Abstract
Background/Aims
Sickle cell disease (SCD) is a life-threatening, autosomal recessive blood disorder prevalent in sub-Saharan Africa. We identified the prevalence of sickle cell trait (SCT) among pregnant women and male partners in Enugu State, Nigeria, and determined the accuracy of self-reported sickle cell status and its reliability for identifying high-risk newborns for targeted screening.
Methods
We conducted a nested cohort study of expectant parents enrolled in the Healthy Beginning Initiative (HBI). HBI is a cluster-randomized trial of a congregation-based approach designed to increase HIV testing. Participants completed a survey regarding self-awareness of their sickle cell genotype and consented to genotype screening by cellulose acetate electrophoresis.
Results
SCT prevalence (HbAS) was 22% (746 of 3,371). Only 50% of participants provided an accurate self-report. Self-report accuracy was significantly different (p<0.0001) between individuals who reported having SCT or SCD (61% accuracy) vs. those who reported not having SCT or SCD (86% accuracy). Demographic variables including gender, age, household size, employment, education, and home location were significantly associated with providing an accurate self-report.
Conclusions
Low numbers of accurate parental self-reports coupled with high SCT prevalence in Nigeria, could limit the efficacy of targeted newborn screening. However, our data indicates that it is feasible to integrate sickle cell screening for pregnant women with existing, community-based, healthcare programs developed by the President’s Emergency Plan for AIDS Relief (PEPFAR), such as HBI. Expanding screening programs could enable development of targeted newborn screening based on maternal genotype that could identify all newborns with SCD in resource-limited settings.
Keywords: sickle cell trait, sickle cell disease, newborn screening, carrier testing, genetic screening, diagnosis, sub-Saharan Africa
Introduction
Sickle cell disease (SCD) is a life-threatening genetic blood disorder that affects over 6 million newborns annually [1,2]. It is an autosomal recessive disorder most commonly caused by homozygosity for the A to T mutation in the sixth codon of the hemoglobin β-subunit (i.e. homozygosity for the S variant of hemoglobin β-subunit; SS) [3]. It can also be caused by compound heterozygosity for the S and C variants (SC) [3]. Individuals with sickle cell trait (SCT) are heterozygous for the S variant (AS) and hence are unaffected carriers.
The incidence of disease is disproportionately high in sub-Saharan Africa, where over 75% of all global SCD births occur [1]. Over 250,000 African infants, predominantly from sub-Saharan Africa, are born annually with SCD [4], and SCD is the most prevalent genetic disease in Africa [5]. Moreover, 30% of the world’s annual SCD and SCT births are located in two countries, both in sub-Saharan Africa: Nigeria and the Democratic Republic of the Congo [1].
Despite the very high burden of SCD, Nigeria does not have a national newborn screening program for SCT and SCD [6]. Diagnostic screening is the key to identifying infants with SCD so that infants and families can promptly receive potentially lifesaving medical and educational interventions. Without routine newborn screening, many children are not identified early enough to receive key preventative care. Accordingly, the implementation of universal newborn SCD screening was a major factor in the significant decrease of pediatric SCD mortality in many developed countries [7–9]. The World Health Organization has recommended newborn screening as a key strategy for reducing pediatric mortality in Africa [5], where infants with SCD face an estimated 50–90% early childhood mortality rate [3]. However, newborn screening programs can be logistically and economically challenging to implement, particularly in sub-Saharan Africa where over 70% of the population has access to little or no health care infrastructure [10]. Many pilot or demonstration programs for universal newborn screening have been debuted in various resource-limited settings throughout Africa, including the Democratic Republic of Congo (DRC) [11], Burkina Faso [12], Ghana [13], Nigeria [14], Angola [15] and Liberia [16]. To date, none of these countries has been able to scale the program to the national level and maintain a long-term national sickle cell newborn screening program.
Targeted newborn screening, in which testing is limited to infants deemed high-risk based on parental sickle cell status (as determined by voluntary self-report or laboratory testing) could be a useful alternative to universal newborn screening in resource-limited settings. Notably, a targeted newborn screening program for SCD was successfully implemented and maintained long-term (16+ years) in the Republic of Benin [17]. In addition, utilizing existing public health programs and infrastructure by incorporating newborn screening into established infant welfare clinics [18], would also decrease cost and increase program sustainability. Similarly, tailoring programs to increase community acceptance could increase the number of parents who consent to screening of their newborn and commit to a comprehensive care regimen [14]. It has been noted that cultural sensitivity and thoughtful adaptation of newborn SCD screening programs to the needs of diverse local communities in sub-Saharan Africa will be a key part of any screening program’s success [19].
As a first step towards development of a newborn screening program relevant to the specific needs of Nigeria, we sought to determine (a) the population prevalence of SCT among pregnant women and their male partners in Enugu State, southeast Nigeria, and (b) the extent and accuracy of parental self-reported sickle cell genotype. We conducted a nested cohort study by integrating sickle cell screening within an existing and community-accepted HIV testing infrastructure, the Healthy Beginning Initiative (HBI). HBI is a cluster-randomized trial designed to increase HIV testing of pregnant women and their male partners [20]. It is a congregation-based program in Enugu State, southeast Nigeria, which was created with heavy participation and leadership from the local community.
