Abstract
The use of naturally-occurring agents to regulate tumorigenesis is on the rise. Several herbal extracts, pure plant-derived active constituents, and food additives have been reported to possess potent anti-cancer properties and cancer-ameliorating effects. The wide-range anti-cancer effects of Nigella sativa, also known as black seed or black cumin, have been extensively studied using different in vitro and in vivo models. Here, we provide a comprehensive, analytical review of the reported anti-cancer properties of N. sativa seed extracts. This review focuses on analyzing experimental findings related to the ability of N. sativa to exert anti-proliferative, pro-apoptotic, anti-oxidant, cytotoxic, anti-mutagenic, anti-metastatic, and NK cytotoxic activity enhancing effects against various primary cancer cells and cancer cell lines. Moreover, we underline the molecular mechanisms of action and the signal transduction pathways implicated in the suppression of tumorigenesis by N. sativa. The major signaling pathway utilized by N. sativa to manifest its anti-cancer activity is the iNOS signaling pathway. This review underscores the recent developments that highlight an effective therapeutic potential of N. sativa to suppress tumor development, reduce tumor incidence, and ameliorate carcinogenesis. In sum, experimental findings reported in the last two decades strongly suggest that N. sativa fractions could serve, alone or in combination with known chemotherapeutic drugs, as effective agents to control tumor initiation, growth, and metastasis, and hence, treatment of a wide range of cancers.
Keywords: Nigella sativa, Anti-cancer, Apoptosis, Cytotoxicity, NK cytotoxic activity
Highlights
-
•
N. sativa exerts cytotoxic, pro-apoptotic, anti-proliferative, anti-oxidant, anti-mutagenic, and anti-metastatic effects.
-
•
Augmentation of NK cytotoxic activity is a one molecular mechanism by which N. sativa manifests its anti-cancer activity.
-
•
The anti-cancer effects of N. sativa are primarily mediated via iNOS, p53, and caspase signaling pathways.
-
•
N. sativa extracts can potentially be employed in the development of effective anti-cancer therapeutic agents.
1. Introduction
Many herbs have been shown to possess therapeutic potential towards several medical conditions, and hence, they are of a substantial medicinal value. For centuries, people around the globe have been using numerous medicinal herbs to alleviate the signs and symptoms of various disorders [1]. Herbal medicine, also known as botanical medicine, phytomedicine, phytotherapy, herbology, and herbalism, is a form of therapy that uses plants or plant extracts to prevent or treat different diseases and to boost the overall health status [1]. Herbal medicine is one of the oldest, if not the oldest, and probably remains to be of growing popularity. It is really intriguing that despite the great advancement in the fields of conventional medicine and drug discovery, the use of herbal formulations is still extremely widespread throughout the world, indicative of peoples' perception of the safety and therapeutic efficacy of such medicinal herbs. Although herbal medicine is more prevalent in Asia, Africa, and to a lesser extent in Europe, the use of medicinal herbs has witnessed a significant, gradual increase in North America [1], [2]. It is most likely the gentle, nourishing, efficacious, synergistic, cost-effective, and safe properties of medicinal herbs that make them an attractive option for many people as therapeutic agents [1], [2]. In fact, the discovery of the vast majority, if not all, conventional drugs is based on the chemical, physiological, and therapeutic actions of the bioactive constituents of many medicinal herbs. The recent advancement in pharma and medicine, manifested by the development of biotechnologies, and mass production of highly specific, chemically-synthesized drugs, has certainly revolutionized the therapeutic approach to health care and disease management worldwide. However, herbal medicine continues to be a primary ideology in many populations today and a very common practice in different parts of the world.
Herbs and spices are known to be major taste enhancers in most cuisines, primarily used as a source of flavor and aroma. Besides their use as food additives, a wide range of herbs and spices have been used to prevent or treat medical conditions including cancer. Over the past few decades, research investigating dietary factors and their effects on various medical conditions has been constantly growing. A large number of studies focused on these naturally-occurring products and reported a plethora of anti-cancer properties manifested through various molecular mechanisms. For instance, an ethanolic extract of Piper nigrum (black pepper) has been shown to induce DNA damage and reduce cell viability in MCF-7 human cancer cells [3]. Treatment with the ethanolic extract of P. nigrum inhibited cell proliferation by 57% and elevated ROS levels by 65%. Moreover, the same extract increased Bax and p53 levels, both of which are key proteins in regulating the cell cycle arrest. Another study used flow cytometric analysis to describe the anti-cancer effects exerted by Fagonia cretica, a tea herb and a food additive [4]. Indeed, the F. cretica extract caused a dose-dependent arrest of the cell cycle at G0/G1 phase and enhanced the rate of apoptosis in MCF-7 and MDA-MB-231 human cancer cells. Another example of a widely used active food constituent is sesamin, a major lignin in sesame seeds. Siao and colleagues showed that sasamin plays a strong preventive role against cancer by modulating apoptotic signaling pathways and restricting angiogenesis [5]. Hence, various herbs and food additives are becoming widely used for the treatment and/or the prevention of acute and chronic conditions ranging from mild allergies to more serious diseases including cancer. Yet, despite the intensive research efforts devoted to the identification of herbs with therapeutic properties, the exact molecular pathways and cellular mechanisms by which these herbs induce their therapeutic effects are not fully understood.
Nigella sativa is an annual flowering plant that is grown almost all over the world but is native to South and Southwest Asia and commonly found in Northern Africa, the Middle East, and Southern Europe [6], [7]. N. sativa is also known as nigella, blackseed, black cumin, black caraway, Roman coriander, fennel flower, nutmeg flower, “kalonji” (in India), “Kalo jeera” (in Bangladesh), “Hak Jung Chou” (in China), and “habbat al-barakah” (in the Middle East). N. sativa belongs to the botanical family Ranunculaceae [8], [9]. The mature plant grows to 20–90 cm of height with finely divided leaves, and white, pale blue, or pale purple delicate flowers containing 5–10 petals [10]. The follicles within the fruits contain many small (2.0–3.5mm × 1.0–2.0 mm) angular, black seeds with whitish interior [10]. Aside from its use as a food flavoring additive, N. sativa seeds oil and extracts have been used since ancient times to treat several diseases and medical conditions. N. sativa plant extracts have been commonly used in various traditional systems of medicine like Ayurveda, Siddha, Unani, Arabic, Islamic, etc. Several N. sativa crude extracts have been popularly used in traditional medicine as appetite stimulants, bronchodilators, liver tonics, and analgesics as well as to treat various conditions like diabetes, asthma, hypertension, cardiovascular disease, liver and kidney diseases, digestive problems, diarrhea, skin disorders, microbial infections, cancer, etc. [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19] Such uses of N. sativa extracts in traditional medicine have been validated by well-designed experiments showing that such extracts possess cardio-protective, anti-microbial, anti-histaminic, anti-diabetic, antihypertensive, anti-hyperlipidemic, anti-diarrheal, hepato-protective, renal protective, gastro-protective, spasmolytic, immunomodulatory, anti-inflammatory, anti-oxidant, and anti-cancer properties [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Hence, traditional medicine uses that are validated by experimental evidence strongly suggest that N. sativa extracts can be of potent therapeutic efficacy in the prevention and treatment of various infectious and non-infectious diseases.
