Abstract
The study aimed to evaluate the anti-Sporothrix sp. activity of the essential oil of Origanum majorana Linn. (marjoram), its chemical analysis, and its cytotoxic activity. A total of 18 fungal isolates of Sporothrix brasiliensis (n: 17) from humans, dogs and cats, and a standard strain of Sporothrix schenckii (n: 1) were tested using the broth microdilution technique (Clinical and Laboratory Standard Institute – CLSI M27-A3) and the results were expressed in minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC). The MIC50 and MIC90 of itraconazole against S. brasiliensis were 2 μg/mL and 8 μg/mL, respectively, and the MFC50 and MFC90 were 2 μg/mL and >16 μg/mL, respectively, with three S. brasiliensis isolates resistant to antifungal. S. schenckii was sensitive at MIC of 1 μg/mL and MFC of 8 μg/mL. For the oil of O. majorana L., all isolates were susceptible to MIC of ≤2.25–9 mg/mL and MFC of ≤2.25–18 mg/mL. The MIC50 and MIC90 were ≤2.25 mg/mL and 4.5 mg/mL, respectively, and the MFC50/90 values were twice more than the MIC. Twenty-two compounds were identified by gas chromatography with a flame ionization detector (CG-FID) and 1,8-cineole and 4-terpineol were the majority. Through the colorimetric (MTT) assay, the toxicity was observed in 70–80% of VERO cells between 0.078 and 5 mg/mL. For the first time, the study demonstrated the satisfactory in vitro anti-Sporothrix sp. activity of marjoram oil and further studies are needed to ensure its safe and effective use.
Keywords: Sporotrichosis, Sporothrix schenckii complex, Antifungal resistance, Marjoram, Lamiaceae
Introduction
Sporotrichosis is a zoonotic mycosis caused by the fungus of Sporothrix schenckii complex, such as S. schenckii var. schenckii, S. schenckii var. luriei, S. brasiliensis, S. globosa, S. mexicana and S. albicans.1, 2 This disease has a worldwide occurrence, mainly in countries of America, such as Brazil, Mexico, Colombia, Uruguay and Peru, and also in South Africa, India, Japan.3, 4 The infection is acquired through the traumatic inoculation by conidial and can be transmitted through scratch and bite of sick cats.5, 6 S. brasiliensis is considered the most virulent among the complex species and with a high prevalence in Brazil, being geographically restricted to this country.7, 8
Limited lesions to skin and subcutaneous tissue may arise in the host and may have lymphatic involvement with systemic clinical signs.9 The treatment of choice is performed with the antifungal itraconazole10, 11, 12; however, the appearance of resistant strains of Sporothrix sp. has been observed because of the indiscriminate use of several antifungals in therapies.2, 13, 14
This problem has stimulated the search for new effective chemical compounds, such as those in medicinal plants, but only approximately 30% of antimicrobial medications in the market are derived from natural products and the resources for elaborate drugs from plants are poorly explored.15 In folk medicine, marjoram (Origanum majorana Linn., synonymous of Majorana hortensis Linn.) is used to treat asthma, indigestion, cramps, headache, dizziness, depression, and rheumatism, and it has diuretic activity.16, 17 This plant has a strong antioxidant activity attributed to its high content of flavonoids and phenolic acids, which are used for food preservation.18 Essential oil of O. majorana L. has shown antimicrobial properties, such as antiviral, antibacterial and antifungal,18, 19, 20, 21, 22 among others.
The pathogenic fungi of human and veterinary medical interest that were sensitive to the essential oil of O. majorana L. are Candida sp., Aspergillus sp., Trichophyton sp., Microsporum sp., Malassezia sp. and Trichosporon sp.21, 23, 24 These results in vitro show its promising activity in fungal diseases, including those with zoonotic potential. In sporotrichosis, no studies with O. majorana L. have been done, which encouraged the development of this work. This study aimed to evaluate the in vitro efficacy of O. majorana L. essential oil against clinical isolates of S. schenckii complex and to analyze its chemical constituents and cytotoxic activity in mammalian cells.
Materials and methods
Plant material
The essential oil of O. majorana Linn., originating from Egypt, was obtained from Ferquima® Indústria e Comércio Ltda. (Vargem Grande Paulista, São Paulo, SP, Brazil), for which quality parameters are described in an accompanying technical report (appearance, color, purity, odor, density – 20 °C, refraction index – 20 °C).