METHODS
Study design and setting
This cohort study was nested within the Healthy Beginning Initiative (HBI). HBI is a large, cluster-randomized, controlled study designed to determine the effectiveness of a congregation-based intervention in increasing HIV testing among pregnant women and their male partners in southeast Nigeria. The study design details of HBI have been described previously [20]. In summary, 40 churches were selected from 40 communities across seven local government areas in Enugu State, southeast Nigeria. Sample collection was randomly selected, as detailed in [20], and was designed to be representative for the population to minimize selection bias and increase generalizability. Church-organized prayers sessions for pregnant women were used to recruit participants early in their pregnancy. Integrated, onsite tests for HIV, hepatitis B, and sickle cell genotype were implemented during church-organized baby showers to reduce stigma associated with HIV-only testing. Church-organized baby receptions were used for post-delivery follow up and linkage to care. Recruitment occurred between January 20, 2013, and August 31, 2013 and follow-up was completed by August 31, 2014. The study follow-up period was 9 months after the last pregnant woman was recruited, with participant contact (baby receptions) held every 3 months.
Data Collection
Between March 2013 and August 2013, two church-based volunteer health advisors (VHAs) who could read and write in English were selected from each participating church and trained on basic research methodology, including how to obtain informed consent and complete the survey instrument. HBI-trained research coordinators with the assistance of the VHAs conducted an investigator-assisted, cross-sectional survey of HBI participants to collect data on self-reported awareness of sickle cell genotype. Pregnant women and their male partners who signed consent to participate in HBI were independently approached to participate in the cross-sectional survey and to complete a 41-item questionnaire containing two specific questions on sickle cell trait: (a) are you aware of your sickle cell trait status, and (b) if yes, what is your genotype. The survey instrument was piloted and validated among 25 post-partum women in the same community. Participants were then offered screening for sickle cell genotype as part of the integrated laboratory testing. This study was approved by the Institutional Review Board of the University of Nevada, Reno and the Nigerian National Health Research Ethics Committee.
Laboratory procedure
Sickle cell screening was conducted by cellulose acetate electrophoresis modified from Evans (1971) [21]. For confirmation, each test was performed twice.
Statistical analysis
Analyses include descriptive statistics for social demographic distributions and genotype prevalence among participants. Chi-square tests were conducted to determine whether the accuracy of sickle cell status self-reports was significantly different between different groups of participants. A binary logit logistic analysis with Fisher’s scoring was used to determine by an odds ratio assessment whether certain demographic variables were significantly associated with the ability to provide an accurate sickle cell status self-report. Analyses were performed with Statistical Analysis System (SAS), version 9.4.The log-likelihood ratio full-enumeration exact test (“HWxtest” package in R, version 3.3.1) was used to determine if there was a significant difference (P < 0.05) between the number of observed SS individuals and the number of SS individuals expected, assuming Hardy-Weinberg Equilibrium [22].
Role of the funding source
The Healthy Beginning Initiative was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH) and the President’s Emergency Plan for AIDS Relief (PEPFAR). Additional support for this study was provided by the HealthySunrise Foundation; TEND Foundation and Mapuije Foundation. These funding agencies played no role in the study conception, design, data collection, data analysis, data interpretation or writing of the report.
RESULTS
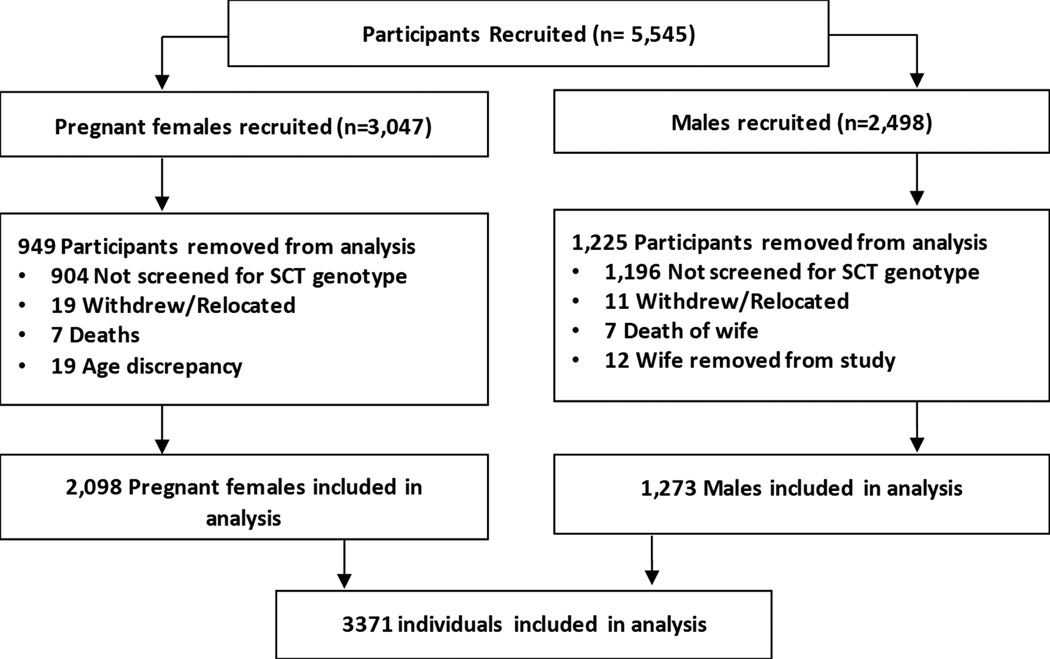
From January to August 2013, we approached and enrolled 3,047 pregnant women and 2,498 male partners participating in HBI. By completion, 45 women and 30 men withdrew, relocated, died or were removed from the study. A total of 69% of women (2,098 of 3,047) and 51% of males (1,273 of 2,498) completed both the questionnaire regarding their self-awareness of their sickle cell status and laboratory screening for sickle cell genotype (Figure 1).
