERT protein using anti-hTERT antibody (β-actin was used as an internal control). hTERT protein was detected in A549, SKOV3 and 293T cells but not in U2OS or HPDLF cells
hTERT promoter drives Fth expression in telomerase-positive cells
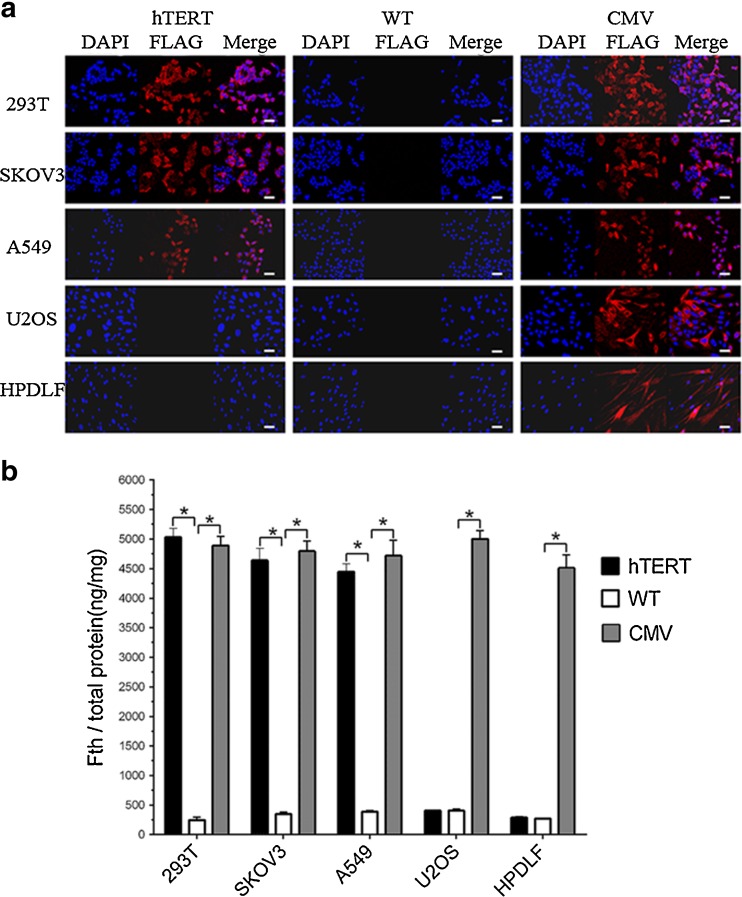
After transfection with Lenti-hTERT-Fth1-3FLAG-Puro, immunofluorescence staining showed that FLAG-tagged protein was present in telomerase-positive cells but absent in telomerase-negative cells. In contrast, FLAG-tagged protein was present in all five cell lines transfected with Lenti-CMV-Fth1-3FLAG-Puro and absent in all five untransfected cell lines (Fig. 2a). Analysis by ELISA showed that Fth protein content in Lenti-hTERT-Fth1-3FLAG-Puro-transfected telomerase-positive cells was significantly greater (4421–5039 ng/mg of total protein) than that in untransfected telomerase-positive cells (246–388 ng/mg of total protein) (p < 0.05, n = 3). In contrast, there was no remarkable difference between the Lenti-hTERT-Fth1-3FLAG-Puro-transfected telomerase-negative cells and the untransfected telomerase-negative cells (270–409 ng/mg of total protein) (p > 0.05, n = 3) (Fig. 2b). By comparison, both telomerase-positive and telomerase-negative cells transfected with Lenti-CMV-Fth1-3FLAG-Puro contained greater Fth protein than their untransfected counterparts (p < 0.05, n = 3) (Fig. 2b).
Fig. 2.