Chemical analysis
Chemical analysis was performed using high-resolution gas chromatography with a flame ionization detector (CG-FID) by HP 7820A (Agilent®) equipped with an HP-5 column (30 m × 0.32 mm × 0.25 mm) at an initial temperature of 70 °C with the addition of 3 °C/min up to 240 °C. The injector temperature was 250 °C, and that of the FID detector was 260 °C. The speed of the drag of hydrogen gas was 3 mL/min, and that of the split was 1:30. The solution of essential oil was diluted with 1% chloroform and injected into the chromatograph at a volume of 1 μL. Data were acquired through the EZChrom Elite Compact® program (Agilent).
Cytotoxicity assay
The cytotoxic effects of O. majorana L. were estimated using the colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay that measures the reduction of the MTT dye to an insoluble and colored formazan. The technique was executed as previously described,25 and tested in mammalian cells of VERO type, which were grown in RPMI-1640 (Roswell Park Memorial Institute medium, Sigma, Steinheim, Germany) supplemented with l-glutamine, without sodium bicarbonate (pH 7.2) and added of penicillin–streptomycin and fungizone in a humidified atmosphere of 5% CO2 at 37 °C. Trypsin was added on to the cell monolayer and the cells were resuspended in RPMI-1640, resulting in a suspension with approximately 2 × 105 cells/mL. Aliquots of this cells suspension were placed in individual wells in 96-well microplate except first well as blank. Cells were incubated in RPMI-1640 medium supplemented with 10% fetal calf serum at CO2 5%, 37 °C by 24 h.
Subsequently, 100 μL of the oil in seven successive concentrations on a logarithmic scale was added in the microplates. Concentrations of 5–0.078 mg/mL of the essential oil diluted in RPMI-1640 were tested in triplicate for 48 h at 37 °C in a humidified atmosphere of 5% CO2 and for control RPMI-1640 only was used. Then, 50 μL MTT solution (2.5 mg/mL) was added in each well and incubated for 2 h and 30 min at 37 °C and 5% CO2. After removal of MTT solution, 50 μL of dimethylsulfoxide (DMSO) was added to each well to dissolve the formazan crystals, and gentle shaking for 5 min. The spectrophotometric absorbance of the samples was measured using a microplate reader at wavelength of 540 nm. The appearance of cells was monitored by inverted microscope and the results were expressed as an inhibition percentage relative to the control cells, which was considered 100%.
Fungal isolates
For the antifungal susceptibility test, seventeen clinical isolates of S. brasiliensis derived from humans (n: 3), dogs (n: 6), and cats (n: 8) with sporotrichosis were used along with a standard strain of S. schenckii (IOC 1226) from human case (n: 1), totaling eighteen tested isolates. The fungal isolates were stored in mycology collection of the Centro de Diagnóstico e Pesquisa em Micologia Veterinária (Universidade Federal de Pelotas – UFPEL – Pelotas, RS, Brazil) and cooled in an average temperature of 4 °C. The mycology identification of S. brasiliensis was analyzed using the polymerase chain reaction – restriction fragment length polymorphism (PCR-RFLP), proposed by Rodrigues et al.26
Antifungal activity
The antifungal susceptibility tests were performed using the broth microdilution technique, according to the M27-A3 guidelines of the Clinical and Laboratory Standards Institute,27 adapted for the use of essential oil. Subcultures in Brain-Heart-Infusion agar (BHI, Acumedia, Lansing, MI, USA) at 35 °C for 48 h were performed. A portion of each fungal colony was transferred to individual tubes containing sterile saline solution and adjusted according to the scale of 1.0 McFarland and in the ultraviolet (UV)–visible spectrophotometer (Spectrum Instruments Co., Shanghai, China) in transmittance at 530 nm and at an absorbance of 80–82%. The suspensions were adjusted in saline solution (1:100) and, then, in RPMI-1640 with 3-morpholinopropane-1-sulfonic acid – MOPS – (1:20) in order to obtain the final inoculum concentration of 5 × 104 CFU/mL.