In this review, the in vitro and in vivo anti-cancer properties of N. sativa extracts are discussed. Special emphasis is given to the molecular and cellular mechanisms that mediate the anti-proliferative, pro-apoptotic, and anti-oxidant effects of N. sativa. Recent advances in the establishment of an effective therapeutic potential of N. sativa extracts, leading to suppressed tumor initiation and progression, are also underscored.
2. Anti-proliferative and pro-apoptotic effects of N. sativa
The potent anti-cancer potential of N. sativa is well established through in vitro and in vivo studies using different cell lines and animal models. Driven by traditional medical practices in Sri Lanka, a decoction (hot-water extract) comprised of N. sativa (seeds), Hemidesmus indicus (roots), and Smilax glabra (rhizome), a polyherbal mixture used to treat different types of cancer, has been shown to ameliorate diethylnitrosamine-induced hepatocarcinogenesis in male Wistar rats at a dose of 4–6 g/kg/day after 10 weeks of oral feeding [20]. The researchers of the aforementioned study indicated that the potential anti-cancer effects of the extracts of the individual plants in the decoction were not examined because only the decoction is traditionally used in cancer chemotherapy [20]. Subsequent studies suggest that flow cytometric analysis conducted using Annexin V and propidium iodide staining demonstrated that HepG2 cells were in the late stage of apoptosis and/or necrosis 24 h post treatment with the polyherbal mixture [21]. Consistently, oral administration (6 g/kg/day) of the polyherbal mixture of N. sativa, H. indicus, and S. glabra led to a long-term protection against diethylnitrosamine-induced hepatocellular adenoma in Wistar rats [22], [23]. In fact, a great deal of literature underscores many in vitro and in vivo effects of pure N. sativa extracts. In an early in vivo study, topical application of N. sativa extract (100 mg/kg) inhibited the two-stage initiation/promotion of skin carcinogenesis and delayed the onset of skin papilloma in mice challenged with 7,12-dimethylbenzanthracene/croton oil [24]. The same study revealed that intraperitoneal administration of N. sativa extract significantly reduced methylcholanthrene (MCA)-induced soft tissue sarcomas in albino mice by about 70% following 30 days of subcutaneous administration of MCA [25]. Aqueous and ethanolic extracts of N. sativa seeds, both separately and in combination, were shown to exert potent anti-proliferative effects on MCF-7 human breast cancer cells in presence and absence of H2O2, which seems to play a synergistic role [26]. In another study, Salim and Fukushima examined the effects of N. sativa oil on the development of colon tumors in a murine model of 1,2-dimethylhydrazine (DMH)-induced colon cancer [27]. Fourteen weeks post DMH challenge, Fischer 344 rats that were treated with N. sativa oil at the initiation and post-initiation stages of colon carcinogenesis displayed significantly reduced DMH-induced aberrant crypt foci (ACF), which are putative pre-neoplastic lesions for colon cancer [27]. Immunohistochemical analysis revealed that N. sativa oil exerted potent anti-proliferative activity in the colonic ACF in rats that were treated with N. sativa oil at both the initiation and post-initiation stages of DMH challenge [27]. Similarly, using 7,12-di-methylbenz(a)anthracene (DMBA)-induced mammary carcinoma model, female Sprague–Dawley rats that were injected with DMBA were subsequently orally treated with N. sativa oil (4 g/kg/day) starting 2 weeks before or at the time of DMBA injection, and the experiment lasted for 3 months [28]. The frequency of mammary papillary, comedo, and cribriform carcinoma was reduced in rats treated with N. sativa oil at the time of DMBA injection, and this frequency was more potently recused in rats that were pre-treated with N. sativa oil for 2 weeks before DMBA challenge [28]. The reduced frequency of mammary carcinoma was associated with reduced serum levels of tumorigenicity markers (total sialic acid (TSA) and lipid-bound sialic acid (LSA)), serum levels of endocrine derangement markers (prolactin, estradiol, and progesterone) and levels of apoptotic markers (serum tumor necrosis factor α (TNFα), tissue caspase-3 activity, and DNA fragmentation) [28]. Using the essential oil, an ethanolic extract, and a butanol extract of N. sativa and different cell lines (P815, IC01, Vero cells, and BSR cells), Ait Mbarek and colleagues demonstrated that the potency of the in vitro anti-cancer activity of N. sativa depends, at least partially, on the tumor cell type [29]. In the aforementioned study, the anti-cancer activity of N. sativa essential oil was also evaluated in vivo. Injection of 30–50 μl (28.5–47.5 mg/mouse) N. sativa essential oil into the tumor site of a DBA2/P815 (H2d) mouse model led to a significant suppression of solid tumor development (more than 10-fold decrease in tumor size) and resulted in a significantly delayed mortality of P815 mastocytoma tumor-bearing mice [29]. Recently, the administration of N. sativa ethanolic extract treatment was shown to improve the histopathological changes in the malignant liver tissue which were caused by diethylnitrosamine (DENA) treatment, without causing any direct cytotoxic effect [30]. In a similar study, the effects of a methanolic extract of N. sativa on the modulation of glyco-regulatory enzymes in an albino rat model of hepatocellular carcinoma were investigated [31]. Hepatocellular carcinoma was induced in albino rats by intraperitoneal injection of DENA and carbon tetrachloride (CCl4), leading to a significant increase in the serum level of α-fetoprotein (AFP), the relative liver weight, and the activities of hexokinase, glyceraldehyde phosphate dehydrogenase, and G6P dehydrogenase in both the serum and liver homogenate of treated rats. Oral administration of a methanolic extract of N. sativa (1 g/kg/day) for 2 weeks prior to induction of hepatocellular carcinogenesis improved the histopathological changes associated with DENA and CCl4 treatment, bringing the physiological and biochemical parameters indicated above back to normal levels [31]. Very recently, an in vitro study demonstrated that an aqueous extract of N. sativa (0.1–1.0% concentration) caused a significant decrease in cell proliferation and varying morphological changes including cell shrinkage and membrane damage in HepG2 cells, accompanied by DNA damage and cell death [32] (Fig. 1).
