Abstract
Context:
An elevated fibroblast growth factor (FGF) 23 is an independent risk factor for cardiovascular disease and mortality in patients with kidney disease. The relationship between FGF23 and cause-specific mortality in the general population is unknown.
Objective:
To investigate the association of elevated FGF23 with the risk of cause-specific mortality in a racially and ethnically diverse urban general population.
Design, Setting, Participants:
The Northern Manhattan Study is a population-based prospective cohort study. Residents who were > 39 years old and had no history of stroke were enrolled between 1993 and 2001. Participants with available blood samples for baseline FGF23 testing were included in the current study (n = 2525).
Main Outcome Measures:
Cause-specific death events.
Results:
A total of 1198 deaths (474 vascular, 612 nonvascular, 112 unknown cause) occurred during a median follow-up of 14 years. Compared to participants in the lowest FGF23 quintile, those in the highest quintile had a 2.07-fold higher risk (95% confidence interval [CI], 1.45, 2.94) of vascular death and a 1.64-fold higher risk (95% CI, 1.22, 2.20) of nonvascular death in fully adjusted models. Higher FGF23 was independently associated with increased risk of mortality due to cancer, but only in Hispanic participants (hazard ratio per 1 unit increase in ln FGF23 of 1.87; 95% CI, 1.40, 2.50; P for interaction = .01).
Conclusions:
Elevated FGF23 was independently associated with increased risk of vascular and nonvascular mortality in a diverse general population and with increased risk of cancer death specifically in Hispanic individuals.
Elevated FGF23 was independently associated with increased risk of vascular and non-vascular mortality in a diverse general population, and with increased risk of cancer death in Hispanics.
Fibroblast growth factor 23 (FGF23) is an endocrine hormone that is primarily secreted by osteocytes (1). FGF23 regulates phosphate homeostasis by increasing renal phosphate excretion and by inhibiting renal production of 1,25-dihydroxyvitamin D and accelerating its degradation (2). Together, the latter effects reduce the efficiency of gastrointestinal absorption of dietary phosphate. In healthy individuals, serum phosphate levels are maintained within a narrow normal range by FGF23 levels that rise and fall in parallel with dietary phosphate intake (3, 4). In patients with chronic kidney disease, FGF23 levels rise progressively beginning early in the course of disease as part of a compensatory response that maintains neutral phosphate balance as patients' capacity for renal phosphate excretion decreases (5, 6).
In contrast to these seemingly beneficial and compensatory physiological effects, prospective studies demonstrated that elevated FGF23 is independently associated with increased risks of cardiovascular disease events and death (7–10). As potential underlying mechanisms, elevated FGF23 has been implicated in the pathogenesis of left ventricular hypertrophy, impaired leukocyte function, and chronic inflammation (11–14). These data suggest that the initially adaptive increases in FGF23 levels in chronic kidney disease may ultimately become maladaptive and contribute to end-organ injury (15).
Most prior clinical outcome studies of FGF23 examined individuals with moderate to severe chronic kidney disease, end-stage renal disease (ESRD), and prevalent cardiovascular disease (7–9). Few studies focused on general populations, and data on the relationships of FGF23 with specific causes of death are scarce (16–20). Furthermore, most prior studies investigated predominantly Caucasian or African American populations. Data on the relationship of FGF23 with mortality in the Hispanic-American population are lacking. We tested the hypothesis that elevated FGF23 is an independent risk factor for death in the Northern Manhattan Study (NOMAS), which is a population-based prospective cohort of a racially and ethnically diverse urban population, including a large proportion of Hispanic Americans.
Subjects and Methods
Study population
NOMAS is a population-based prospective cohort study of stroke risk factors, incidence, and prognosis in a racially and ethnically diverse urban population. The study details have been published previously (21). Briefly, eligible participants were stroke-free, > 39 years old, and residents of Northern Manhattan, New York, for at least 3 months in a household with a telephone. Audits and Surveys, Inc., performed random digit dialing using dual-frame sampling to invite individuals to an in-person baseline interview and assessment. The initial telephone response rate was 91%, and a total of 3298 participants ultimately enrolled between 1993 and 2001, representing an overall participation rate of 68%. The current study was limited to the 2525 participants with available blood samples for measurements of circulating FGF23 and creatinine concentrations at baseline. Characteristics of participants in whom FGF23 could be tested were generally similar to those of the overall cohort (10). All participants provided written informed consent, and the institutional review boards at Columbia University Medical Center and the University of Miami approved the study.