In a microplate with ninety-six wells containing 100 μL of RPMI-1640 with MOPS in each well, 100 μL of essential oil of O. majorana L. containing 1% Tween 80 was added to the column corresponding to a higher concentration of the product, and serial dilutions were performed. The oil plant was tested in the concentrations of 72–2.25 mg/mL. Then, 100 μL of the inoculum was added to all wells except in the negative control, where 100 μL of the marjoram essential oil was added. Itraconazole was used as the positive control and was prepared in dimethyl sulfoxide and diluted in RPMI-1640 medium with MOPS in order to obtain the final concentrations of 16–0.0313 μg/mL. The microplates were incubated on a rotatory shaker (Certomat® BS-1, B. Sartorius, Göttingen, Germany) at 35 °C for 72 h, and the minimal inhibitory concentration (MIC) was visually compared with that of the negative control and was defined as the lowest concentration of the tested product inhibiting the visible growth of the fungus (100% inhibition).
For the minimal fungicidal concentration (MFC), 10 μL of aliquots of the wells with no fungal growth was transferred to Petri dishes containing Sabouraud dextrose agar (Acumedia, Lansing, MI, USA) and incubated at 35 °C for 72 h to visualize fungal growth. MFC was determined to be the lowest concentration able to eliminate fungal growth. All experiments were performed in duplicate.
Statistical analysis
The analysis of variance and comparison of geometric means were performed according to Tukey test using the statistical software BioEstat®, 5.3 version, and value p < 0.05 was considered significant.
Results
Antifungal activity
According to the results of the anti-Sporothrix sp. activity of the essential oil of O. majorana L. and itraconazole (Table 1), no significant difference existed between the results in the MIC values; however, the MFC values differed statistically (p = 0.05) between itraconazole and essential oil, in which O. majorana L. presented better results. All tested isolates were susceptible to the essential oil of marjoram with fungistatic activity being observed at concentrations of ≤2.25–9 mg/mL, while fungicidal activity occurred between ≤2.25 and 18 mg/mL. The concentrations of the oil up to 9 mg/mL showed antifungal activity against all S. brasiliensis, the MIC50 and MIC90 being observed at the concentrations ≤2.25 mg/mL and 4.5 mg/mL, respectively. At the minimal concentration tested of the oil, 66.7% (2/3) of the humans isolates, 83.3% (5/6) of the isolates from dogs and 50% (4/8) from cats were sensitive (MIC ≤2.25 mg/mL), as well as the standard strain of S. schenckii. In the fungicidal activity, the MFC50 and MFC90 values of overall S. brasiliensis were 4.5 mg/mL and 9 mg/mL, respectively, and were twice the MIC50/90 values, whereas the standard strain was more sensitive (MFC ≤2.25 mg/mL). In relation to the itraconazole, all isolates were sensitive to the fungistatic activity between the MIC values ≤0.03 and 16 μg/mL, which MIC50 was 1 μg/mL and MIC90 was 16 μg/mL. However, the fungicidal activity was observed between ≤0.03 and >16 μg/mL, where MFC50 was 2 μg/mL, but the MFC90 was >16 μg/mL, indicating that 17.6% (3/17) of the S. brasiliensis were resistant to the maximal concentration tested of this antifungal.
Table 1.
Minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of the Origanum majorana Linn. essential oil and itraconazole against Sporothrix brasiliensis and Sporothrix schenckii.
| Origin of the isolates of Sporothrix schenckii complex |
Origanum majorana L. (mg/mL) |
Itraconazole (μg/mL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC |
MFC |
MIC |
MFC |
|||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |
| Sporothrix brasiliensis | ||||||||||||
| Humans (n: 3) | ≤2.25–4.5 | – | – | ≤2.25–4.5 | – | – | ≤0.03–0.5 | – | – | ≤0.03 to 2 | – | – |
| Dogs (n: 6) | ≤2.25–4.5 | ≤2.25 | ≤2.25 | ≤2.25–18 | 4.5 | 9 | 0.5–8 | 1 | 2 | 1 to >16 | 2 | 16 |
| Cats (n: 8) | ≤2.25–9 | ≤2.25 | 4.5 | ≤2.25–9 | 4.5 | 9 | ≤0.03–16 | 2 | 16 | 0.25 to >16 | 2 | >16 |
| Overall (n: 17) | ≤2.25–9 | ≤2.25 | 4.5 | ≤2.25–18 | 4.5 | 9 | ≤0.03–16 | 2 | 8 | ≤0.03 to >16 | 2 | >16 |
| Sporothrix schenckii | ||||||||||||
| IOC 1226 (n: 1) | ≤2.25 | – | – | ≤2.25 | – | – | 1 | – | – | 8 | – | – |
| Overall (n: 18) | ≤2.25–9 | ≤2.25 | 4.5 | ≤2.25–18 | 4.5 | 9 | ≤0.03–16 | 1 | 16 | ≤0.03 to >16 | 2 | >16 |
50%, MIC/MFC at which 50% of isolates were inhibited/eliminated; 90%, MIC/MFC at which 90% of isolates were inhibited/eliminated.