Figure 1.
Flow chart of participants included in the data analysis. Participants included males and females recruited by the Healthy Beginning Initiative program in Enugu, Nigeria.
Cohort demographics
The men and women in our study were predominantly 25–35 years old, married, and had completed their secondary education (Supplementary Table 1). The majority of the study population lived in rural areas, with 27% living greater than 10km from the nearest health care facility (894 of 3,328 reports; 43 did not report), and the remainder equally divided amongst those who lived 5–10km and those who lived 0–5km from the nearest health care facility (Supplementary Table 1). Households with 3–6 people accounted for 70% of study participants (2,342 of 3,333 reports; 38 did not report) (Supplementary Table 1).
Prevalence of SCT and SCD among expectant parents
The prevalence of SCT, as defined by heterozygosity for the S variant of hemoglobin β-subunit (AS), was 22% among our participants (746 of 3,371) (Table 1). SCT prevalence was similar among female and male participants (21% and 23%, respectively, Supplementary Tables 2 and 3). Heterozygosity for the C variant of hemoglobin β-subunit was rare, as is expected for southeastern Nigeria [23]; only four of the 3,371 study participants were genotyped as AC (Table 1). No individuals were compound heterozygous for the hemoglobin S and C variants.
Table 1.
Lab-determined sickle cell genotype vs. self-reported status.
| Genotype | Self-reported status | No self-report | Total | |||
|---|---|---|---|---|---|---|
| AA | AS | SS | Other | |||
| AA | 1465 | 143 | 2 | 4 | 1003 | 2617 |
| AS | 235 | 232 | 2 | 1 | 276 | 746 |
| SS | 3 | 0 | 0 | 0 | 1 | 4 |
| AC | 1 | 0 | 0 | 0 | 3 | 4 |
| Total | 1704 | 375 | 4 | 5 | 1283 | 3371 |
Participant genotype was determined by cellulose acetate electrophoresis on hemolysate from venous blood samples. Shaded cells contain individuals who self-reported their sickle cell status correctly.
Estimates of SCD prevalence due to hemoglobin S homozygosity are commonly calculated from the observed frequency of the S allele in a study population and by assuming Hardy-Weinberg Equilibrium (HWE) [1]. Accordingly, the prevalence of SCD due to hemoglobin S homozygosity in our study population is expected to be 1% (42 of 3,371 participants). However, only four participants in this study were homozygous for hemoglobin S, which equates to an SCD prevalence of 0.1% (Table 1). This 10-fold deviation between the expected and observed SCD prevalence is significantly different (log-likelihood ratio full-enumeration exact test, P = 0.000000).
Reliability of self-reported sickle cell genotype among expectant parents
We found that 62% of participants (2,088 of 3,371) stated that they were aware of their sickle cell status. A greater proportion of females (69%; 1,447 of 2,098) than males (50%; 641 of 1,273) stated that they knew their sickle cell status. Furthermore, among all females and males who reported their sickle cell status, 81% were correct (1,697 of 2,088), as determined by laboratory testing for hemoglobin A, S, and C (see Methods and Table 1). The accuracy of female self-reports on sickle cell status was similar to that of male self-reports (Supplementary Tables 2 and 3).
The proportion of participants who accurately self-reported their status varied depending on the genotype that the individual self-reported. We found that 86% of all female and male participants who self-reported their genotype as AA, which is the most common genotype, were correct (1,465 of 1,704). But only 61% (235 of 384) of those who reported being a non-AA genotype (i.e. AS or “Other”) were correct (Table 1). This phenomenon was similar for both females and males (Supplementary Tables 2 and 3). Furthermore, none of the nine individuals who reported as SS or “Other” were correct; six of these nine individuals were actually AA and the remaining three were AS (Table 1). The difference in accuracy between all individuals (female and male) who self-reported having SCD or SCT (i.e. SS, AS, or AC) and all individuals who reported being unaffected (i.e. AA) was significant (Chi-squared analysis, p<0.0001, Supplementary Table 4).
When including the participants that were not aware of their sickle cell genotype, only 50% of all participants were able to provide an accurate sickle cell status self-report (Table 2). However, only 11% (239 of 2,088) of all participants who said they were aware of their sickle cell status were wrong in a manner that would have endangered the ability of a targeted screen based on parental self-reported status to identify infants with SCD (i.e. parent self-reported as AA when she or he was actually AS, AC, or SS) (Table 1). This proportion was similar among female and male participants (11% vs. 13%, Supplementary Tables 2 and 3).
Table 2.