Baseline data collection and measurements
Data on sociodemographic characteristics, risk factors for stroke and cardiovascular disease, and other medical conditions were collected through in-person interviews by trained research personnel using standardized data collection instruments. Physical examination was conducted by study physicians. All assessments were conducted in English or Spanish, depending on participants' primary language. Race and ethnicity were determined based on self-identification using questions modeled after the U.S. census (22). Standardized questions, adapted from the Behavioral Risk Factor Surveillance System by the Center for Disease Control and Prevention, were used to obtain information regarding cigarette smoking, hypertension, diabetes, hypercholesterolemia, and prevalent cardiovascular disease (23). Standard techniques were used to measure blood pressure, height, and weight. Morning fasting blood samples were obtained.
Baseline blood specimens were spun immediately at room temperature and stored at −80°C until processing. Plasma FGF23 was measured in duplicate in batches of stored samples at the University of Miami FGF23 Core Laboratory using the second generation C-terminal enzyme linked immunosorbent assay (Immutopics). The intra-assay coefficient of variation was 3.6%; the interassay coefficients of variation evaluated with quality-control samples from 26 independent assay runs were 2.7% at a concentration of 31 relative units (RU)/mL and 4.4% at a level of 272 RU/mL. Levels of 25-hydroxyvitamin D were measured using liquid chromatography mass spectrometry (intra-assay, 5.7% for 25-hydroxyvitamin D2, and 5.1% for 25-hydroxyvitamin D3; interassay, 5.2% for 25-hydroxyvitamin D2, and 6.9% for 25-hydroxyvitamin D3). Serum phosphate was measured spectrophotometrically, intact (1–84) PTH was measured by electrochemiluminescence using the manufacturer's reagents (Roche Diagnostics), and serum creatinine was measured using the kinetic alkaline picrate assay (Jaffé reaction). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation (24).
Mortality outcomes and cause of death
The outcomes were all-cause mortality and cause-specific death events. All participants or their family members underwent annual telephone follow-up to assess their vital status. Participants were followed until the occurrence of death, voluntary withdrawal from the study, loss to follow-up, or January 2015 when the database was locked for this analysis. Only two participants from the NOMAS cohort were completely lost to follow-up after their baseline examination, and the average annual contact rate was 99%. Cause of death was adjudicated using medical records, death certificates, and interviews with family members and health care providers, as needed. Study physicians resolved uncertain cases. The study team first evaluated and classified all deaths as either vascular or nonvascular. Vascular causes included stroke, myocardial infarction, congestive heart failure, pulmonary embolism, arrhythmia, and sudden death. Nonvascular causes included cancer, chronic obstructive pulmonary disease, infection, and other. Deaths without sufficient records were deemed to be of unknown etiology.
Statistical analysis
We used descriptive statistics to compare demographic and clinical characteristics across quintiles of baseline FGF23 levels. Continuous variables were summarized as mean with standard deviation or median with interquartile range (IQR). Categorical variables were expressed as proportions. When analyzed as a continuous variable, FGF23 was natural log-transformed (ln) to follow a normal distribution. We used Cox proportional hazards regression to examine the relationships between baseline plasma FGF23 levels and all-cause and cause-specific mortality. Model 1 was adjusted for demographic factors (age, sex, and race/ethnicity). Model 2 was adjusted further for traditional cardiovascular disease risk factors (cigarette smoking, body mass index, hypertension, diabetes, hypercholesterolemia, and prevalent cardiovascular disease) and eGFR. Model 3 was adjusted further for mineral metabolism markers (serum phosphate, PTH, 25-hydroxyvitamin D, calcium, and albumin). The proportionality assumption was met for cause-specific mortality, but it was violated for all-cause mortality. To accommodate nonproportional hazards of all-cause mortality, we included time-by-covariate interaction terms in the multivariable Cox models of all-cause mortality (25). We tested whether race/ethnicity, impaired kidney function, prevalent cardiovascular disease, and baseline 25-hydroxyvitamin D modified the effect of FGF23 on mortality by testing the significance of interaction terms (candidate effect modifier × ln FGF23). Analyses were performed using SAS version 9.4 (SAS Institute). All statistical tests were two-sided, and P values < .05 were considered statistically significant.
Results
Baseline characteristics of the 2525 participants are presented in Table 1, overall and by FGF23 quintiles. The mean age was 68.9 ± 9.9 years; 36.4% of participants were men, 53.6% were Hispanic, and 23.4% were non-Hispanic black. One fifth of the participants had a history of cardiovascular disease, the prevalence of which increased with ascending FGF23 quintiles. The mean eGFR was 79.7 ± 22.4 mL/min/1.73 m2; 16.4% of participants had eGFR < 60 mL/min/1.73 m2.