Analysis of FLAG-tagged Fth expression in cells transfected with or without lentiviral vector. a Immunofluorescence staining of cells with anti-FLAG antibody. After transfection with Lenti-hTERT-Fth1-3FLAG-Puro, FLAG-tagged protein was identified in immunofluorescence images of A549, SKOV3 and 293T cells but not U2OS or HPDLF cells. Red fluorescence represents FLAG-tagged protein; blue fluorescence represents cell nuclei. Scale bar 50 μm. b ELISA for Fth protein in all cell types. In A549, SKOV3 and 293T cells, but not U2OS or HPDLF cells, transfected with Lenti-hTERT-Fth1-3FLAG-Puro, Fth protein level was significantly increased compared to the untransfected cells. Error bars indicate standard deviations of triplicate samples. *p value was statistically significant (p < 0.05). hTERT Lenti-hTERT-Fth1-3FLAG-Puro-transfected cells, CMV Lenti-CMV-Fth1-3FLAG-Puro-transfected cells, WT wild-type cells
More iron was accumulated in cells with Fth expression
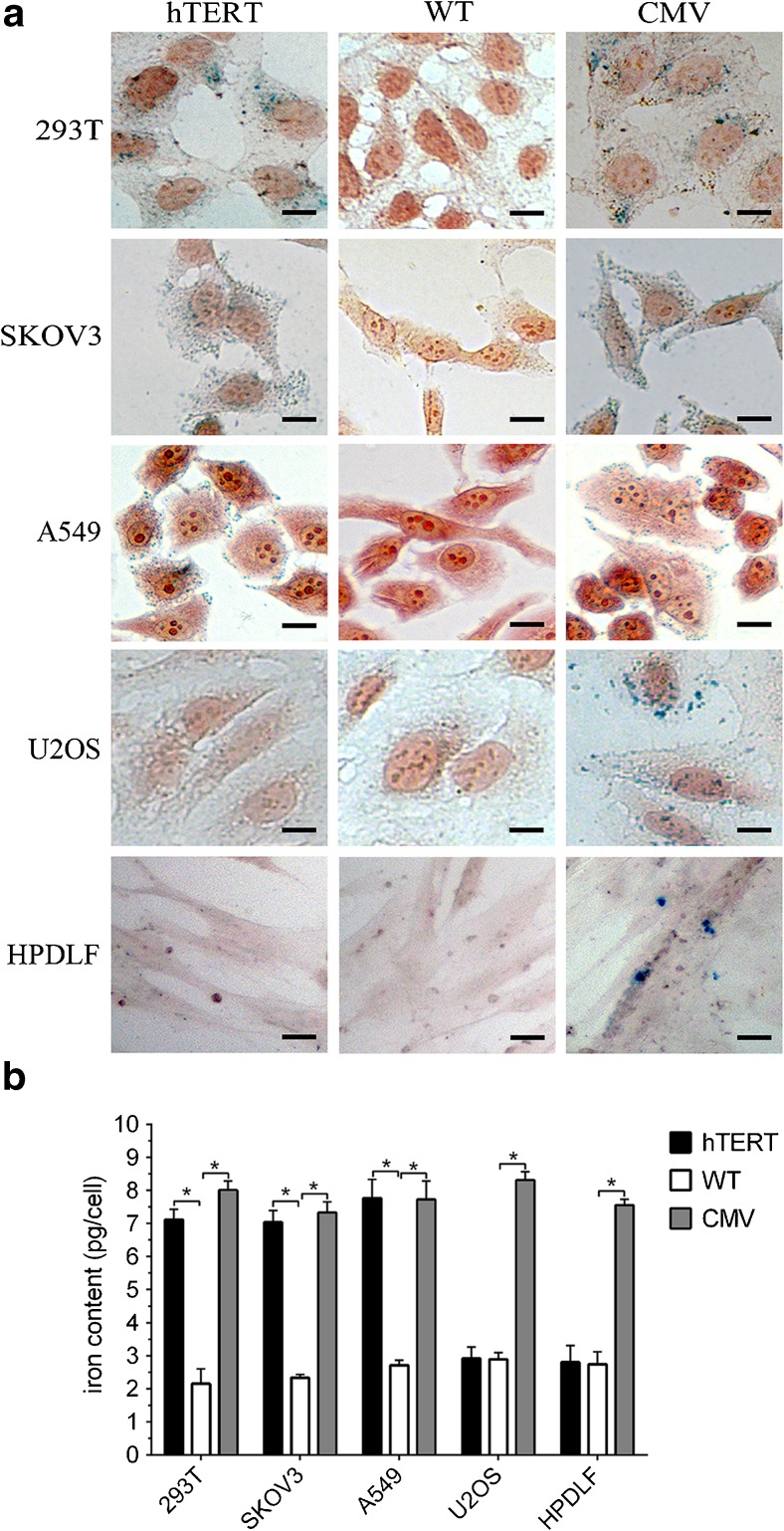
To verify the ability of Fth expression to augment iron storage, Prussian blue staining was performed. Blue granules of accumulated iron were detected in telomerase-positive cells but not in telomerase-negative cells transfected with Lenti-hTERT-Fth1-3FLAG-Puro. Moreover, blue granules were not observed in any untransfected cells (Fig. 3a). The iron content assay revealed that the iron content of Lenti-hTERT-Fth1-3FLAG-Puro-transfected telomerase-positive cells (7.033–7.766 pg/cell) was significantly higher than that of untransfected telomerase-positive cells (2.152–2.712 pg/cell) (p < 0.05, n = 3), but this difference was not observed in telomerase-negative cells (2.740–2.914 pg/cell) (p > 0.05, n = 3) (Fig. 3b). In contrast, all cells transfected with Lenti-CMV-Fth1-3FLAG-Puro showed blue granules and had significantly higher iron content than their untransfected counterparts (p < 0.05, n = 3).