Demographic distribution of participants based on self-reported status
| Demographic variable |
Participant response |
Correct self- report (%) |
Incorrect or no self-report (%) |
Total |
|---|---|---|---|---|
| Gender | Female | 1184 (56) | 914 (44) | 2098 |
| Male | 513 (40) | 760 (60) | 1273 | |
| Total | 1697 | 1674 | 3371 | |
| Age | 16–24.9 | 239 (49) | 252 (51) | 491 |
| 25–34.9 | 902 (53) | 787 (47) | 1689 | |
| ≥35 | 546 (47) | 608 (53) | 1154 | |
| Totala | 1687 | 1647 | 3334 | |
| Marital status | Married | 1645 (51) | 1589 (49) | 3234 |
| Divorced | 1 (100) | 0 (0) | 1 | |
| Separated | 3 (30) | 7 (70) | 10 | |
| Single | 48 (38) | 78 (62) | 126 | |
| Total | 1697 | 1674 | 3371 | |
| Education level | None/Primary | 369 (33) | 735 (67) | 1104 |
| Secondary | 953 (55) | 791 (45) | 1744 | |
| Tertiary | 370 (75) | 123 (25) | 493 | |
| Totalb | 1692 | 1649 | 3341 | |
| Employment | Full-time | 810 (54) | 690 (46) | 1500 |
| Part-time | 380 (47) | 435 (53) | 815 | |
| Unemployed | 490 (49) | 502 (51) | 992 | |
| Totalc | 1680 | 1627 | 3307 | |
| Household size | ≤2 | 279 (54) | 236 (46) | 515 |
| 3–6 | 1210 (52) | 1132 (48) | 2342 | |
| ≥7 | 198 (42) | 278 (58) | 476 | |
| Totald | 1687 | 1646 | 3333 | |
| Distance to healthcare facility |
0–5km | 585 (49) | 609 (51) | 1194 |
| 5–10km | 641 (52) | 599 (48) | 1240 | |
| 10–15km | 291 (50) | 287 (50) | 578 | |
| ≥15km | 168 (53) | 148 (47) | 316 | |
| Totale | 1685 | 1643 | 3328 | |
| Area | Rural | 1116 (45) | 1338 (55) | 2454 |
| Urban | 576 (65) | 310 (35) | 886 | |
| Totalf | 1692 | 1648 | 3340 |
37 participants did not provide age.
30 participants did not provide education status.
64 participants did not provide employment status.
38 participants did not provide household size.
43 participants did not provide distance to healthcare facility.
31 participants did not provide household area descriptor.
Influence of demographic variables on accuracy of self-reported sickle cell status
Participant demographics were analyzed by self-report accuracy (correct self-reports vs. incorrect report or inability to provide a report) (Table 2). We also performed a binary logit logistic analysis with Fisher’s scoring to determine whether specific demographic variables were significantly associated with participants’ ability to provide an accurate report of their sickle cell status (Table 3). The largest effects in odds ratio came from participant gender, education level, and household location. Our data indicate that females were less likely to provide an inaccurate self-report or to be unable to provide a self-report as compared to males (Table 2), and this difference was statistically significant (odds ratio 0.442; 95% C.I. 0.369–0.53) (Table 3). Similarly, participants who lived in urban areas or who had completed tertiary education were also significantly more likely to provide an accurate self-report (Table 3). Smaller but still statistically significant effects in odds ratio were seen with age, employment status, and household size. Participants older than 35 years were significantly more likely to provide an accurate self-report than participants aged 25–34.9yrs, as were participants who were employed full-time vs. unemployed and participants from households with fewer than 7 people vs. households with 7 or more people (Table 3). The marital status of participants and the distance of their home to the nearest healthcare facility were not significantly associated with a difference in ability to provide an accurate self-report of sickle cell status (Table 3).
Table 3.
Certain demographic variables significantly effect the ability of participants to provide an accurate self-report of sickle cell status.
| Effect | Odds Ratio | 95% Confidence Limits | |
|---|---|---|---|
| Gender: | Female vs. Male | * 0.442 | (0.369–0.53) |
| Age: | ≥35 vs. 25–34.9 | * 0.813 | (0.677–0.977) |
| 16–24.9 vs. 25–34.9 | 1.238 | (0.989–1.548) | |
| Marital status: | Married vs. Single | 0.675 | (0.453–1.006) |
| Separated vs. Single | 1.646 | (0.389–6.972) | |
| Household size: | 3–6 vs. ≥7 | * 0.78 | (0.626–0.973) |
| ≤2 vs. ≥7 | * 0.699 | (0.527–0.929) | |
| Employment: | Full-Time vs. Unemployed | * 0.765 | (0.637–0.919) |
| Part-Time vs. Unemployed | 0.945 | (0.772–1.157) | |
| Education: | None/Primary vs. Tertiary | * 4.033 | (3.124–5.207) |
| Secondary vs. Tertiary | * 1.913 | (1.506–2.43) | |
| Area: | Rural vs. Urban | * 1.738 | (1.455–2.075) |
| Distance to healthcare facility: |
0–5km vs. 5–10 km | 1.052 | (0.886–1.248) |
| 10–15 km vs. 5–10 km | 1.028 | (0.83–1.273) | |
| ≥15 km vs. 5–10 km | 0.962 | (0.736–1.258) |
Logistic analysis with Fisher’s scoring was used to determine the odds ratio for the difference in the risk of participants providing an inaccurate self-report or of being unable to provide a sickle cell status self-report vs. providing an accurate self-report. Odds ratio <1 indicates lower risk of providing an inaccurate self-report or of failing to provide a self-report.