Chemical composition
Chemical analysis found twenty-two compounds, and 1,8-cineole was the majority component, followed by 4-terpineol, γ-terpinene, p-cymene, sabinene, and others (Table 2).
Table 2.
Chemical constituents identified in the essential oil of Origanum majorana L. and respective concentrations through high resolution gas chromatography in a flame ionization detector (CG-FID).
| Constituents | Retention rate | Area | Concentration (%) |
|---|---|---|---|
| α-Thujene | 932 | 281,814 | 1.1 |
| α-Pinene | 932 | 494,138 | 2.0 |
| Camphene | 941 | 96,074 | 0.4 |
| Sabinene | 946 | 1,669,329 | 6.7 |
| β-Pinene | 975 | 470,225 | 1.9 |
| Myrcene | 984 | 408,894 | 1.6 |
| α-Phellandrene | 1003 | 226,808 | 0.9 |
| α-Terpinene | 1015 | 1,152,102 | 4.6 |
| p-Cymene | 1019 | 1,755,163 | 7.0 |
| Limonene | 1023 | 1,316,921 | 5.3 |
| 1,8-Cineole | 1027 | 5,222,929 | 20.9 |
| β-Ocimene | 1056 | 34,661 | 0.1 |
| γ-Terpinene | 1089 | 2,130,943 | 8.5 |
| Trans sabinene hydrate | 1094 | 564,967 | 2.3 |
| Linalool | 1099 | 1,096,294 | 4.4 |
| Camphor | 1142 | 48,346 | 0.2 |
| Terpinen-4-ol | 1175 | 5,100,062 | 20.4 |
| α-Terpineol | 1188 | 1,162,894 | 4.7 |
| Linalyl acetate | 1261 | 438,735 | 1.8 |
| Bornila acetate | 1285 | 212,333 | 0.8 |
| β-Caryophyllene | 1421 | 546,553 | 2.2 |
| Humulene | 1453 | 41,993 | 0.2 |
| Others | 517,426 | 2.1 |
Cytotoxic effects
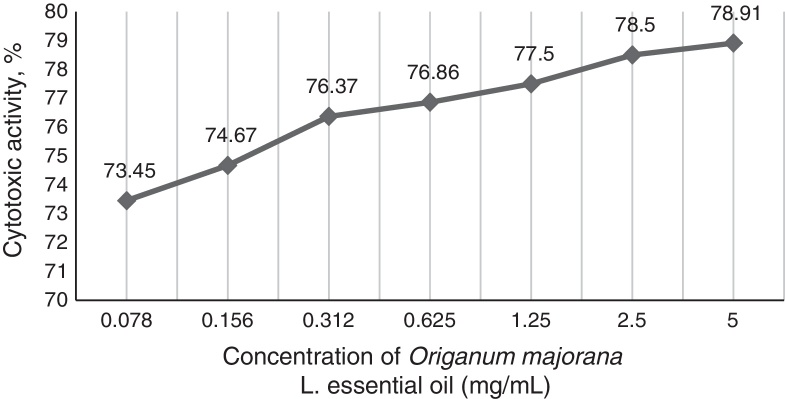
In the observed values of MIC50 and MIC90 for total isolates, the cytotoxicity of O. majorana L. occurred between 77.5% and 78.91%. The toxicity activity in mammalian cells was directly proportional to tested concentrations and decreased to 73.45% at a concentration of 0.078 mg/mL (Fig. 1).
Fig. 1.
Cytotoxic percentage (%) of the essential oil of Origanum majorana L. in VERO cells tested through MTT assay in the concentration of 0.078–5 mg/mL.