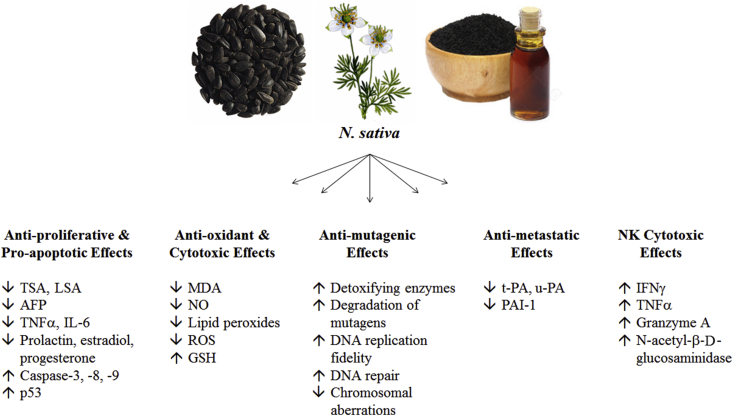
Fig. 1.
A brief summary of the known molecular and cellular mechanisms underlying the anti-proliferative, pro-apoptotic, anti-oxidant, cytotoxic, anti-mutagenic, anti-metastatic, and NK-mediated cytotoxic effects of N. sativa. (TSA: total sialic acid, LSA: lipid-bound sialic acid, AFP: α-fetoprotein, TNFα: tumor necrosis factor α, IL-6: interleukin-6, MDA: malondialdehyde, NO: nitric oxide, ROS, reactive oxygen species, GSH: glutathione, t-PA: tissue-type plasminogen activator, u-PA: urokinase-type plasminogen activator, PAI-1: plasminogen activator inhibitor type 1, IFNγ: interferon γ).
3. Anti-oxidant and cytotoxic effects of N. sativa
Among the first reports pointing to the potential anti-cancer properties of N. sativa, Swamy and Tan demonstrated that an aqueous extract and an ethyl acetate chromatographic fraction of N. sativa seeds (50 μg/ml) caused significant cytotoxic effects against various types of cancer cell lines (HepG2, MOLT4, and LL/2), but not against normal, non-cancerous human umbilical cord endothelial cells [33]. Aside from their anti-proliferative effects, both aqueous as well as ethanolic extracts of N. sativa seeds were found to induce significant cytotoxic effects on MCF-7 cells in presence and absence of H2O2 [26]. However, the ethanolic extract of N. sativa exerted more potency against MCF-7 cells compared to the aqueous extract (LC50 values in presence of H2O2 were 377 μM and 725 μM, respectively). Also, the aforementioned anti-cancer polyherbal mixture, which is comprised of N. sativa (seeds), H. indicus (roots), and S. glabra (rhizome), was shown to exert cytotoxic effects in human hepatoma HepG2 cell line at 5–50 mg/ml concentration [21]. In fact, the three individual plant extracts exerted cytotoxic efficacy in the order N. sativa > H. indicus > S. glabra [21]. Such anti-cancer effects were confirmed by Samarakoon and colleagues who demonstrated that both the aqueous and ethanolic extracts of the polyherbal mixture of N. sativa, H. indicus, and S. glabra caused strong dose-dependent cytotoxicity in HepG2 cells [22]. However, most of these studies do not yield insightful results since N. sativa extracts were used in combination with H. indicus and S. glabra extracts, making it challenging to draw plausible conclusions regarding the anti-cancer activity of N. sativa itself. Nonetheless, several studies examined the effects of N. sativa and its extracts on various cell lines. An in vitro cytotoxic study showed that a crude methanolic extract of N. sativa caused about 50% cytotoxicity in Ehrlich ascites carcinoma (EAC), Dalton's lymphoma ascites (DLA), and Sarcoma-180 cells (S-180 cells) [24]. Another in vivo study demonstrated that 6-month oral administration of N. sativa seeds (0.2 g/rat/day) provided protective effects against methylnitrosourea-induced oxidative stress and colon carcinogenesis in Sprague Dawely rats due to reduced expression of malondialdehyde (MDA), a biomarker of lipid peroxidation, and nitric oxide (NO) [34]. Zaoui and colleagues examined the possible biochemical and histopathological effects of N. sativa fixed oil in Iops of a mice and Wistar-Kyoto rats [35]. Acute toxicity of N. sativa fixed oil was assessed in mice that received a single oral or intraperitoneal dose, and the LD50 values were determined to be 28.8 ml/kg and 2.06 ml/kg, respectively [35]. The chronic toxicity of N. sativa fixed oil was assessed in rats receiving a daily oral dose of 2 ml/kg for a period of 12 weeks. It was demonstrated that chronic treatment with N. sativa fixed oil did not affect the level or catalytic activity of key hepatic enzymes including aspartate-aminotransferase, alanine-aminotransferase, and gamma-glutamyltransferase, nor it had any marked histopathological effects in the heart, liver, kidney, and pancreatic tissues [35]. The very low toxicity of the chronic treatment with N. sativa fixed oil was evidenced by biochemical stability and high LD50 values, suggesting that the indicated doses are sub-toxic and do not raise major safety concerns. Moreover, Islam and colleagues demonstrated that N. sativa oil exerts cytotoxic effects against a panel of four human cancer cell lines (SCL, SCL-6, SCL-37′6, and NUGC-4) and 3T6 fibroblast mouse cell line with LC50 values 155.02 ± 10.4, 185.77 ± 2.9, 120.40 ± 20.5, 384.53 ± 12.1, and 286.83 ± 23.3 μg/ml, respectively, with no significant cytotoxic effects on normal cells [36]. In another study, Ali assessed the ability of N. sativa oil to ameliorate the nephrotoxicity associated with gentamicin, an antibiotic, in rats [37]. Intramuscular injection of gentamicin was associated with proximal tubular damage, histopathological and biochemical signs of nephrotoxicity, elevated levels of creatinine and urea, as well as decreased level of glutathione (GSH) and total anti-oxidant status [37]. Such effects were abrogated by oral administration of N. sativa oil (1–2 ml/kg/day) for 10 days, without any detectable overall toxicity [37]. Similarly, oral N. sativa treatment (4 g/kg/day) of rats with DMBA-induced mammary carcinoma for a period of 3 months resulted in reduced tissue levels of oxidative stress markers (NO and lipid peroxides) [28]. Intragastric administration of N. sativa oil for 12 days in male albino rats potently reduced the hepatic and overall toxic effects associated with intraperitoneal administration of cyclophosphamide, an anti-cancer drug that causes a high degree of lipid peroxidation and reactive oxygen species (ROS) over-production [38]. Similarly, oral administration of N. sativa oil (90 mg/kg/day) in albino rats for 30 and 60 days significantly ameliorated, in a time-dependent manner, the toxic effects and pathological tissue damage in the spleen and thymus resulting from treatment with chloramphenicol, a potent antibiotic [39]. These findings suggest that N. sativa oil co-treatment could potentially reduce the toxicity-related side effects that accompany the bactericidal and anti-cancer chemotherapy. In a recent study, the hepatotoxic effects of N. sativa were evaluated in Spargue Dawley rats by measuring the catalytic activity of key liver enzymes (ALT and AST) and by histopathological assessment of liver tissue [40]. Rats were fed diet supplemented with 0.01–1 g/kg/day of N. sativa seeds powder for 28 days. It was demonstrated that N. sativa powder supplementation did not lead to a significant change in the catalytic activity of ALT and AST, histopathological abnormalities, inflammation, or necrosis in the liver tissue even at the highest dose of 1 g/kg/day [40]. This study showed that 0.01–1 g/kg/day doses of N. sativa seeds powder caused no marked toxic effects on liver function in rats and they are considered safe. Very recently, Hadi and colleagues performed a clinical trial to assess the anti-oxidant effects of N. sativa oil in patients with rheumatoid arthritis (RA) [41]. It was revealed that a daily dose of 1 g N. sativa oil for 8 weeks significantly reduced the serum levels of MDA and NO, suggesting that N. sativa can potentially be employed in the treatment of RA due to its ability to suppress RA-associated oxidative stress responses [41] (Fig. 1).