Significant difference between the compared demographic groups’ ability to provide an accurate self-report of sickle cell status.
DISCUSSION
Building on existing infrastructure for HIV testing
Substantial progress has been made in the fight against HIV infection through the partnership between the governments of the United States and Nigeria. From 2004 through 2011, the United States President’s Emergency Plan For AIDS Relief (PEPFAR) invested close to $2.5 billion, including $488 million dollars in 2011 alone, to support Nigeria as it built the infrastructure to fight the HIV/AIDS epidemic [24]. The results of this partnership include 1) an increase in available HIV testing sites from 1,074 in 2009 to 7,075 in 2013, 2) an increase in individuals >15 years of age who have been tested for HIV from 1.7 million in 2009 to 4.08 million in 2013, and 3) an increase in the number of pregnant women tested for HIV from 907,387 in 2010 to 1.7 million in 2013 [25]. The sickle cell screening program used in this study was built on this foundational infrastructure. To our knowledge, our study is the first to integrate sickle cell screening with HIV testing for expectant parents in Nigeria. Our data indicate that acceptance of sickle cell screening for expectant parents and their newborns is very high and that screening for expectant parents and their infants can be readily incorporated into existing, community-based, HIV testing programs and established public health infrastructure, such as those developed by PEPFAR, in resource-limited areas.
Generalizability
Our cohort study was nested within the HBI study, which itself is a cluster-randomized controlled trial. Sample collection for HBI was randomly selected, as detailed in [20], and was designed to be representative for the population in southeastern Nigeria to minimize selection bias and increase generalizability. Furthermore, for HBI and consequently this cohort study, churches were used as convenience venues in the community to identify pregnant women, implement intervention, and conduct post-delivery follow up. This is similar to the use of CVS, Walgreens, etc. for influenza immunization in the United States [26]. These neighborhood stores are used for immunization campaigns in the United States because they are easily accessible, widely distributed and as highly patronized as worship centers in most resource-limited settings. Accordingly, we anticipate that our findings will likely be generalizable for other resource-limited settings beyond southeastern Nigeria. We note that for these same reasons, the HBI model of patient contact via churches in southeastern Nigeria is currently being adapted for implementation in Mosques in Northern Nigeria and Hindu Temples in India, where these venues serve a similar function.
Discordance between SCT and SCD prevalence
The recruitment of such a large cohort enabled us to determine the population prevalence of SCT and SCD among women of childbearing age and their male partners in southeast Nigeria. Importantly, the high prevalence of SCT detected amongst Nigerians in this study is in stark contrast to the low prevalence of SCD (22% vs. 0.1%, respectively) (Table 1). Moreover, the 0.1% SCD prevalence observed in our study population of expectant parents is significantly different than the 1% SCD prevalence expected for these adults, given the observed frequency of the Hemoglobin S allele in our study population and assuming Hardy-Weinberg equilibrium (HWE). This 10-fold difference between observed and expected SCD prevalence suggests excess mortality for individuals with SCD within the birth cohort represented by our adult study population.
Furthermore, a recent analysis of newborn SCD screening surveys throughout Africa determined that deviations from HWE equilibrium are common [22]. Specifically, the observed prevalence of SCD in screened newborns is typically much higher than that expected based on HWE [22]. This implies that within the birth cohort represented by the adult, expectant parents in our study, the 1% SCD prevalence expected based on HWE may also be an underestimate. In this case, the difference between the expected and observed SCD prevalence in our study group would be larger than 10-fold and would suggest an even more serious excess mortality rate for newborns with SCD within the birth cohort represented by our adult study population. This would be consistent with the estimated 50–90% early childhood mortality rate reported for infants with SCD in sub-Saharan Africa [3].
Extent and accuracy of parental self-reported sickle cell status and implications for its use in targeted newborn screening
Targeted newborn screening is less expensive and labor-intensive than universal newborn screening. If effective, it could be a useful public health strategy in resource-limited settings. To be successful, targeted newborn screens based on parental self-reported sickle cell status will require expectant parents to accurately report their sickle cell status.
Individuals may not be able to report their status because they have never been tested or because they do not remember the test results. A cross-sectional survey of Ghanaian women found that only 47% of women who reported being tested for sickle cell also reported that they knew their test results [27]. In our study group, 69% of expectant mothers (1,447 of 2,098) stated that they knew their status, which implies that many more were tested previously. Interestingly, the proportion of women who reported their status in our study is much higher than the 29% that was observed in a previous study of post-partum mothers in Nigeria [14]. This difference may be due to our study’s larger sample size (3,371 vs. 630) or to a general increase in sickle cell testing in Nigeria since Odunvbun, et al. collected their data in 2000 [14]. From the perspective of developing sickle cell screening programs in resource-limited settings, we note that improvements in communication of genetic test results between patients and clinicians could increase individuals’ recollection of their sickle cell status [28–30] and improve the effectiveness of targeted newborn screens based on parental self-reported status without increasing the number of adult diagnostic tests performed (i.e. without increasing the cost of the program).