Table 1.
Baseline Characteristics Overall and by FGF23 Quintiles
| Overall | FGF23 |
|||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| n | 2525 | 504 | 506 | 505 | 505 | 505 |
| FGF23, RU/mL | 57 (44–81) | 36 (32–39) | 47 (44–49) | 57 (54–60) | 73 (68–81) | 131 (104–233) |
| Demographics | ||||||
| Age, y | 68.9 (9.9) | 66.6 (9.4) | 67.8 (9.5) | 67.7 (9.6) | 70.1 (10.0) | 72.3 (10.1) |
| Male, n (%) | 919 (36.4) | 245 (48.6) | 209 (41.3) | 184 (36.4) | 133 (26.3) | 148 (29.3) |
| Race/ethnicity, n (%) | ||||||
| Hispanic | 1353 (53.6) | 270 (53.6) | 295 (58.3) | 281 (55.6) | 262 (51.9) | 245 (48.5) |
| Non-Hispanic black | 590 (23.4) | 121 (24.0) | 107 (21.1) | 92 (18.2) | 119 (23.6) | 151 (29.9) |
| Non-Hispanic white | 521 (20.6) | 104 (20.6) | 93 (18.4) | 116 (23.0) | 112 (22.2) | 96 (19.0) |
| Non-Hispanic other | 61 (2.4) | 9 (1.8) | 11 (2.2) | 16 (3.2) | 12 (2.4) | 13 (2.6) |
| CVD risk factors | ||||||
| BMI, kg/m2 | 27.8 (5.4) | 26.7 (4.4) | 27.3 (5.1) | 27.8 (5.3) | 29.0 (5.9) | 28.2 (5.9) |
| Cigarette smoking, n (%) | 426 (16.9) | 79 (15.7) | 73 (14.4) | 85 (16.8) | 86 (17.0) | 103 (20.4) |
| Prevalent CVD, n (%) | 566 (22.4) | 73 (14.5) | 100 (19.8) | 97 (19.2) | 137 (27.1) | 159 (31.5) |
| Hypertension, n (%) | 1875 (74.3) | 323 (64.1) | 359 (70.9) | 371 (73.5) | 411 (81.4) | 411 (81.4) |
| Diabetes, n (%) | 535 (21.2) | 96 (19.0) | 97 (19.2) | 91 (18.0) | 113 (22.4) | 138 (27.3) |
| Hypercholesterolemia, n (%) | 1164 (46.1) | 215 (42.7) | 236 (46.6) | 245 (48.5) | 245 (48.5) | 223 (44.2) |
| Biomarkers | ||||||
| Phosphate, mg/dL | 3.1 (0.5) | 3.0 (0.5) | 3.0 (0.4) | 3.0 (0.5) | 3.1 (0.5) | 3.2 (0.6) |
| Calcium, mg/dL | 9.1 (0.5) | 9.1 (0.4) | 9.0 (0.6) | 9.1 (0.4) | 9.1 (0.7) | 9.1 (0.5) |
| Albumin, g/dL | 4.4 (0.3) | 4.5 (0.3) | 4.5 (0.3) | 4.5 (0.3) | 4.4 (0.3) | 4.3 (0.4) |
| 25-hydroxyvitamin D, ng/mL | 22.4 (14.0) | 22.3 (11.4) | 22.0 (12.9) | 24.2 (18.7) | 22.9 (13.9) | 20.6 (11.4) |
| PTH, pg/mL | 58.9 (40.9) | 48.7 (19.4) | 53.8 (20.2) | 55.9 (22.8) | 59.9 (26.1) | 76.4 (77.1) |
| Creatinine, mg/dL | 1.0 (0.4) | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.3) | 0.9 (0.3) | 1.1 (0.7) |
| eGFR, mL/min/1.73 m2 | 79.7 (22.4) | 85.8 (21.3) | 83.4 (19.8) | 79.6 (18.5) | 78.4 (21.8) | 71.5 (27.1) |
| eGFR < 60, n (%) | 413 (16.4) | 45 (8.9) | 46 (9.1) | 55 (10.9) | 96 (19.0) | 171 (33.9) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease. Continuous variables are reported as mean (SD) or median (IQR).