Fig. 3.
Analysis of iron accumulation in cells treated with FAC (1 mM) for 48 h. a Prussian blue staining of cells showed positive results in A549, SKOV3 and 293T cells, but not in U2OS or HPDLF cells, transfected with Lenti-hTERT-Fth1-3FLAG-Puro. Scale bar 5 μm. b Average iron content in each cell sample measured by an iron assay kit. A549, SKOV3 and 293T cells, but not U2OS or HPDLF cells, transfected with Lenti-hTERT-Fth1-3FLAG-Puro accumulated more iron than the untransfected cells. Error bars indicate standard deviations of triplicate samples. *p value was statistically significant (p < 0.05). hTERT Lenti-hTERT-Fth1-3FLAG-Puro-transfected cells, CMV Lenti-CMV-Fth1-3FLAG-Puro-transfected cells, WT wild-type cells
hTERT promoter-mediated Fth reporter gene expression induces specific MRI contrast in telomerase-positive cells in vitro
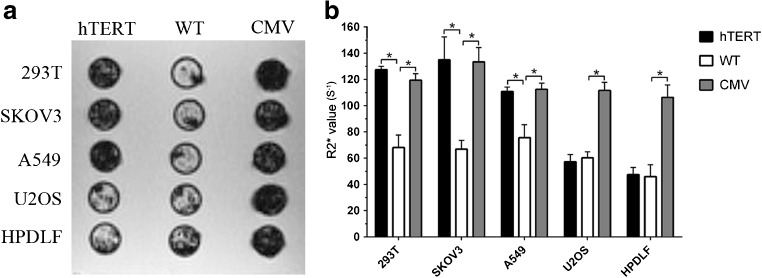
The T2*WI signal intensity of Lenti-hTERT-Fth1-3FLAG-Puro-transfected telomerase-positive cells was lower than that of untransfected telomerase-positive cells, but this difference was not observed in telomerase-negative cells (Fig. 4a). In comparison, all of the Lenti-CMV-Fth1-3FLAG-Puro-transfected cells showed signal loss compared to their untransfected counterparts (Fig. 4a). Furthermore, these signal changes were in agreement with the increase in cellular R2*. The R2* value of Lenti-hTERT-Fth1-3FLAG-Puro-transfected telomerase-positive cells (110.8–134.9 s−1) was higher than that of untransfected ones (66.83–75.61 s−1) (p < 0.05), but this difference was not observed in telomerase-negative cells (54.28–58.84 s−1) (p > 0.05) (Fig. 4b).
Fig. 4.
In vitro MRI analysis of cells treated with FAC (1 mM) for 48 h. a T2*-weighted images of cells. Remarkable hypointensity was detected in A549, SKOV3 and 293T cells, but not U2OS or HPDLF cells, transfected with Lenti-hTERT-Fth1-3FLAG-Puro. b R2* values of cells were measured from R2* maps. Error bars indicate standard deviations of R2* within three regions of interest on the parametric map. *p value was statistically significant (p < 0.05). hTERT Lenti-hTERT-Fth1-3FLAG-Puro-transfected cells, CMV Lenti-CMV-Fth1-3FLAG-Puro-transfected cells, WT wild-type cells
hTERT promoter-mediated Fth reporter gene expression induces specific MRI contrast in telomerase-positive tumours in vivo
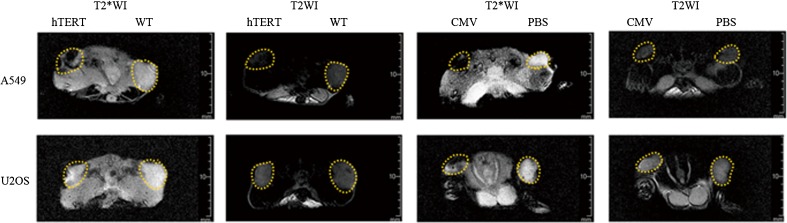
A549 tumours showed hypointensity on T2*WI after injection with Lenti-hTERT-Fth1-3FLAG-Puro, while the U2OS tumours did not (Fig. 5). In contrast, both A549 and U2OS tumours showed hypointensity on T2*WI after injection with Lenti-CMV-Fth1-3FLAG-Puro (Fig. 5). There was no apparent difference in signal intensity between the PBS-injected tumours and the WT (wild type) tumours (Fig. 5).