Discussion
Antifungal activity
The drug of choice in the treatment of sporotrichosis was effective against several S. brasiliensis, being similar to the findings of Marimon et al.,2 that showed in vitro activity between 0.5 and 2 μg/mL against twenty-three isolates of S. brasiliensis, with MIC50 and MIC90 of 0.5 μg/mL and 1 μg/mL, respectively. In our work, the MIC50 and MIC90 found were 2 μg/mL and 8 μg/mL, demonstrating that the fungal isolates of S. brasiliensis originating from humans and animals in the south of Brazil were twice more resistant.
In isolates from humans, no resistance was observed, and according Kauffman et al.28 and Yamada et al.,29 the resistance was lower compared to that of animals, mainly cats, due to greater care during human therapy in relation to their own health. In relation to feline sporotrichosis, it is known to have a high incidence in the Southeast and Southern regions of Brazil.8, 11, 30, 31 MIC50 of itraconazole against S. brasiliensis isolated from cats were 2 μg/mL, but the MIC90 was 16 μg/mL, demonstrating its lower susceptibility to antifungal. In the overall S. brasiliensis tested, one clinical isolate from a dog and two isolates from cats were resistant to the fungicidal activity (MFC >16 μg/mL).
This observation may be a reflection of previous factors that favor the emergence of antifungal resistance. It is noticed that the antifungal administered in animals may not always be the same used in humans and its efficacy may be affected due to pharmacokinetics parameters and kind of formulation, among others,32 and a difference in the pharmaceutical technology between human and veterinary drugs may occur,33 which may influence the therapeutic antifungal response. Besides, the irregularities in veterinary therapeutic management by animal owners are common due to the difficulties in the oral administration and high cost of antifungals and this often culminates in the abandonment of therapy, particularly when there is improvement of skin lesions.34
The resistance of Sporothrix spp. to itraconazole has been evidenced by Rodrigues et al.,14 and this problem reflects in an alert because sporotrichosis is important in the public health. Interestingly, the same resistant isolates were susceptible to essential oil in concentrations equal to or less than 9 mg/mL, thus suggesting that this plant can be used for further studies in the treatment of sporotrichosis. In accordance with our study, Souza et al.23 showed the promising activity of the commercial essential oil of O. majorana L. at 160 μL/mL against pathogenic fungi, including in strains of Candida albicans, C. tropicalis, Cryptococcus neoformans, T. mentagrophytes, M. gypseum and A. flavus, which were resistant to conventional antifungal, as amphotericin B, 5-fluorocytosine and fluconazole. This observation reflects in the promising use of this essential oil in antifungal therapies.
No valid criteria exist for the minimal antifungal concentrations in vitro tests with plant extracts,35 but O. majorana L. oil showed satisfactory activity with MIC50 and MIC90 of ≤2.25 mg/mL and 4.5 mg/mL, respectively, including in resistant isolates to itraconazole (n: 3), that were sensitive to the plant at MIC of ≤2.25 mg/mL for one isolate from dog and one from cat, and at MIC of 9 mg/mL for one isolate from cat. No studies were found on the activity of O. majorana L. in S. schenckii complex, and for the first time, the in vitro sensibility of S. brasiliensis to this plant was studied.
Similar to our study, the resistance of S. schenckii and S. brasiliensis to itraconazole also was reported as sensitive to plant extracts from the Pterocaulon genus35 and Camellia sinensis L.,36 respectively, indicating the promising use of the several plants in the sporotrichosis. Furthermore, resistance of bacteria and yeasts to antimicrobial drugs was sensitive to the essential oils of Rosmarinus officinalis L.37 and Origanum vulgare L.38, 39 showing that, as found in this study with O. majorana L., other medicinal plants also are potential candidates as new antimicrobial agents. Given this fact, it is necessary to explore the studies for their promising use in the treatment of sporotrichosis.
Chemical composition
The chemical compounds were similar to those scientifically described.18, 21, 24, 40 However, our data differed from the findings by Marino et al.,41 in which thymol and carvacrol were prevalent. The presence of phenolic compounds in plants is highly correlated to antimicrobial properties,40, 42 but the identified compounds were predominated by terpenoids, which were related to antimicrobial activity when tested alone.43 The plant causes an increase in the permeability of the cytoplasmatic fungal membrane, destroying the physical structure.44 However, Souza et al.23 suggested that the essential oil of O. majorana L. acts as an inhibitor of the microorganism's cell wall, although other mechanisms for expressing their anti-Sporothrix sp. activity may be involved.