4. Anti-mutagenic effects of N. sativa
A few studies have examined the potential of N. sativa to exert anti-mutagenic activity against N-methyl-N′-nitro-N-nitrosoguanidine (MNNG), a directly acting mutagen. Although an aqueous extract of N. sativa had no cytoprotective nor anti-mutagenic activity against MMNG in primary rat hepatocytes [42], an ethanolic extract of N. sativa exerted an inhibitory effect against MNNG mutagenicity due to significantly reduced chromosomal aberrations in primary rat hepatocytes [43]. The anti-mutagenic activity of the ethanolic extract of N. sativa was observed in MNNG-challenged primary rat hepatocytes that were pre-treated, co-treated, or post-treated with the extract, without inducing direct apoptosis [43]. Such anti-mutagenic effects against MNNG were attributed to possible induction of detoxifying enzymes that degrade MNNG, chemical interaction with or absorption of MNNG (or its electrophilic degradation products), enhanced fidelity of DNA replication, and/or improved DNA repair [43]. Such factors that prevent or reduce chromosomal aberrations [43]. Although some findings provide evidence of a potent anti-mutagenic activity of N. sativa, research is still in its early stages of establishing a direct link between the specific ingredients in N. sativa extracts and the anti-mutagenic activity of N. sativa (Fig. 1).
5. Anti-metastatic effects of N. sativa
Awad investigated the effect of N. sativa oil on HT1080 human fibrosarcoma cell lines with regard to their fibrinolytic potential, a hallmark of malignant tumors [44]. N. sativa oil (25–200 μg oil/ml) caused a significant dose-dependent down-regulation of key fibrinolytic products including tissue-type plasminogen activator (t-PA), urokinase-type plasminogen activator (u-PA), and plasminogen activator inhibitor type 1 (PAI-1), both in sub-confluent and confluent cell cultures [44]. This study highlights the ability of N. sativa to hinder local tumor invasion and metastasis. Ait Mbarek and colleagues reported similar findings, whereby injection of 30–50 μl (28.5–47.5 mg/mouse) N. sativa essential oil into the tumor site of a DBA2/P815 (H2d) mouse model resulted in inhibition of liver metastasis even after 30 days of treatment [29] (Fig. 1).
6. Effects of N. sativa on natural killer (NK) cytotoxic activity
Enhancement of NK cytotoxic activity has been proposed by several research groups to serve as a mechanism underlying the anti-cancer effects of N. sativa [45], [46], [47], [48], [49], [50]. In an in vivo study performed on healthy volunteers, El-Kadi and colleagues showed that ingestion of N. sativa oil for 4 weeks enhanced the ratio of helper to suppressor T cells and significantly improved NK cytotoxic function [46]. In agreement, an in vivo study performed in mice revealed that 1-week oral administration of an aqueous extract of N. sativa caused a significant increase in the number of splenic NK cells and a significant enhancement of splenic NK cytotoxic activity against YAC-1 tumor cells [48]. These in vivo findings were supported by in vitro studies. Abuharfeil and colleagues demonstrated that a fresh aqueous extract of N. sativa (50 and 100 μg/ml) led to a significant increase in splenic NK cytotoxic activity against YAC-1 tumor cells (% cytotoxicity 43.7 ± 3.6 and 62.7 ± 5.6 at 200:1 E:T ratio, 45.7 ± 5.7 and 44.6 ± 6.2 at 100:1 E:T ratio, and 13.6 ± 2.7 and 18.3 ± 3.1 at 50:1 E:T ratio, respectively) [47]. Indeed, the fresh aqueous extract of N. sativa appeared to be more potent in inducing NK cytotoxic activity compared to the old dried aqueous extract or the ethanolic extract [47]. A similar study from our laboratory provided further in vitro experimental evidence indicating that an aqueous extract of N. sativa (50–100 μg/ml) significantly enhanced killing of YAC-1 tumor cells due to augmented NK cytotoxic activity leading to 14% (3 folds) and 23% (4.5 folds) cytotoxicity 200:1 E:T ratio at 50 μg/ml and 100 μg/ml concentrations, respectively [50]. Importantly, enhanced killing of YAC-1 tumor cells is due to the ability of N. sativa extract to improve NK cytotoxic activity rather than inducing an immediate cytotoxic effect. This is evidenced by the findings that N. sativa extract had no significant, direct cytotoxic effect against YAC-1 tumor cells in absence of NK cells [50]. We have reported similar observations in which aqueous extracts (100 μg/ml) of black pepper (P. nigrum) and cardamom (Elettaria cardamomum) caused a significant increase (35% and 45% cytotoxicity, respectively) in the NK cytotoxic activity against YAC-1 tumor cells [51]. Therefore, it seems that boosting the cytotoxic potential of NK cells against tumor cells is at least one mechanism exploited by several plant extracts to exert their tumoricidal action. Interestingly, Abuharfeil and colleagues assessed NK cytotoxic activity in the presence of the aqueous extract of N. sativa using splenocytes obtained from BALB/c mice [47], whereas in our study the NK cytotoxic activity was assessed using splenocytes obtained from C57/BL6 mice [50]. Although the enhancement of NK cytotoxic activity caused by N. sativa does not seem to be strain-specific, more studies are required to confirm this argument using splenocytes from a wide range of mice strains and even different animal models. Along the same lines, an aqueous extract of N. sativa (10–500 μg/ml) was shown to significantly enhance the cytotoxic activity (26.6–67.7% cytotoxicity) of NK cells isolated from human blood against K562 tumor cells in vitro [49]. The improved cytotoxic potential of NK cells was primarily due to the ability of N. sativa extract to significantly enhance the production of interferon γ (IFNγ) and TNFα, immunostimulatory cytokines with potent tumoricidal activity, from NK cells [49]. Moreover, treatment of NK cells with N. sativa extract led to a significant increase in the release, and hence activity, of granzyme A and N-acetyl-β-d-glucosaminidase, key proteolytic enzymes involved in target cell killing [49]. These findings suggest that augmentation of NK cytotoxic activity against tumor cells serves as an effective immunomodulatory mechanism that may explain, at least partially, the reported in vitro and in vivo anti-cancer effects of N. sativa. An early in vivo study, however, demonstrated that intraperitoneal injection of N. sativa oil (100 μg/100 ml/mouse) for 7 days caused a significant decrease in the number of splenic NK cells in non-infected and CMV-infected BALB/c mice [52]. Interestingly, although N. sativa oil treatment had no effect on NK cytotoxic activity in non-infected mice, it caused a significant suppression of NK cytotoxic activity in CMV-infected mice [52]. The same study revealed that in vitro treatment of splenic NK cells isolated from non-infected mice with N. sativa oil (100 μg/ml) significantly decreased their cytotoxic activity against YAC-1 tumor cells [52]. These findings are inconsistent with those reported by El-Kadi and his colleagues [46] regarding the effects of N. sativa oil on NK cytotoxic effects against cancer cells. These inconsistent findings are most likely due to different experimental conditions including the dose of N. sativa extract or oil, cell type, species, incubation time, and method of detection. It is worth mentioning that N. sativa oil may exert toxic effects against NK cells, which could be another factor influencing the outcome of the reported experiments. Future studies, with a carefully-designed experimental approach that addresses the raised possible experimental variables, are required to shed more light on the potential modulatory effects of N. sativa oil on NK cytotoxic activity.