The median FGF23 for the overall population was 56.9 (IQR, 43.9–81.0) RU/mL. There were no significant differences between race/ethnicity groups: Hispanic, 55.1 (IQR, 43.9–79.1) RU/mL; non-Hispanic black, 59.7 (IQR, 43.8–91.3) RU/mL; non-Hispanic white, 57.7 (IQR, 43.7–78.6) RU/mL; and non-Hispanic other, 56.5 (IQR, 47.1–77.4) RU/mL. Compared to individuals in the lowest FGF23 quintile, participants in the highest quintile were older, more frequently were women, and had a higher prevalence of hypertension and diabetes and lower eGFR.
Mortality outcomes
A total of 1198 deaths occurred during a median follow-up of 14 (IQR, 9–16) years. The cause of death was vascular in 474 participants, nonvascular in 612, and unknown in 112. Leading causes of vascular death were sudden death (n = 226), stroke (n = 60), myocardial infarction (n = 56), heart failure (n = 47), and other (n = 85). The leading causes of nonvascular death were cancer (n = 233), chronic obstructive pulmonary disease (n = 116), infection (n = 111), and other (n = 152).
FGF23 and risk of all-cause mortality
Because the proportionality assumption was violated for all-cause mortality, we used Cox models with time-by-covariate interaction terms to examine associations between FGF23 and all-cause mortality. In the fully adjusted model with covariates and their corresponding time-by-covariate interaction terms, higher FGF23, expressed on a continuous scale, was independently associated with increased risk of all-cause mortality (hazard ratio [HR], 2.71 per 1 U increase in ln FGF23; 95% confidence interval [CI], 1.30, 5.65).
FGF23 and risk of specific causes of death
Higher FGF23, expressed as a continuous variable or in quintiles, was independently associated with increased risk of mortality due to vascular causes and nonvascular causes, including cancer in all multivariable-adjusted models (Table 2). In contrast, higher FGF23 was associated with increased risk of death due to infection (HR, 1.55 per 1 U increase in ln FGF23; 95% CI, 1.16, 2.07) when it was analyzed on a continuous scale, but not when it was analyzed categorically (data not shown).
Table 2.
HRs of FGF23 for Cause-Specific Mortality
| Deaths, n | Incidencea | Model 1c |
Model 2d |
Model 3e |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Vascular death | ||||||||
| ln FGF23b | 474 | 15.0 | 1.54 (1.36, 1.74) | <.001 | 1.43 (1.25, 1.63) | <.001 | 1.38 (1.19, 1.60) | <.001 |
| Quintile 1 | 69 | 9.7 | Reference | Reference | Reference | |||
| Quintile 2 | 80 | 12.1 | 1.29 (0.94, 1.79) | .120 | 1.22 (0.88, 1.69) | .24 | 1.32 (0.92, 1.91) | .133 |
| Quintile 3 | 88 | 13.4 | 1.39 (1.01, 1.90) | .044 | 1.28 (0.93, 1.76) | .137 | 1.27 (0.88, 1.84) | .194 |
| Quintile 4 | 92 | 14.7 | 1.27 (0.92, 1.75) | .143 | 1.02 (0.74, 1.42) | .89 | 1.00 (0.69, 1.47) | .98 |
| Quintile 5 | 145 | 29.2 | 2.55 (1.89, 3.43) | <.001 | 2.07 (1.53, 2.81) | <.001 | 2.07 (1.45, 2.94) | <.001 |
| P for trend | <.001 | <.001 | <.001 | |||||
| Nonvascular death | ||||||||
| ln FGF23b | 612 | 19.4 | 1.59 (1.44, 1.77) | <.001 | 1.52 (1.36, 1.69) | <.001 | 1.43 (1.27, 1.62) | <.001 |
| Quintile 1 | 106 | 14.8 | Reference | Reference | Reference | |||
| Quintile 2 | 99 | 15.0 | 1.06 (0.80, 1.39) | .70 | 1.03 (0.78, 1.36) | .82 | 1.03 (0.76, 1.39) | .85 |
| Quintile 3 | 97 | 14.8 | 1.06 (0.80, 1.39) | .71 | 1.05 (0.79, 1.38) | .76 | 1.02 (0.75, 1.38) | .92 |
| Quintile 4 | 147 | 23.5 | 1.49 (1.15, 1.92) | .002 | 1.42 (1.09, 1.85) | .010 | 1.40 (1.05, 1.88) | .024 |
| Quintile 5 | 163 | 32.8 | 2.07 (1.60, 2.66) | <.001 | 1.92 (1.48, 2.49) | <.001 | 1.64 (1.22, 2.20) | .001 |
| P for trend | <.001 | <.001 | <.001 | |||||
| Cancer death | ||||||||
| ln FGF23b | 233 | 7.4 | 1.42 (1.19, 1.71) | <.001 | 1.34 (1.11, 1.62) | .002 | 1.32 (1.08, 1.62) | .010 |
| Quintile 1 | 41 | 5.7 | Reference | Reference | Reference | |||
| Quintile 2 | 45 | 6.8 | 1.27 (0.83, 1.94) | .27 | 1.26 (0.82, 1.93) | .29 | 1.31 (0.82, 2.08) | .26 |
| Quintile 3 | 35 | 5.3 | 1.02 (0.65, 1.61) | .92 | 1.00 (0.63, 1.58) | .99 | 1.04 (0.63, 1.70) | .89 |
| Quintile 4 | 55 | 8.8 | 1.64 (1.08, 2.47) | .019 | 1.64 (1.07, 2.51) | .022 | 1.72 (1.08, 2.74) | .022 |
| Quintile 5 | 57 | 11.5 | 2.11 (1.40, 3.19) | <.001 | 2.02 (1.32, 3.09) | .001 | 1.88 (1.17, 3.04) | .010 |
| P for trend | <.001 | <.001 | .010 | |||||
Incidence per 1000 person-years.