Cytotoxic effects
O. majorana L. oil presented a cytotoxicity between 73.45% and 78.91% in the tested concentrations, which were high, but the gradual reduction of the cytotoxicity to the lower tested concentration should be noted. In overall isolates, 66.67% (12/18) of tested Sporothrix spp. were sensitive to the MIC ≤2.25 mg/mL, indicating that the lower concentrations may present inhibitory activity with lower cytotoxicity. Although the maximal non-toxic concentration of O. majorana L. essential oil in VERO cells was 3.2 μg/mL,22 this value was four times lower than the minimal tested concentration. However, it is noticed that the use of the O. majorana L. oil against sporotrichosis need to be studied at its effective antifungal concentrations and, also, our study was performed in vitro.
According to Nogueira and Andrade,45 the mammalian cells in the MTT test are more susceptible in comparison to the in vivo test due to the direct exposure of the product, whereas to the in vivo the product is influenced by the route of administration under consideration, that can suffer internal actions by oral administration, as well as influence by topical absorption, and may present lower toxic potential. The dose and frequency of administration can influence the degree of toxicity of medicinal plants,46 and low doses can cause an allergic reaction in sensitive patients as well as skin irritations.47, 48 Studies on the toxic effects of this plant in vivo should be conducted in order to evaluate the influence of its dose and the route of administration in patients with sporotrichosis.
Conclusion
The in vitro activity of O. majorana L. essential oil against S. brasiliensis and S. schenckii, including in resistant isolates to itraconazole, encourages greater studies on antifungal potential of this promising plant, that had 1,8-cineole as majority compound among chemical constituents identified. However, the cytotoxic activity of O. majorana L. oil was observed between 70% and 80% of mammalian cells in all concentrations tested. Further studies are needed to ensure its safe and effective use.
Conflict of interest
The authors declare having no conflict of interest in this study.
Acknowledgments
We thank Zoilo Pires de Camargo (Universidade Federal de São Paulo, UNIFESP, São Paulo, SP, Brazil) for the biomolecular analysis of the clinical isolates and CNPq, CAPES and FAPERGS for financial support and providing scholarships.
Associate Editor: Carlos Pelleschi Taborda
References
- 1.Marimon R., Cano J., Gené J., Sutton D.A., Kawasaki M., Guarro J. Sporothrix brasiliensis, S. globosa and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marimon R., Serena C., Géne J., Cano J., Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix schenckii. Antimicrob Agents Chemother. 2008;52:732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva M.B.T., Costa M.M.M., Torres C.C.S. Urban sporotrichosis: a neglected epidemic in Rio de Janeiro, Brazil. Cad Saúde Pública. 2012;28(10):1867–1880. doi: 10.1590/s0102-311x2012001000006. [DOI] [PubMed] [Google Scholar]
- 4.Carrada-Bravo T., Olvera-Macías M.I. New observations on the ecology and epidemiology of Sporothrix schenckii and sporotrichosis. Rev Latinoam Patol Clin. 2013;60:5–24. [Google Scholar]
- 5.Xavier M.O., Nobre M.O., Sampaio D.P., Jr. Esporotricose felina com envolvimento humano na cidade de Pelotas, RS, Brasil. Ciênc Rural. 2004;34(6):1961–1963. [Google Scholar]
- 6.Cruz L.C.H. Complexo Sporothrix schenckii. Revisão de parte de literatura e considerações sobre diagnóstico e a epidemiologia. Vet Zootec. 2011;20:8–28. [Google Scholar]
- 7.Rodrigues A.M., de Melo T.M., de Hoog G.S. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montenegro H., Rodrigues A.M., Dias M.A.G., da Silva E.A., Bernardi F., de Camargo Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: an emerging animal infection in São Paulo, Brazil. BMC Vet Res. 2014;10:269. doi: 10.1186/s12917-014-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson C.E. Esporotricose. Braz J Vet Res Anim Sci. 2011;48(3):250–259. [Google Scholar]