Fig. 5.
In vivo MRI analysis of tumours injected with vectors. T2*-weighted imaging of tumours shows remarkable hypointensity in A549 tumours, but not U2OS tumours, injected with Lenti-hTERT-Fth1-3FLAG-Puro and in A549 and U2OS tumours injected with Lenti-CMV-Fth1-3FLAG-Puro. No apparent signal loss in T2-weighted images of the tumours was observed. hTERT Lenti-hTERT-Fth1-3FLAG-Puro-injected tumours, CMV Lenti-CMV-Fth1-3FLAG-Puro-injected tumours, WT wild-type tumours, PBS PBS-injected tumours
Immunohistologic analysis of tumours
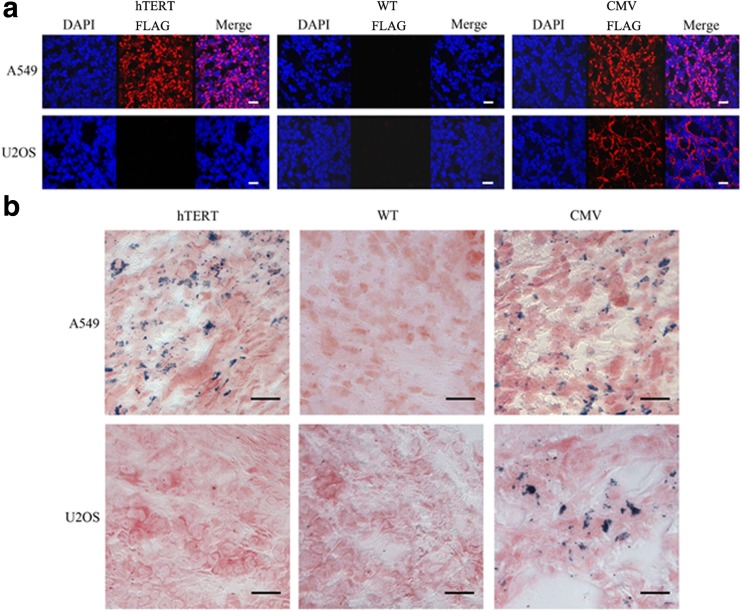
Immunofluorescence staining of tumours with or without lentivirus injection was performed using anti-FLAG antibody. After injection with Lenti-hTERT-Fth1-3FLAG-Puro, FLAG-tagged Fth proteins were only expressed in A549 tumours (Fig. 6). In contrast, FLAG-tagged Fth proteins were detected in both tumour types after injection with Lenti-CMV-Fth1-3FLAG-Puro. Accordingly, notable positive Prussian blue staining was observed in Lenti-hTERT-Fth1-3FLAG-Puro-injected A549 tumours and Lenti-CMV-Fth1-3FLAG-Puro-injected A549 and U2OS tumours, but not in other tumours. These results were in line with those of the cell experiments described above.
Fig. 6.
Histological analyses of tumour sections. a Immunofluorescence staining of FLAG protein in tumours with anti-FLAG antibody. Red fluorescence represents FLAG-tagged proteins; blue fluorescence represents cell nuclei. Scale bar 50 μm. b Prussian blue staining of iron deposits in tumour tissues. Scale bar 10 μm. hTERT Lenti-hTERT-Fth1-3FLAG-Puro-injected tumours, CMV Lenti-CMV-Fth1-3FLAG-Puro-injected tumours, WT wild-type tumours
Discussion
In recent years, great efforts have been devoted to designing molecular targeting reagents for cancer diagnosis. The research focus of many studies is on conjugating exogenous contrast agents with a specific ligand/antibody, so that the agents target the cells of interest and, thus, alter the MR signal intensity of those cells [17, 18]. However, there are many limitations to this method, including the decay of receptor–ligand or antigen–antibody binding activity in vivo, the limited retention time of exogenous contrast agents in cells due to cellular division, and the background caused by nonspecific uptake of exogenous contrast agents by the reticuloendothelial system, which influences the visualization and analysis of cancer lesions.