Improved patient-clinician communication as well as SCT community outreach education could also be used to increase the accuracy of parental self-reported status. We found that the accuracy of parental self-reporting was significantly different when parents reported being unaffected (genotype AA) vs. having SCD or SCT (non-AA genotype) (p-value < 0.0001). In our dataset, expectant parents who reported being AA were much more likely to be correct than individuals who reported having a non-AA genotype (86% vs. 61% accuracy; Table 1). Similar results were recently observed for a cohort of African American adults in the United States, in which 100% of those who reported not having SCD were correct that they did not have SCD (although 12% actually had SCT), yet only 6% of those who reported having SCD were actually SS or SC (63% of these had SCT and 25% were genotype AA) [31]. Together, our results and those of Bean, et al. indicate that self-awareness of one’s sickle cell status is a challenging problem common to both developed nations with robust medical infrastructure and resource-limited areas, such as Nigeria. Furthermore, these findings also suggest that the communication of negative sickle cell test results has been more effective than communication of positive test results (i.e. individual has SCD or SCT).
Our analysis of the influence of participant demographics on the accuracy of parental sickle cell self-reports identified several demographic groups that were significantly less likely to provide an accurate sickle cell self-report. Consequently, communication and education outreach efforts tailored to these subpopulations could be particularly useful. In addition, gender had a large effect on the odds ratio, with males at significantly higher risk of failing to provide an accurate self-report. It is possible that this gender difference could be due to increased contact between females and medical staff due to pregnancy and delivery, which are events that are more likely than others to prompt discussion of sickle cell status and the pattern of sickle cell disease inheritance. Regardless, our data indicate that the inclusion of males from all demographic groups should be a high priority for future sickle cell community outreach educational efforts.
Our data indicates that 11% of all parental self-reports in our study were wrong in a manner that would have endangered the ability of a targeted newborn screen based on parental self-reported status to identify infants with SCD (i.e. parent self-reported as AA when she or he was actually AS, AC, or SS) (Table S2 and Table 1). Thus in regions where resources are limited, yet there is still sufficient public health infrastructure to track and document patient test results, we propose that instead of targeting sickle cell screening to only the infants of parents who do not report their status or who report as a non-AA genotype, the preferred screening strategy would be two-step laboratory testing of i) first-time mothers, and ii) high-risk infants, where highrisk infants would be defined as infants from laboratory-tested, non-AA genotype mothers. In such a strategy, expectant primipara women would be offered sickle cell genotype testing either prenatally or at the time of delivery. Ideally, all tested women would then receive a document with their sickle cell status and be entered into a secure maternal sickle cell genotype database. Preferably, such a database would be accessible to authorized health care providers in both urban and rural Nigerian health care clinics, since an estimated 95 million people (53% of the population) reside in rural areas [32]. Mobile phones and mobile phone coverage are extensive throughout rural Nigeria, and a mobile phone application could be one solution that would enable authorized providers access to the data even in rural areas with frequent electricity interruptions and no ground internet infrastructure [33]. Furthermore, integrating two-step screening within an existing community-based, public health infrastructure, as we did by integrating sickle cell screening as part of the Healthy Beginning Initiative’s HIV testing program, could increase community acceptance and parental commitment while simultaneously increasing program affordability and sustainability. We note that all women who participated in this sickle cell study, which represented 69% of women participating in the HBI community-based HIV testing program, also gave consent for sickle cell screening of their infants, which underscores the feasibility of integrating both primipara and newborn screening for sickle cell with established HIV testing programs for expectant parents.
Testing all primiparas and all infants from non-AA mothers would be more logistically complex to implement than screens based simply on parental self-reported status. But it would eliminate the issue of inaccurate parental sickle cell status self-reports or the lack of parental self-reports. To limit the number of adult diagnostic tests, ideally women would be tested only once, and their genotype results would be stored for use with subsequent pregnancies. For primiparas, this strategy would require 22% more diagnostic tests (adult and newborn tests combined) than a universal newborn screening program that did not test mothers. But for second pregnancies where the maternal genotype could be retrieved from the secure database, this strategy would require only three-quarters of the total diagnostic tests of universal newborn screening (first and second pregnancy; adult and newborn tests combined), and would achieve the same SCD identification rate as universal screening. For third pregnancies, this strategy would require only 55% of the total diagnostic tests required for universal screening (first, second and third pregnancy; adult and newborn tests combined). The cost-savings of implementing this strategy as compared to universal screening continue to improve with additional pregnancies. In Nigeria, the average fertility rate is 6.0 per woman [33], which makes the cost-savings of this strategy vs. universal newborn screening highly attractive.
In summary, we conclude that sickle cell screening of expectant parents and their infants has high public acceptability in Nigeria and can be readily integrated into the existing healthcare infrastructure. The high prevalence of SCT among expectant parents in Nigeria coupled with the unexpectedly low prevalence of SCD among these adults highlights the urgent need for a newborn screening system to identify infants with SCD early so they can receive prompt, lifesaving medical care. Moreover, the high proportion of expectant parents who inaccurately report having SCT or SCD indicates the need for better community education on SCD as well as the need for any targeted newborn screening strategies to be based on parental laboratory tests. Increasing access to SCD diagnosis by implementing routine newborn screening in Nigeria and other resource-limited regions could increase the number of patients who receive timely treatment and help decrease the devastating early mortality rate in children with SCD.