Although some of the signaling molecules involved in mediating the immunostimulatory effects of N. sativa extracts in NK cells have been identified, the exact signaling pathways and molecular targets implicated in these pathways are largely unknown. Future in vitro and in vivo studies should focus on elucidating the targeted receptors and intracellular/extracellular factors involved in the signal transduction pathways that are modulated in NK cells by N. sativa extracts. Furthermore, we suggest that the stimulatory potential of N. sativa toward NK cytotoxic activity be further confirmed by in vitro and in vivo studies using a wide range of primary and transformed NK cells against numerous primary tumors and cancer cell lines (Fig. 1).
7. Signaling pathways underlying the anti-cancer effects of N. sativa
Several in vitro and in vivo studies were conducted in an attempt to elucidate the molecular and cellular mechanisms underlying the anti-cancer activity of N. sativa. The key mechanisms underlying the documented anti-cancer effects of N. sativa have been largely attributed to their ability to modulate the activity of key enzymes [31], [43], [44], [49], [53], [54], [55], [56], [57], [58]. suppress inflammation [8], [50], [53], [55], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], and induce apoptosis in tumor cells [21], [41], [54], [55], [72], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101].
One mechanism that is implicated in tumorigenesis involves the inducible nitric oxide synthase (iNOS) pathway. NO, which is synthesized by iNOS or other nitric oxide synthase (NOS) isoforms during physiological reactions including inflammation, is an endogenous radical implicated in predisposition to tumor development. In a recent study, Fathy and Nikaido investigated the effect of an ethanolic extract of N. sativa on modulating the iNOS pathway in rats with DENA-induced hepatocarcinogenesis [30]. Oral administration of N. sativa ethanolic extract (250 mg/kg/day) for 5 days led to a significant reduction in the serum levels of AFP, NO, interleukin-6 (IL-6), and TNFα, factors whose production was significantly increased after treatment with DENA [30]. Very recently, Alhamzi and colleagues demonstrated that a methanolic extract of N. sativa seeds (50–100 μl/ml) induced apoptosis in MCF-7 cells in a time- and dose- dependent manner, as judged by TUNEL assay [97]. The methanolic extract of N. sativa led to a significant time- and dose-dependent increase in the expression of apoptotic factors including caspase-3, caspase-8, caspase-9, and p53 in MCF-7 cells, indicating that N. sativa manifests its anti-cancer activity by targeting the p53 and caspase signaling pathways [97].
A brief summary about the reported in vitro and in vivo anti-cancer activities of N. sativa is given in Table 1.
Table 1.
A brief summary of the reported in vitro and in vivo anti-cancer activities of N. sativa.
| Activity | N. sativa |
|---|---|
| Anti-proliferative and pro-apoptotic effects |
|
| Anti-oxidant and cytotoxic effects |
|
| Anti-mutagenic effects | |
| Anti-metastatic effects | |
| Effects on NK cytotoxic activity |
|
8. Anti-cancer effects of N. sativa phytoconstituents
Many of the anti-cancer activities of N. sativa have been attributed to its major active constituent, thymoquinone (TQ). TQ has been shown to exert anti-proliferative, pro-apoptotic, anti-oxidant, anti-oxidant, anti-mutagenic, anti-angiogenic, and anti-metastatic effects against cancer cells [6], [12], [14], [16], [17], [18], [19], [38], [53], [55], [63], [64], [66], [70], [71], [77], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100]. TQ seems to mediate its anti-cancer effects by targeting a number of cellular pathways involving p53, NF-κB, PPARγ, STAT3, MAPK, and PI3K/AKT transducing signals [67], [69], [72], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100]. Besides TQ, other phytoconstituents of N. sativa have also been shown to contribute to the anti-cancer potential of N. sativa extracts. α-hederin is a pentacyclic triterpene saponin found in N. sativa seeds that exerts effective anti-cancer effects, both in vitro and in vivo [54], [102], [103], [104], [105], [106], [107]. Moreover, thymol, thymohydroquinone, dithymoquinone, nigellimine-N-oxide, nigellicine, nigellidine, and carvacrol are phytoconstituents of N. sativa that have been demonstrated to play anti-cancer and cytotoxic functions [13], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117]. Yet, the exact molecular mechanisms underlying the anti-cancer effects of these phytoconstituents are not fully known, and future studies are needed to elucidate the detailed mechanisms of action that mediate the anti-cancer effects of N. sativa phytoconstituents.
9. Conclusions
N. sativa is among the most commonly used herb in the history of mankind. N. sativa is considered by many to be a “miracle” herb due to its effective therapeutic potential to alleviate signs and symptoms of many diseases including cancer. The anti-cancer properties of N. sativa have been mainly attributed to its ability to exert potent anti-proliferative, pro-apoptotic, anti-oxidant, anti-mutagenic, and anti-metastatic roles. The protective effects of N. sativa against tumor initiation and progression have also been attributed, at least in part, to their ability to suppress inflammation and exert immune-boosting effects. Enhancement of NK cytotoxic activity against cancer cells and regulation of signaling pathways, such as iNOS, p53, and caspases, mediate the potential of N. sativa to subdue tumorigenesis and cancer. In vitro and in vivo experimental findings suggest that N. sativa extracts can potentially be employed in the development of effective therapeutic agents that can be employed in the regulation of various stages of tumorigenesis and treatment of many types of cancer. Further studies are definitely needed to shed more light on the molecular and cellular mechanisms underlying the anti-cancer effects of N. sativa. Such research endeavors will hopefully elucidate the exact signaling pathways implicated in the suppressive role that N. sativa extracts play in tumorigenesis and cancer. Moreover, although the preclinical, experimental evidence suggesting potent anti-cancer effects of various N. sativa extracts is compelling, preventive and clinical studies that directly point to the anti-cancer potential of N. sativa extracts are still lacking. Future studies should focus on establishing a direct link between the reported anti-cancer effects of N. sativa extracts and cancer prevention/treatment in preclinical and clinical settings.