Ferritin possesses a superparamagnetic moment and can shorten the T2 relaxation time of protons nearby [19]. The use of ferritin as a reporter gene to produce endogenous contrast can avoid some of the limitations of exogenous contrast agents. Cohen et al. first demonstrated that the introduced expression of ferritin heavy chain under tetracycline (TET) regulation resulted in an elevation of R1 and R2 relaxation rates in C6 cells, and they detected C6 tumours expressing ferritin heavy chain gene on T2WI [20]. Soon after, this team generated transgenic mice expressing the ferritin heavy chain under the control of the vascular endothelial cadherin promoter, and specifically visualized endothelial cells by MR imaging [21]. To date, a variety of promoters have been explored and used to regulate target gene expression, e.g. promoters selected on the basis of their potential to target cancer, such as the prostate-specific antigen promoter [22], α-fetoprotein promoter [23] and the prolactin promoter [24].
In the present study, we used the hTERT promoter to drive ferritin heavy chain expression, since it is functional in the majority of cancer cells regardless of their histological types, but is silent in somatic or benign cells. We constructed a FLAG-tagged ferritin heavy chain reporter gene expression lentiviral vector Lenti-hTERT-Fth1-3FLAG-Puro and showed that FLAG-tagged Fth was specifically expressed in telomerase-positive cells both in vitro and in vivo. In addition, Prussian blue staining for iron was observed in Fth-expressing cells and tumours. With the increase in ferritin protein and iron accumulation, the R2* value of cells was significantly increased and signal loss was observed on T2*-weighted images. However, we found that the results obtained above did not correspond very well with each other, possibly because of the contribution of many factors to R2*, such as the mode of nucleation of iron, the aggregation of ferritin in lysosomes [25], different levels of promoter activity and different levels of ferritin expression in different cell lines. As shown in Fig. 5, signal loss was observed on GRE T2*-weighted images of tumours with Fth reporter gene expression but not on FSE T2-weighted images, suggesting that a GRE sequence is likely more sensitive to iron than FSE sequence [26].
Moreover, the Fth-expressing tumours also showed heterogeneous signal intensity on T2*-weighted images. There are several possible explanations for this finding: (1) the uneven distribution of iron particles in the tumour tissues; (2) the manner of lentivirus injection, which may cause cells near the injection site to be transfected with more vector particles than those distant from the injection site, resulting in a higher ferritin expression level in the cells near the injection site; and (3) the maldistribution of telomerase-positive cells in the lesions, as the hTERT promoter can only drive ferritin expression in them.
In summary, we used an MR molecular imaging technology to specifically detect cancer cells both in vitro and in vivo. We demonstrate for the first time that MR reporter imaging mediated by the hTERT promoter could be a novel and broad-spectrum imaging tool for cancer research and diagnosis.
Acknowledgments
The study was supported by the National Science Foundation of China (no.81471635) and the Science and Technology Commission of ChongQing City, China (no.cstc2013jjB10010). The scientific guarantor of this publication is Zhang Dong. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Approval from the Institutional Animal Care and Use Committee of the Third Military Medical University was obtained.
Abbreviations
- CMV
Cytomegalovirus
- ELISA
Enzyme-linked immunosorbent assay
- FAC
Ferric ammonium citrate
- FSE
Fast spin echo
- Fth
Ferritin heavy chain
- hTERT
Human telomerase reverse transcriptase
- GRE
Gradient recalled echo
- MRI
Magnetic resonance imaging
- TE
Time to echo
- TR
Time to repetition
- T2*WI
T2*-weighted imaging
- T2WI
T2-weighted imaging
Contributor Information
Li Wen, Phone: +86-023-68774036, Email: cqzdwl@163.com.
Dong Zhang, Phone: +86-023-68774036, Email: hszhangd@163.com.