- 10.Honse C.O., Rodrigues A.M., Gremião I.D.F., Pereira A.S., Schubach T.M. Use of local hyperthermia to treat sporotrichosis in a cat. Vet Rec. 2010;166(7):208–209. doi: 10.1136/vr.b4768. [DOI] [PubMed] [Google Scholar]
- 11.Madrid I.M., Mattei A.S., Martins A.F., Nobre M.O., Meireles M.C.A. Feline sporotrichosis in the southern region of Rio Grande do Sul, Brazil: clinical, zoonotic and therapeutic aspects. Zoonoses Public Health. 2010;57(2):151–154. doi: 10.1111/j.1863-2378.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 12.Pereira S.A., Passos S.R.L., Silva J.N. Response to azolic antifungal agents for treating feline sporotrichosis. Vet Rec. 2010;166:290–294. doi: 10.1136/vr.166.10.290. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Galhardo M.C., Zancopé-Oliveira R.M., Monzón A., Rodriguez-Tudela J.L., Cuenca-Estrella M. Antifungal susceptibility profile in vitro of Sporothrix schenckii in two growth phases and by two methods: microdilution and E-test. Mycoses. 2010;53(3):227–231. doi: 10.1111/j.1439-0507.2009.01701.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues A.M., de Hoog G.S., Pires D.C. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis. 2014;14:219. doi: 10.1186/1471-2334-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chattopadhyay D., Chawla-Sarkar M., Chatterjee T. Recent advancements for the evaluation of anti-viral activities of natural products. N Biotechnol. 2009;25(5):348–365. doi: 10.1016/j.nbt.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Den Broucke C.O., Lemli J.Á. Antispasmodic activity of Origanum compactum. Planta Med. 1980;38:317–331. doi: 10.1055/s-2008-1074884. [DOI] [PubMed] [Google Scholar]
- 17.Jun W.J., Han B.K., Yu K.W. Antioxidant effects of Origanum majorana L. on superoxide anion radicals. Food Chem. 2001;75:439–444. [Google Scholar]
- 18.Vági E., Simándi B., Suhajda Á., Héthelyi É. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res Int. 2005;38:51–57. [Google Scholar]
- 19.Daferera D.J., Ziogas B.N., Polissiou M.G. GC–MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J Agric Food Chem. 2000;48:2576–2581. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- 20.Nostro A., Blanco A.R., Cannatelli M.A. Susceptibility of methicillin-resistant Staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol Lett. 2004;230:191–195. doi: 10.1016/S0378-1097(03)00890-5. [DOI] [PubMed] [Google Scholar]
- 21.Busatta C., Vidal R.S., Popiolski A.S. Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiol. 2008;25:207–211. doi: 10.1016/j.fm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Erdoğan I.O., Ozcelik B., Kartal M., Kan Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk J Biol. 2012;36:239–246. [Google Scholar]
- 23.Souza N.A.B., Lima E.O., Guedes D.N., Pereira F.O., Souza E.L., Sousa F.B. Efficacy of Origanum essential oils for inhibition of potentially pathogenic fungi. Braz J Pharm Sci. 2010;46(3):499–508. [Google Scholar]
- 24.Santin R. Faculdade de Veterinária, UFRGS; Porto Alegre, RS, Brasil: 2013. Potencial antifúngico e toxicidade de óleos essenciais da família Lamiaceae. (Thesis) 104 p. [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues A.M., de Hoog G.S., Camargo Z.P. Genotyping species of the Sporothrix schenckii complex by PCR-RFLP of calmodulin. Diagn Microbiol Infect Dis. 2014;78:383–387. doi: 10.1016/j.diagmicrobio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 27.CLSI – Clinical and Laboratory Standards Institute . 3rd ed. 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. M27-A3 Guideline. Approved Standard. [Google Scholar]
- 28.Kauffman C.A., Bustamante B., Chapman S.W., Pappas P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K., Zaitz C., Framil V.M.S., Muramatu L.H. Cutaneous sporotrichosis treatment with potassium iodide. A 24 year experience in São Paulo State, Brazil. Rev Inst Med Trop São Paulo. 2007;53(2):89–93. doi: 10.1590/s0036-46652011000200006. [DOI] [PubMed] [Google Scholar]