Sources of support
None.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Barrett B., Kiefer D., Rabago D. Assessing the risks and benefits of herbal medicine: an overview of scientific evidence. Altern Ther Health Med. 1999;5:40–49. [PubMed] [Google Scholar]
- 2.Donaldson K. Introduction to the healing herbs. ORL Head Neck Nurs. 1998;16:9–16. [PubMed] [Google Scholar]
- 3.de Souza Grinevicius V.M., Kviecinski M.R., Santos Mota N.S., Ourique F., Porfirio Will Castro L.S., Andreguetti R.R. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J Ethnopharmacol. 2016;189:139–147. doi: 10.1016/j.jep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Lam M., Carmichael A.R., Griffiths H.R. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS One. 2012;7(6):e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siao A.C., Hou C.W., Kao Y.H., Jeng K.C. Effect of sesamin on apoptosis and cell cycle arrest in human breast cancer mcf-7 cells. Asian Pac J Cancer Prev. 2015;16(9):3779–3783. doi: 10.7314/apjcp.2015.16.9.3779. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S., Azmi A.S., Padhye S., Singh M.W., Baruah J.B., Philip P.A. Structure-activity studies on therapeutic potential of thymoquinone analogs in pancreatic cancer. Pharm Res. 2010;27(6):1146–1158. doi: 10.1007/s11095-010-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M.A., Chen H.C., Tania M., Zhang D.Z. Anticancer activities of Nigella sativa (black cumin) Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):226–232. doi: 10.4314/ajtcam.v8i5S.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali B.H., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 9.Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt M.S., Sultan M.T. Nigella sativa: reduces the risk of various maladies. Crit Rev Food Sci Nutr. 2010;50(7):654–665. doi: 10.1080/10408390902768797. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S., Padhye S., Azmi A., Wang Z., Philip P.A., Kucuk O. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62(7):938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randhawa M., Alghamdi M. Anticancer activity of Nigella sativa (black seed) - a review. Am J Chin Med. 2011;39(6):1075–1091. doi: 10.1142/S0192415X1100941X. [DOI] [PubMed] [Google Scholar]
- 14.Woo C.C., Kumar A.P., Sethi G., Tan K.H. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83(4):443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Shabana A., El-Menyar A., Asim M., Al-Azzeh H., Al Thani H. Cardiovascular benefits of black cumin (Nigella sativa) Cardiovasc Toxicol. 2013;13(1):9–21. doi: 10.1007/s12012-012-9181-z. [DOI] [PubMed] [Google Scholar]
- 16.Abukhader M.M. Thymoquinone in the clinical treatment of cancer: fact or fiction? Pharmacogn Rev. 2013;7(14):117–120. doi: 10.4103/0973-7847.120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider-Stock R., Fakhoury I.H., Zaki A.M., El-Baba C.O., Gali-Muhtasib H.U. Thymoquinone: fifty years of success in the battle against cancer models. Drug Discov Today. 2014;19(1):18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Rahmani A.H., Alzohairy M.A., Khan M.A., Aly S.M. Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evid Based Complement Altern Med. 2014;2014:724658. doi: 10.1155/2014/724658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majdalawieh A.F., Fayyad M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. 2015;28:295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Iddamaldeniya S.S., Wickramasinghe N., Thabrew I., Ratnatunge N., Thammitiyagodage M.G. Protection against diethylnitrosoamine-induced hepatocarcinogenesis by an indigenous medicine comprised of Nigella sativa, Hemidesmus indicus and Smilax glabra: a preliminary study. J Carcinog. 2003;2(1):6. doi: 10.1186/1477-3163-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thabrew M.I., Mitry R.R., Morsy M.A., Hughes R.D. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus and Smilax glabra on human hepatoma HepG2 cells. Life Sci. 2005;77(12):1319–1330. doi: 10.1016/j.lfs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Samarakoon S.R., Thabrew I., Galhena P.B., De-Silva D., Tennekoon K.H. A comparison of the cytotoxic potential of standardized aqueous and ethanolic extracts of a polyherbal mixture comprised of Nigella sativa (seeds), Hemidesmus indicus (roots) and Smilax glabra (rhizome) Pharmacogn Res. 2010;2:335–342. doi: 10.4103/0974-8490.75451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iddamaldeniya S.S., Thabrew M.I., Wickramasinghe S.M., Ratnatunge N., Thammitiyagodage M.G. A long-term investigation of the anti-hepatocarcinogenic potential of an indigenous medicine comprised of Nigella sativa, Hemidesmus indicus and Smilax glabra. J Carcinog. 2006;5:11. doi: 10.1186/1477-3163-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomi N.J., Nair S.C., Jayawardhanan K.K., Varghese C.D., Panikkar K.R. Antitumour principles from Nigella sativa seeds. Cancer Lett. 1992;63:41–46. doi: 10.1016/0304-3835(92)90087-c. [DOI] [PubMed] [Google Scholar]
- 25.Salomi M.J., Nair S.C., Panikkar K.R. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- 26.Farah I.O., Begum R.A. Effect of Nigella sativa (N. sativa L.) and oxidative stress on the survival pattern of MCF-7 breast cancer cells. Biomed Sci Instrum. 2003;39:359–364. [PubMed] [Google Scholar]
- 27.Salim E.I., Fukushima S. Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. Nutr Cancer. 2003;45(2):195–202. doi: 10.1207/S15327914NC4502_09. [DOI] [PubMed] [Google Scholar]
- 28.Abd El-Aziz M.A., Hassan H.A., Mohamed M.H., Meki A.M.A., Abdel-Ghaffar S.K.H., Hussein M.R. The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigella sativa in mammary carcinoma: an animal model. Int J Exp Pathol. 2005;86(6):383–396. doi: 10.1111/j.0959-9673.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ait Mbarek M., Ait Mouse H., Elabbadi N., Bensalah M., Gamouh A., Aboufatima R. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Braz J Med Biol Res. 2007;40:839–847. doi: 10.1590/s0100-879x2006005000108. [DOI] [PubMed] [Google Scholar]
- 30.Fathy M., Nikaido T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Env Health Prev Med. 2013;18(5):377–385. doi: 10.1007/s12199-013-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel-Hamid N.M., Abdel-Ghany M.I., Nazmy M.H., Amgad S.W. Can methanolic extract of Nigella sativa seed affect glyco-regulatory enzymes in experimental hepatocellular carcinoma? Environ Health Prev Med. 2013;18(1):49–56. doi: 10.1007/s12199-012-0292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan F., Kalamegam G., Gari M., Abuzenadah A., Chaudhary A., Al Qahtani M. Evaluation of the effect of Nigella sativa extract on human hepatocellular adenocarcinoma cell line (HepG2) in vitro. BMC Genomics. 2014;15(Suppl. 2):63. [Google Scholar]