Per 1 U increases in ln FGF23.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: adjusted for covariates in model 1 plus traditional cardiovascular disease risk factors (cigarette smoking, body mass index, hypertension, diabetes, hypercholesterolemia, prevalent cardiovascular disease) and eGFR.
Model 3: adjusted for covariates in models 1 and 2 plus mineral metabolism markers (phosphate, PTH, 25-hydroxyvitamin D, calcium, and albumin).
FGF23 and risk of mortality: effect modification by clinical factors
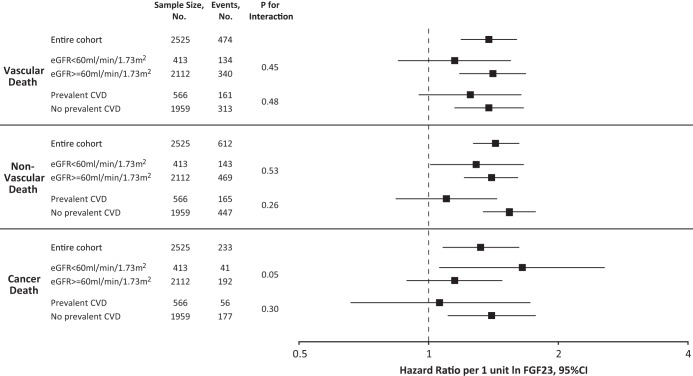
The relationship between FGF23 and all-cause mortality was not significantly modified by impaired kidney function (eGFR < 60 mL/min/1.73 m2; P for interaction = .85), prevalent cardiovascular disease (P for interaction = .37), or baseline 25-hydroxyvitamin D levels (P for interaction = .47). Similarly, these factors did not significantly modify the associations between elevated FGF23 and vascular, nonvascular, and cancer death (Figure 1).
Figure 1.
HRs of FGF23 for cause-specific mortality, stratified by kidney function and prevalent cardiovascular disease. Multivariable-adjusted risks of vascular, nonvascular, and cancer death, stratified by kidney function and prevalent cardiovascular disease. The model was adjusted for age, sex, cigarette smoking, body mass index, hypertension, diabetes, hypercholesterolemia, prevalent cardiovascular disease, eGFR, phosphate, PTH, 25-hydroxyvitamin D, calcium, and albumin. HR per 1 U increase in natural log-transformed levels of FGF23. Error bars indicate 95% CI. CVD, cardiovascular disease.