- 30.Schubach T.M., Schubach A., Okamoto T. Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998–2001) J Am Vet Med Assoc. 2004;224:1623–1629. doi: 10.2460/javma.2004.224.1623. [DOI] [PubMed] [Google Scholar]
- 31.Madrid I.M., Mattei A.S., Fernandes C.G., Nobre M.O., Meireles M.C.A. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173(4):265–273. doi: 10.1007/s11046-011-9509-4. [DOI] [PubMed] [Google Scholar]
- 32.Mawby D.I., Whittemore J.C., Genger S., Papich M.G. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med. 2014;28:72–77. doi: 10.1111/jvim.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham F., Elliott J., Lees P. Springer Science & Business Media; Berlin, Germany: 2010. Comparative and Veterinary Pharmacology. [Google Scholar]
- 34.Chaves A.R., Campos M.P., Barros M.B.L. Treatment abandonment in feline sporotrichosis – study of 147 cases. Zoonoses Public Health. 2013;60:149–153. doi: 10.1111/j.1863-2378.2012.01506.x. [DOI] [PubMed] [Google Scholar]
- 35.Stopiglia C.D.O., Vianna D.R., Meirelles G.C., Teixeira H., Von Poser G.L., Scroferneker M.L. Antifungal activity of Pterocaulon species (Asteraceae) against Sporothrix schenckii. J Mycol Med. 2011;21(3):169–172. doi: 10.1016/j.mycmed.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Waller S.B., Madrid I.M., Serra E.F., Gomes A.R., Cleff M.B., Faria R.O. In vitro susceptibility of the Sporothrix brasiliensis to aqueous extracts of green-tea (Camellia sinensis L. Kuntze) Acta Vet Bras. 2015;9(4):342–347. [Google Scholar]
- 37.Luqman S., Dwivedi G.R., Darokar M.P., Kalra A., Khanuja S.P.S. Potential of Rosemary oil to be used in drug-resistant infections. Altern Ther Health Med. 2007;13(5):54–59. [PubMed] [Google Scholar]
- 38.Cleff M.B., Meinerz A.R.M., Schuch L.F.D., Rodrigues M.R.A., Meireles M.C.A., Mello J.R.B. In vitro activity of the essential oil of Origanum vulgare against Sporothrix schenckii. Arq Bras Med Vet Zootec. 2008;60(2):513–516. [Google Scholar]
- 39.Maida I., Lo Nostro A., Pesavento G. Exploring the anti-Burkholderia cepacia complex activity of essential oils: a preliminary analysis. Evid Based Complement Altern Med. 2014 doi: 10.1155/2014/573518. Article ID 573518, 10 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sfeir J., Lefrançois C., Baudoux D., Derbré S., Licznar P. In vitro antibacterial activity of essential oils against Streptococcus pyogenes. Evid Based Complement Altern Med. 2013 doi: 10.1155/2013/269161. Article ID 269161, 9 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marino M., Bersani C., Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol. 2001;67:187–195. doi: 10.1016/s0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara L.K., Montesanto D., Chiantese C. Origanum marjoran L. in medicine and foods. Ingred Aliment. 2003;2:23–25. [Google Scholar]
- 43.Kurekci C., Padmanabha J., Bishop-Hurley S.L., Hassan E., Al-Jassim R.A., McSweeney C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int J Food Microbiol. 2013;166(3):450–457. doi: 10.1016/j.ijfoodmicro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Ultee A., Smid E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int J Food Microbiol. 2001;64:373–378. doi: 10.1016/s0168-1605(00)00480-3. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira R.M.B., Andrade S.F. Ed. I. Roca; Brasil: 2011. Manual de Toxicologia Veterinária. [Google Scholar]
- 46.Veiga V.F., Jr., Pinto A.C., Maciel M.A.M. Plantas Medicinais: Cura Segura? Quím Nova. 2005;28(3):519–528. [Google Scholar]
- 47.Baričevič D., Bartol T. The biological/pharmacological activity of the oregano genus. In: Kintzios S., editor. Oregano: The Genera Origanum and Lippia, Medicinal and Aromatic Plants – Industrial Profiles. Taylor & Francis; London, England: 2002. pp. 177–214. [Google Scholar]
- 48.Lorenzi H., Matos F.J. Editora Instituto Plantarum; Nova Odessa, SP: 2006. Plantas Medicinais no Brasil: Nativas e Exóticas Cultivadas. [Google Scholar]