References
- 1.Dhaene K, Van Marck E, Parwaresch R. Telomeres, telomerase and cancer: an up-date. Virchows Arch. 2000;437:1–16. doi: 10.1007/s004280000189. [DOI] [PubMed] [Google Scholar]
- 2.Saldanha SN, Andrews LG, Tollefsbol TO. Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Anal Biochem. 2003;315:1–21. doi: 10.1016/S0003-2697(02)00663-2. [DOI] [PubMed] [Google Scholar]
- 3.Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8:137–142. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 4.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilad AA, Winnard PT, Jr, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 6.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 7.Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Keith WN, Vassaux G. Noninvasive imaging of the transcriptional activities of human telomerase promoter fragments in mice. Cancer Res. 2004;64:4906–4911. doi: 10.1158/0008-5472.CAN-04-0426. [DOI] [PubMed] [Google Scholar]
- 8.Yu ST, Yang YB, Liang GP, et al. An optimized telomerase-specific lentivirus for optical imaging of tumors. Cancer Res. 2010;70:2585–2594. doi: 10.1158/0008-5472.CAN-09-3841. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Lee SH, Biswal S. Magnetic resonance reporter gene imaging. Theranostics. 2012;2:403–412. doi: 10.7150/thno.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa S, Furukawa T, Saga T. Molecular MR imaging of cancer gene therapy: ferritin transgene reporter takes the stage. Magn Reson Med Sci. 2010;9:37–47. doi: 10.2463/mrms.9.37. [DOI] [PubMed] [Google Scholar]
- 11.Davis HE, Morgan JR, Yarmush ML. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys Chem. 2002;97:159–172. doi: 10.1016/S0301-4622(02)00057-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Cho HR, Choi SH, Woo JS, Moon WK. In vivo imaging of tumor transduced with bimodal lentiviral vector encoding human ferritin and green fluorescent protein on a 1.5T clinical magnetic resonance scanner. Cancer Res. 2010;70:7315–7324. doi: 10.1158/0008-5472.CAN-10-0241. [DOI] [PubMed] [Google Scholar]
- 13.Braunstein I, Cohen-Barak O, Shachaf C, et al. Human telomerase reverse transcriptase promoter regulation in normal and malignant human ovarian epithelial cells. Cancer Res. 2001;61:5529–5536. [PubMed] [Google Scholar]
- 14.Fujii S, Maeda H, Wada N, Kano Y, Akamine A. Establishing and characterizing human periodontal ligament fibroblasts immortalized by SV40T-antigen and hTERT gene transfer. Cell Tissue Res. 2006;324:117–125. doi: 10.1007/s00441-005-0101-4. [DOI] [PubMed] [Google Scholar]
- 15.Irving J, Wang Z, Powell S, et al. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11:174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan P, Otero J, Ray P, et al. Visualization of telomerase reverse transcriptase (hTERT) promoter activity using a trimodality fusion reporter construct. J Nucl Med. 2006;47:270–277. [PMC free article] [PubMed] [Google Scholar]
- 17.Ren J, Wang F, Wei G, et al. MRl of prostate cancer antigen expression for diagnosis and lmmunotherapy. PLoS ONE. 2012;7:e38350. doi: 10.1371/journal.pone.0038350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang HM, Park CW, Woo MA, et al. HER2/neu antibody conjugated poly(amino acid)-coated iron oxide nanoparticles for breast cancer MR imaging. Biomacromolecules. 2010;11:2866–2872. doi: 10.1021/bm100560m. [DOI] [PubMed] [Google Scholar]
- 19.Brooks RA, Vymazal J, Goldfarb RB, Bulte JW, Aisen P. Relaxometry and magnetometry of ferritin. Magn Reson Med. 1998;40:227–235. doi: 10.1002/mrm.1910400208. [DOI] [PubMed] [Google Scholar]
- 20.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7:109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen B, Ziv K, Plaks V, et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007;13:498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 22.Willhauck MJ, Sharif Samani BR, Gildehaus FJ, et al. Application of 188rhenium as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
- 23.Kim KI, Lee YJ, Lee TS, et al. In vitro radionuclide therapy and in vivo scintigraphic imaging of alpha-fetoprotein-producing hepatocellular carcinoma by targeted sodium iodide symporter gene expression. Nucl Med Mol Imaging. 2013;47:1–8. doi: 10.1007/s13139-012-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirland JA, Seymour ZC, Windeatt S, et al. Real-time imaging of gene promoter activity using an adenoviral reporter construct demonstrates transcriptional dynamics in normal anterior pituitary cells. J Endocrinol. 2003;178:61–69. doi: 10.1677/joe.0.1780061. [DOI] [PubMed] [Google Scholar]
- 25.Cohen B, Ziv K, Plaks V, Harmelin A, Neeman M. Ferritin nanoparticles as magnetic resonance reporter gene. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:181–188. doi: 10.1002/wnan.11. [DOI] [PubMed] [Google Scholar]
- 26.Juchems MS, Cario H, Schmid M, Wunderlich AP. Liver iron content determined by MRI: spin-echo vs. gradient-echo. Röfo. 2012;184:427–431. doi: 10.1055/s-0031-1299320. [DOI] [PubMed] [Google Scholar]