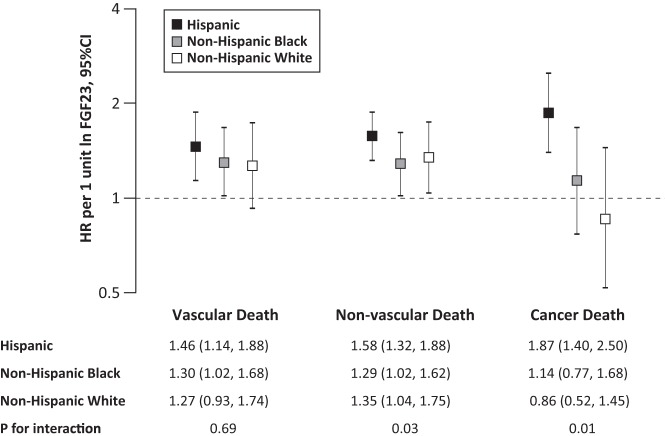
FGF23 and risk of mortality: effect modification by race and ethnicity
Black race did not modify the relationship between FGF23 and risk of all-cause mortality (P for interaction = .18), but there was significant interaction between FGF23 and Hispanic ethnicity (P for interaction = .02). The overall crude death rate was higher among non-Hispanic (55.9/1000 person-years for non-Hispanic black and 52.3/1000 person-years for non-Hispanic white) than Hispanic participants (26.3/1000 person-years). The magnitude of vascular-related mortality risk associated with FGF23 was comparable among Hispanic (HR, 1.46 per 1 U increase in ln FGF23; 95% CI, 1.14, 1.88) and non-Hispanic participants (HR, 1.30 per 1 U increase in ln FGF23; 95% CI, 1.02, 1.68, for non-Hispanic black; and HR, 1.27 per 1 U increase in ln FGF23; 95% CI, 0.93, 1.74, for non-Hispanic white; P for interaction = .69; Figure 2). In contrast, the magnitude of the risk of nonvascular mortality associated with FGF23 was significantly higher in Hispanic (HR, 1.58 per 1 U increase in ln FGF23; 95% CI, 1.32, 1.88) vs non-Hispanic participants (HR, 1.29 per 1 U increase in ln FGF23; 95% CI, 1.02, 1.62, for non-Hispanic black; and HR, 1.35 per 1 U increase in ln FGF23; 95% CI, 1.04, 1.75, for non-Hispanic white; P for interaction = .03; Figure 2). This was primarily driven by cancer death. Elevated FGF23 was associated with significantly increased risk of cancer death among Hispanic participants (HR, 1.87 per 1 U increase in ln FGF23; 95% CI, 1.40, 2.50), but not among non-Hispanic participants (HR, 1.14 per 1 U increase in ln FGF23; 95% CI, 0.77, 1.68, for non-Hispanic black; and HR, 0.86 per 1 U increase in ln FGF23; 95% CI, 0.52, 1.45, for non-Hispanic white; P for interaction = .01; Figure 2).
Figure 2.
HRs of FGF23 for cause-specific mortality by race/ethnicity. Multivariable-adjusted risks of vascular, nonvascular, and cancer death per 1 U increase in natural log-transformed levels of FGF23. The model was adjusted for age, sex, cigarette smoking, body mass index, hypertension, diabetes, hypercholesterolemia, prevalent cardiovascular disease, eGFR, phosphate, PTH, 25-hydroxyvitamin D, calcium, and albumin. Error bars indicate 95% CI.
Discussion
Our findings from the population-based and racially and ethnically diverse NOMAS extend prior work on elevated FGF23 levels and risk of mortality. We confirm that elevated FGF23 is a risk factor for all-cause and cardiovascular mortality in the general population as it is in chronic kidney disease, but we now provide new evidence for an independent relationship between elevated FGF23 and increased risk of mortality due to nonvascular causes, including cancer, in the general population. Enabled by the unique demographics of the NOMAS population, we also report that the association between elevated FGF23 and the risk of nonvascular mortality appeared to be driven primarily by the significantly higher risk of cancer death among the Hispanic participants. Additional studies are needed to corroborate our findings on FGF23 and the risk of nonvascular death and to investigate potential mechanisms for the higher risk of FGF23-associated cancer mortality among individuals of Hispanic ethnicity.
Our findings of strong associations between elevated FGF23 and increased risks of all-cause mortality and vascular mortality are consistent with the results of previous studies of patients with moderate to severe chronic kidney disease, patients with ESRD undergoing hemodialysis, and individuals with prevalent cardiovascular disease (7–9, 26, 27). Although studies from the general population are relatively sparse and yielded less consistent results, most concluded that elevated FGF23 was independently associated with increased risk of all-cause mortality and cardiovascular disease events (16–20). These prior studies evaluated populations composed of older and primarily Caucasian individuals, whereas the NOMAS population was younger and more racially and ethnically diverse. Additionally, NOMAS collected information on specific causes of death, which had not been analyzed previously.