Supplementary Material
Acknowledgments
The Healthy Beginning Initiative was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH) and the President’s Emergency Plan for AIDS Relief (PEPFAR) under award number R01HD075050 to Echezona Ezeanolue, MD. Additional support for this study was provided by the HealthySunrise Foundation, TEND Foundation and Mapuije Foundation. The funding agencies played no role in the study conception, design, data collection, data analysis, data interpretation or writing of the report. We are grateful to the Catholic Bishop of Awgu diocese, Anglican Bishop of Enugu; Catholic Bishop of Enugu; Anglican Bishop of Oji-River. Their support was instrumental to the successful implementation of HBI. HBI implementation would not have been possible without the support and tireless effort of the priests in the participating churches. The church-based Volunteer Health Advisors took ownership of the program and made the process of recruitment and implementation smooth for our study team and participants. This study would have been impossible to conduct without the support of PeTR-GS (our PEPFAR-supported partner) staff and volunteers. The authors are grateful to Thomas Kozel, PhD and Betty Pace, MD for their insightful comments on the manuscript.
Footnotes
Contributions
EEE conceptualized the study. EEE, MCO, COE and WY contributed to the study design and trial protocol. Patient recruitment and acquisition of data was done by EEE, MCO, AO, AGO, AH and DP. AB-M analyzed the data. AB-M and WY performed the statistical analysis. AB-M and EEE wrote the manuscript. All authors subsequently reviewed the output, made revisions and approved the content of the manuscript.
Declaration of Interests
The authors declare that they have no conflicts of interest.
Contributor Information
Amanda R. Burnham-Marusich, Email: burnham-marusich@medicine.nevada.edu.
Chinenye O. Ezeanolue, Email: anolue76@yahoo.com.
Michael C Obiefune, Email: mobiefune.ihv@gmail.com.
Wei Yang, Email: weiyang@unr.edu.
Alice Osuji, Email: aaghams.petrgs@gmail.com.
Amaka G. Ogidi, Email: aogidi.petrgs@gmail.com.
Aaron T. Hunt, Email: aaron.hunt@unlv.edu.
Dina Patel, Email: dina.patel@unlv.edu.
Echezona E. Ezeanolue, Email: echezona.ezeanolue@unlv.edu.
References
- 1.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piel FB, Howes RE, Patil AP, Nyangiri OA, Gething PW, Bhatt S, Williams TN, Weatherall DJ, Hay SI. The distribution of haemoglobin c and its prevalence in newborns in africa. Scientific reports. 2013;3:1671. doi: 10.1038/srep01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in africa: A neglected cause of early childhood mortality. American journal of preventive medicine. 2011;41:S398–S405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Regional Office for Africa. Geneva, Switzerland: WHO; 2010. Sickle-cell disease: A strategy for the who african region. Report Number AFR/RC60/8. [Google Scholar]
- 6.Galadanci N, Wudil BJ, Balogun TM, Ogunrinde GO, Akinsulie A, Hasan-Hanga F, Mohammed AS, Kehinde MO, Olaniyi JA, Diaku-Akinwumi IN, Brown BJ, Adeleke S, Nnodu OE, Emodi I, Ahmed S, Osegbue AO, Akinola N, Opara HI, Adegoke SA, Aneke J, Adekile AD. Current sickle cell disease management practices in nigeria. Int Health. 2014;6:23–28. doi: 10.1093/inthealth/iht022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vichinsky E, Hurst D, Earles A, Kleman K, Lubin B. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics. 1988;81:749–755. [PubMed] [Google Scholar]
- 8.Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, Zarkowsky H, Vichinsky E, Iyer R, Lobel JS, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A. The New England journal of medicine. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 9.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girosi F, Olmsted SS, Keeler E, Hay Burgess DC, Lim YW, Aledort JE, Rafael ME, Ricci KA, Boer R, Hilborne L, Derose KP, Shea MV, Beighley CM, Dahl CA, Wasserman J. Developing and interpreting models to improve diagnostics in developing countries. Nature. 2006;444(Suppl 1):3–8. doi: 10.1038/nature05441. [DOI] [PubMed] [Google Scholar]
- 11.Tshilolo L, Aissi LM, Lukusa D, Kinsiama C, Wembonyama S, Gulbis B, Vertongen F. Neonatal screening for sickle cell anaemia in the democratic republic of the congo: Experience from a pioneer project on 31 204 newborns. Journal of clinical pathology. 2009;62:35–38. doi: 10.1136/jcp.2008.058958. [DOI] [PubMed] [Google Scholar]
- 12.Kafando E, Sawadogo M, Cotton F, Vertongen F, Gulbis B. Neonatal screening for sickle cell disorders in ouagadougou, burkina faso: A pilot study. Journal of medical screening. 2005;12:112–114. doi: 10.1258/0969141054855300. [DOI] [PubMed] [Google Scholar]