- 33.Swamy S.M., Tan B.K. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70:1–7. doi: 10.1016/s0378-8741(98)00241-4. [DOI] [PubMed] [Google Scholar]
- 34.Mabrouk G.M., Moselhy S.S., Zohny S.F., Ali E.M., Helal T.E., Amin A.A. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J Exp Clin Cancer Res. 2002;21:341–346. [PubMed] [Google Scholar]
- 35.Zaoui A., Cherrah Y., Mahassini N., Alaoui K., Amarouch H., Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9(1):69–74. doi: 10.1078/0944-7113-00084. [DOI] [PubMed] [Google Scholar]
- 36.Islam S.N., Begum P., Ahsan T., Huque S., Ahsan M. Immunosuppressive and cytotoxic properties of Nigella sativa. Phytother Res. 2004;18:395–398. doi: 10.1002/ptr.1449. [DOI] [PubMed] [Google Scholar]
- 37.Ali B.H. The effect of Nigella sativa oil on gentamicin nephrotoxicity in rats. Am J Chin Med. 2004;32:49–55. doi: 10.1142/S0192415X04001710. [DOI] [PubMed] [Google Scholar]
- 38.Alenzi F.Q., El-Bolkiny Yel-S., Salem M.L. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br J Biomed Sci. 2010;67(1):20–28. doi: 10.1080/09674845.2010.11730285. [DOI] [PubMed] [Google Scholar]
- 39.Ebaid H., Dkhil M., Zahran W., Feki M., Gabry M. Role of Nigella sativa in ameliorating chloramphenicol induced tissue damage in rats. J Med Plants Res. 2011;5(2):280–288. [Google Scholar]
- 40.Dollah M.A., Parhizkar S., Latiff L.A., Bin Hassan M.H. Toxicity effect of Nigella sativa on the liver function of rats. Adv Pharm Bull. 2013;3(1):97–102. doi: 10.5681/apb.2013.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadi V., Kheirouri S., Alizadeh M., Khabbazi A., Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed. 2016;6(1):34–43. [PMC free article] [PubMed] [Google Scholar]
- 42.Khader M., Eckl P.M., Bresgen N. Effects of aqueous extracts of medicinal plants on MNNG-treated rat hepatocytes in primary cultures. J Ethnopharmacol. 2007;112(1):199–202. doi: 10.1016/j.jep.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Khader M., Bresgen N., Eckl P.M. Antimutagenic effects of ethanolic extracts from selected Palestinian medicinal plants. J Ethnopharmacol. 2010;127(2):319–324. doi: 10.1016/j.jep.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Awad E.M. In vitro decreases of the fibrinolytic potential of cultured human fibrosarcoma cell line, HT1080, by Nigella sativa oil. Phytomedicine. 2005;129(1–2):100–107. doi: 10.1016/j.phymed.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Elkadi A., Kandil O. Effect of Nigella sativa (the black seed) on immunity. Proceeding of the 4th International Conference on Islamic Medicine, Kuwait, Bull Islamic Med. 1986;4:344–348. [Google Scholar]
- 46.El-Kadi A., Kandil O., Tabuni A.M. Nigella sativa and cell mediated immunity. Arch AIDS Res. 1989;1:232–233. [Google Scholar]
- 47.Abuharfeil N.M., Maraqa A., Von Kleist S. Augmentation of natural killer cell activity in vitro against tumor cells by wild plants from Jordan. J Ethnopharmacol. 2000;71:55–63. doi: 10.1016/s0378-8741(99)00176-2. [DOI] [PubMed] [Google Scholar]
- 48.Abuharfeil N.M., Salim M., Von Kleist S. Augmentation of natural killer cell activity in vivo against tumour cells by some wild plants from Jordan. Phytother Res. 2001;15:109–113. doi: 10.1002/ptr.692. [DOI] [PubMed] [Google Scholar]
- 49.Shabsoug B., Khalil R., Abuharfeil N. Enhancement of natural killer cell activity in vitro against human tumor cells by some plants from Jordan. J Immunotoxicol. 2008;5:279–285. doi: 10.1080/15376510802312027. [DOI] [PubMed] [Google Scholar]
- 50.Majdalawieh A.F., Hmaidan R., Carr R.I. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol. 2010;131:268–275. doi: 10.1016/j.jep.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Majdalawieh A.F., Carr R.I. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum) J Med Food. 2010;13:371–381. doi: 10.1089/jmf.2009.1131. [DOI] [PubMed] [Google Scholar]
- 52.Salem M.L., Hossain M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22:729–740. doi: 10.1016/s0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 53.Houghton P.J., Zarka R., de las Heras B., Hoult J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 54.Swamy S.M., Huat B.T. Intracellular glutathione depletion and reactive oxygen species generation are important in alpha-hederin-induced apoptosis of P388 cells. Mol Cell Biochem. 2003;245:127–139. doi: 10.1023/a:1022807207948. [DOI] [PubMed] [Google Scholar]
- 55.Chehl N., Chipitsyna G., Gong Q., Yeo C.J., Arafat H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB Oxf. 2009;11(5):373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rastogi L., Feroz S., Pandey B.N., Jagtap A., Mishra K.P. Protection against radiation-induced oxidative damage by an ethanolic extract of Nigella sativa L. Int J Radiat Biol. 2010;86(9):719–731. doi: 10.3109/09553002.2010.484480. [DOI] [PubMed] [Google Scholar]
- 57.Velho-Pereira R., Kumar A., Pandey B., Mishra K., Jagtap A. Radioprotection by macerated extract of Nigella sativa in normal tissues of fibrosarcoma bearing mice. Indian J Pharm Sci. 2012;74(5):403–414. doi: 10.4103/0250-474X.108415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sultan M.T., Butt M.S., Karim R., Ahmad N., Ahmad R.S., Ahmad W. Nigella sativa fixed and essential oil improves antioxidant status through modulation of antioxidant enzymes and immunity. Pak J Pharm Sci. 2015;28(2):589–595. [PubMed] [Google Scholar]
- 59.Nieto N., Torres M.I., Fernandez M.I., Giron M.D., Rios A., Suarez M.D. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. 2000;45:1820–1827. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- 60.Koch T.R., Yuan L.X., Stryker S.J., Ratliff P., Telford G.L., Opara E.C. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig Dis Sci. 2000;45:1814–1819. doi: 10.1023/a:1005517824877. [DOI] [PubMed] [Google Scholar]
- 61.Choudhary S., Keshavarzian A., Yong S., Wade M., Bocckino S., Day B.J. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig Dis Sci. 2001;46:2222–2230. doi: 10.1023/a:1011975218006. [DOI] [PubMed] [Google Scholar]