To our knowledge, our finding of higher risk of cancer death among the Hispanic participants in NOMAS is the first report of an independent association between elevated FGF23 and increased risk of cancer death. Although our study was not capable of investigating specific cancer types or potential underlying mechanisms, we can speculate on several possibilities. In the kidney, FGF23 exerts its effects on phosphate and vitamin D homeostasis by activating heterodimeric receptor complexes that consist of α-Klotho and FGF receptors (FGFRs) 1, 3, and 4 (28). In contrast, in cells that do not express α-Klotho, for example, cardiac myocytes and hepatocytes, FGF23 specifically activates FGFR4 in an α-Klotho-independent manner (12, 14). A single nucleotide polymorphism in the coding region of the FGFR4 gene that results in the rs351855G/A substitution leads to constitutive FGFR4 activation and is associated with accelerated cancer progression and poor prognosis in multiple cancer types, including breast, colon, and lung carcinoma (29, 30). In “knock-in” mice that are genetically engineered to express the mutant rs351855G/A FGFR4 in its natural position in the genome, “second hits” that stimulate FGFR4 are required to induce carcinogenesis (31). Interestingly, Hispanic American populations were more likely to carry the oncogenic FGFR4 rs351855G/A allele than other ethnic groups in some, but not all, reports and public databases (32, 33). Whether elevated FGF23 levels in the setting of more frequent expression of the rs351855G/A risk allele could explain the increased risk of cancer death among Hispanics that we observed will require additional studies to further investigate potential gene-environment interactions, such as between FGFR4 alleles and elevated FGF23 levels.
Another possible mechanism of increased susceptibility to cancer death among Hispanic populations could relate to differences in vitamin D regulation. Epidemiological studies suggest that higher vitamin D levels are linked to lower incidence of cancers, perhaps due to antiproliferative effects of 1,25-dihydroxyvitamin (34). Because FGF23 inhibits expression of renal CYP27B1 (1-α-hydroxylase enzyme) and stimulates expression of CYP24A1 (24-hydroxylase enzyme) (35), perhaps ethnic differences in the effects of FGF23 on systemic or local cellular regulation of vitamin D could modify the risk of cancer death across ethnic groups. Future studies should test whether Hispanic ethnicity modifies the association between vitamin D status and malignancy outcomes.
We observed an independent association between elevated FGF23 and increased risk of infectious death, but only when it was expressed as a continuous variable. In contrast, the HEMO study reported a more robust and significant association between elevated FGF23 and increased risk of infectious death among patients with ESRD undergoing hemodialysis (36). Recent data showing that FGF23 directly inhibits chemokine-induced integrin activation on leukocytes, and thereby impairs neutrophil recruitment and host defense, may explain this finding (13). Furthermore, the HEMO study reported that individuals with the highest FGF23 and lowest 25-hydroxyvitamin D levels were at the highest risk of infectious death. One potential mechanistic explanation is that FGF23 may inhibit extrarenal CYP27B1 and stimulate extrarenal CYP24A1 in immune cells as it does in the kidney (37). In monocytes and macrophages, decreased intracellular 1,25-dihydroxyvitamin D levels reduce production of the intracellular antimicrobial peptide cathelicidin, which is a critical component of the innate immune response (38). Although we found no significant interaction between baseline levels of FGF23 and 25-hydroxyvitamin D for any death outcomes in NOMAS participants, differences between the general population and individuals with ESRD who participated in the HEMO study could yield discrepant results.
Strengths of our study include its population-based prospective design, long follow-up period, large proportion of Hispanic participants, and adjudication of specific causes of death. We also acknowledge several limitations. First, we only tested the association of baseline FGF23 measurements with mortality. However, we previously reported that FGF23 levels remain stable within individuals who maintain relatively stable kidney function over time (39) and that there is a minimal diurnal variability (40). We expect that potential misclassification of the exposure over time due to potential longitudinal changes in FGF23 levels would tend to bias our results toward the null. Second, 54% of our study population were Hispanic, which may limit generalizability to other populations. In addition, because the Hispanic population that enrolled in NOMAS was predominantly from the Dominican Republic and Puerto Rico, our results may not generalize to Hispanic individuals from other countries of origin. Third, we cannot rule out misclassification of the cause of death, which was obtained from medical records, death certificates, and interviews of family members and health care providers, all of which may introduce inaccuracies. Finally, although we used multiple statistical approaches to reduce bias as much as possible, residual confounding is a limitation of all observational studies.
In conclusion, elevated FGF23 was independently associated with increased risk of all-cause mortality and death due to both vascular and nonvascular causes in a racially and ethnically diverse general population, regardless of the presence or absence of a history of cardiovascular disease or kidney disease at baseline. Elevated FGF23 was also associated with increased risk of cancer death specifically among Hispanic Americans, a rapidly growing segment of the U.S. population in which there have been limited data on FGF23 and clinical outcomes. Further research is needed to validate our findings in other Hispanic populations that include individuals from different countries of origin and genetic ancestry, and to evaluate potential mechanisms of how elevated FGF23 levels might contribute to increased risks of specific causes of death.