- 13.Ohene-Frempong K, Oduro J, Tetteh H, Nkrumah F. Screening newborns for sickle cell disease in ghana. Pediatrics. 2008;121:S120–S121. [Google Scholar]
- 14.Odunvbun ME, Okolo AA, Rahimy CM. Newborn screening for sickle cell disease in a nigerian hospital. Public Health. 2008;122:1111–1116. doi: 10.1016/j.puhe.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 15.McGann PT, Ferris MG, Ramamurthy U, Santos B, de Oliveira V, Bernardino L, Ware RE. A prospective newborn screening and treatment program for sickle cell anemia in luanda, angola. Am J Hematol. 2013;88:984–989. doi: 10.1002/ajh.23578. [DOI] [PubMed] [Google Scholar]
- 16.Tubman VN, Marshall R, Jallah W, Guo D, Ma C, Ohene-Frempong K, London WB, Heeney MM. Newborn screening for sickle cell disease in liberia: A pilot study. Pediatr Blood Cancer. 2016 doi: 10.1002/pbc.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahimy MC, Gangbo A, Ahouignan G, Alihonou E. Newborn screening for sickle cell disease in the republic of benin. Journal of clinical pathology. 2009;62:46–48. doi: 10.1136/jcp.2008.059113. [DOI] [PubMed] [Google Scholar]
- 18.Omotade OO, Kayode CM, Falade SL, Ikpeme S, Adeyemo AA, Akinkugbe FM. Routine screening for sickle cell haemoglobinopathy by electrophoresis in an infant welfare clinic. West Afr J Med. 1998;17:91–94. [PubMed] [Google Scholar]
- 19.Tshilolo L, Kafando E, Sawadogo M, Cotton F, Vertongen F, Ferster A, Gulbis B. Neonatal screening and clinical care programmes for sickle cell disorders in sub-saharan africa: Lessons from pilot studies. Public Health. 2008;122:933–941. doi: 10.1016/j.puhe.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Ezeanolue EE, Obiefune MC, Yang W, Obaro SK, Ezeanolue CO, Ogedegbe GG. Comparative effectiveness of congregation- versus clinic-based approach to prevention of mother-to-child hiv transmission: Study protocol for a cluster randomized controlled trial. Implementation science : IS. 2013;8:62. doi: 10.1186/1748-5908-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DI. Haemoglobin electrophoresis on cellulose acetate using whole blood samples. Journal of clinical pathology. 1971;24:877–878. doi: 10.1136/jcp.24.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piel FB, Adamkiewicz TV, Amendah D, Williams TN, Gupta S, Grosse SD. Observed and expected frequencies of structural hemoglobin variants in newborn screening surveys in africa and the middle east: Deviations from hardy-weinberg equilibrium. Genet Med. 2016;18:265–274. doi: 10.1038/gim.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akinyanju OO. A profile of sickle cell disease in nigeria. Annals of the New York Academy of Sciences. 1989;565:126–136. doi: 10.1111/j.1749-6632.1989.tb24159.x. [DOI] [PubMed] [Google Scholar]
- 24.Nigeria USDMt. U.S. President's emergency plan for aids relief [Google Scholar]
- 25.(NACA) NAfCoA. Federal republic of nigeria: Global aids response country progress report. Abuja, Nigeria: 2014. [Google Scholar]
- 26.Ferris AH, McAndrew TM, Shearer D, Donnelly GF, Miller HA. Embracing the convenient care concept. Postgrad Med. 2010;122:7–9. doi: 10.3810/pgm.2010.01.2093. [DOI] [PubMed] [Google Scholar]
- 27.Ross PT, Lypson ML, Ursu DC, Everett LA, Rodrigues O, Campbell AD. Attitudes of ghanaian women toward genetic testing for sickle cell trait. Int J Gynaecol Obstet. 2011;115:264–268. doi: 10.1016/j.ijgo.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Hayeems RZ, Bytautas JP, Miller FA. A systematic review of the effects of disclosing carrier results generated through newborn screening. J Genet Couns. 2008;17:538–549. doi: 10.1007/s10897-008-9180-1. [DOI] [PubMed] [Google Scholar]
- 29.Goldsmith JC, Bonham VL, Joiner CH, Kato GJ, Noonan AS, Steinberg MH. Framing the research agenda for sickle cell trait: Building on the current understanding of clinical events and their potential implications. Am J Hematol. 2012;87:340–346. doi: 10.1002/ajh.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrell MH, Christopher SA, Tluczek A, Kennedy-Parker K, La Pean A, Eskra K, Collins J, Hoffman G, Panepinto J, Farrell PM. Improving communication between doctors and parents after newborn screening. WMJ. 2011;110:221–227. [PMC free article] [PubMed] [Google Scholar]
- 31.Bean CJ, Hooper WC, Ellingsen D, DeBaun MR, Sonderman J, Blot WJ. Discordance between self-report and genetic confirmation of sickle cell disease status in african-american adults. Public Health Genomics. 2014;17:169–172. doi: 10.1159/000360260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Division UNDoEaSAP. World urbanization prospects: The 2014 revision, highlights (st/esa/ser.A/352) 2014 [Google Scholar]
- 33.World Health Organization. Geneva: WHO Press; 2014. World health statistics 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.