Acknowledgments
The Northern Manhattan Study was supported by Grants R01NS29993 and R01HL108623 from the National Institutes of Health, and this study was additionally supported by R01DK076116, and K24DK093723 from the National Institutes of Health.
Disclosure Summary: T.I. has served as a consultant for Ultragenyx. M.W. has served as a consultant or received honoraria from Amgen, Diasorin, Keryx, Pfizer, Sanofi, and Ultragenyx. All other authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- eGFR
- estimated glomerular filtration rate
- ESRD
- end-stage renal disease
- FGF
- fibroblast growth factor
- FGFR
- FGF receptor
- HR
- hazard risk
- IQR
- interquartile range
- ln
- natural log
- NOMAS
- Northern Manhattan Study
- RU
- relative units.
References
- 1. Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21(9):1427–1435. [DOI] [PubMed] [Google Scholar]
- 2. Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78(10):975–980. [DOI] [PubMed] [Google Scholar]
- 3. Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90(3):1519–1524. [DOI] [PubMed] [Google Scholar]
- 4. Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91(8):3144–3149. [DOI] [PubMed] [Google Scholar]
- 5. Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. [DOI] [PubMed] [Google Scholar]
- 6. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152(10):640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright CB, Dong C, Stark M, et al. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS). Neurology. 2014;82(19):1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22(6):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossaint J, Oehmichen J, Van Aken H, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126(3):962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease [published online July 22, 2016]. Kidney Int. 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf M. Mineral (mal)adaptation to kidney disease–Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol. 2015;10(10):1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lutsey PL, Alonso A, Selvin E, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3:e000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor EN, Rimm EB, Stampfer MJ, Curhan GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161(5):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ärnlöv J, Carlsson AC, Sundström J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83(1):160–166. [DOI] [PubMed] [Google Scholar]
- 20. Westerberg PA, Tivesten Å, Karlsson MK, et al. Fibroblast growth factor 23, mineral metabolism and mortality among elderly men (Swedish MrOs). BMC Nephrol. 2013;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32(8):1725–1731. [DOI] [PubMed] [Google Scholar]
- 22. Wallman KK, Hodgdon J. Race and ethnic standards for Federal statistics and administrative reporting. Stat Report. 1977;77–110:450–454. [PubMed] [Google Scholar]
- 23. Gentry EM, Kalsbeek WD, Hogelin GC, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1(6):9–14. [PubMed] [Google Scholar]
- 24. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 25. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. [DOI] [PubMed] [Google Scholar]
- 26. Brandenburg VM, Kleber ME, Vervloet MG, et al. Fibroblast growth factor 23 (FGF23) and mortality: the Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis. 2014;237(1):53–59. [DOI] [PubMed] [Google Scholar]
- 27. Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22(10):1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 29. Ulaganathan VK, Sperl B, Rapp UR, Ullrich A. Germline variant FGFR4 p.G388R exposes a membrane-proximal STAT3 binding site. Nature. 2015;528(7583):570–574. [DOI] [PubMed] [Google Scholar]
- 30. Spinola M, Leoni V, Pignatiello C, et al. Functional FGFR4 Gly388Arg polymorphism predicts prognosis in lung adenocarcinoma patients. J Clin Oncol. 2005;23(29):7307–7311. [DOI] [PubMed] [Google Scholar]
- 31. Seitzer N, Mayr T, Streit S, Ullrich A. A single nucleotide change in the mouse genome accelerates breast cancer progression. Cancer Res. 2010;70(2):802–812. [DOI] [PubMed] [Google Scholar]
- 32. Cross DS, Ivacic LC, Stefanski EL, McCarty CA. Population based allele frequencies of disease associated polymorphisms in the Personalized Medicine Research Project. BMC Genet. 2010;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Center for Biotechnology Information. The Single Nucleotide Polymorphism database, Short Genetic Variations. Reference SNP Cluster Report: rs351855. http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=351855 Accessed May 25, 2016.
- 34. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. [DOI] [PubMed] [Google Scholar]
- 35. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. [DOI] [PubMed] [Google Scholar]
- 36. Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol. 2016;27(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bai X, Miao D, Xiao S, et al. CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J Clin Invest. 2016;126(2):667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bacchetta J, Sea JL, Chun RF, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Isakova T, Xie H, Barchi-Chung A, et al. Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol. 2012;7(5):820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]