- 62.Al-Ghamdi M.S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76:45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 63.El-Dakhakhny M., Madi N.J., Lembert N., Ammon H.P. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81:161–164. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 64.Mahgoub A.A. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 2003;143:133–143. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 65.Chakrabarty A., Emerson M.R., LeVine S.M. Heme oxygenase-1 in SJL mice with experimental allergic encephalomyelitis. Mult Scler. 2003;9:372–381. doi: 10.1191/1352458503ms928oa. [DOI] [PubMed] [Google Scholar]
- 66.Mansour M., Tornhamre S. Inhibition of 5-lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. J Enzyme Inhib Med Chem. 2004;19:431–436. doi: 10.1080/14756360400002072. [DOI] [PubMed] [Google Scholar]
- 67.Mohamed A., Afridi D.M., Garani O., Tucci M. Thymoquinone inhibits the activation of NF-kappaB in the brain and spinal cord of experimental autoimmune encephalomyelitis. Biomed Sci Instrum. 2005;41:388–393. [PubMed] [Google Scholar]
- 68.El-Gouhary I., Mohamed A., Suleiman S., Benghuzzi H. Comparison of the amelioration effects of two enzyme inducers on the inflammatory process of experimental allergic encephalitis (EAE) using immunohistochemical technique. Biomed Sci Instrum. 2005;41:376–381. [PubMed] [Google Scholar]
- 69.Sayed A.A., Morcos M. Thymoquinone decreases AGE-induced NF-kappaB activation in proximal tubular epithelial cells. Phytother Res. 2007;21:898–899. doi: 10.1002/ptr.2177. [DOI] [PubMed] [Google Scholar]
- 70.El Gazzar M.A. Thymoquinone suppresses in vitro production of IL-5 and IL-13 by mast cells in response to lipopolysaccharide stimulation. Inflamm Res. 2007;56:345–351. doi: 10.1007/s00011-007-7051-0. [DOI] [PubMed] [Google Scholar]
- 71.Sayed A.A. Thymoquinone protects renal tubular cells against tubular injury. Cell Biochem Funct. 2008;26:374–380. doi: 10.1002/cbf.1454. [DOI] [PubMed] [Google Scholar]
- 72.Sethi G., Ahn K.S., Aggarwal B.B. Targeting nuclear factor-{kappa}B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 73.Nikakhlagh S., Rahim F., Hossein F., Aryani N., Syahpoush A., Brougerdnya M. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryng. 2001;32(5):402–407. doi: 10.1016/j.amjoto.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 74.Duncker S.C., Philippe D., Martin-Paschoud C., Moser M., Mercenier A., Nutten S. Nigella sativa (black cumin) seed extract alleviates symptoms of allergic diarrhea in mice, involving opioid receptors. PLoS One. 2012;7(6):e39841. doi: 10.1371/journal.pone.0039841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yousefi M., Barikbin B., Kamalinejad M., Abolhasani E., Ebadi A., Younespour S. Comparison of therapeutic effect of topical Nigella with betamethasone and eucerin in hand eczema. J Eur Acad Dermatol Venereol. 2013;27(12):1498–1504. doi: 10.1111/jdv.12033. [DOI] [PubMed] [Google Scholar]
- 76.Abdel-Aziz M., Abass A., Zalata K., Abd Al-Galel T., Allam U., Karrouf G. Effect of dexamethasone and Nigella sativa on inducible nitric oxide synthase in the lungs of a murine model of allergic asthma. Iran J Allergy Asthma Immunol. 2014;13(5):324–334. [PubMed] [Google Scholar]
- 77.Keyhanmanesh R., Pejman L., Omrani H., Mirzamohammadi Z., Shahbazfar A.A. The effect of single dose of thymoquinone, the main constituents of Nigella sativa, in Guinea pig model of asthma. Bioimpacts. 2014;4(2):75–81. doi: 10.5681/bi.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keyhanmanesh R., Nazemiyeh H., Mazouchian H., Bagheri Asl M.M., Karimi Shoar M., Alipour M.R. Nigella sativa pretreatment in Guinea pigs exposed to cigarette smoke modulates in vitro tracheal responsiveness. Iran Red Crescent Med J. 2014;16(7):e10421. doi: 10.5812/ircmj.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahmood M.S., Gilani A.H., Khwaja A., Rashid A., Ashfaq M.K. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res. 2003;17(8):921–924. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- 80.Al-Naggar T.B., Gómez-Serranillos M.P., Carretero M.E., Villar A.M. Neuropharmacological activity of Nigella sativa L. extracts. J Ethnopharmacol. 2003;88(1):63–68. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 81.Ghannadi A., Hajhashemi V., Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 2005;8(4):488–493. doi: 10.1089/jmf.2005.8.488. [DOI] [PubMed] [Google Scholar]
- 82.Vaillancourt F., Silva P., Shi Q., Fahmi J., Fernandes C., Benderdour M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. Cell Biochem. 2001;112:107–117. doi: 10.1002/jcb.22884. [DOI] [PubMed] [Google Scholar]
- 83.Shoieb A.M., Elgayyar M., Dudrick P.S., Bell J.L., Tithof P.K. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–113. [PubMed] [Google Scholar]
- 84.Gali-Muhtasib H., Diab-Assaf M., Boltze C., Al-Hmaira J., Hartig R., Roessner A. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- 85.Gali-Muhtasib H., Kuester D., Mawrin C., Bajbouj K., Diestel A., Ocker M. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68:5609–5618. doi: 10.1158/0008-5472.CAN-08-0884. [DOI] [PubMed] [Google Scholar]
- 86.Gali-Muhtasib H., Ocker M., Kuester D., Krueger S., El-Hajj Z., Diestel A. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gali-Muhtasib H.U., Abou Kheir W.G., Kheir L.A., Darwiche N., Crooks P.A. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15:389–399. doi: 10.1097/00001813-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 88.Hoque A., Lippman S.M., Wu T.T., Xu Y., Liang Z.D., Swisher S. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: a potential target for prevention. Carcinogenesis. 2005;26:785–791. doi: 10.1093/carcin/bgi026. [DOI] [PubMed] [Google Scholar]
- 89.El-Mahdy M.A., Zhu Q., Wang Q.E., Wani G., Wani A.A. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 90.Roepke M., Diestel A., Bajbouj K., Walluscheck D., Schonfeld P., Roessner A. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 91.Kaseb A.O., Chinnakannu K., Chen D., Sivanandam A., Tejwani S., Menon M. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782–7788. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 92.El-Najjar N., Chatila M., Moukadem H., Vuorela H., Ocker M., Gandesiri M. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15(2):183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 93.Badr G., Alwasel S., Ebaid H., Mohany M., Alhazza I. Perinatal supplementation with thymoquinone improves diabetic complications and T cell immune responses in rat offspring. Cell Immunol. 2011;267(2):133–140. doi: 10.1016/j.cellimm.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Badr G., Mohany M., Abu-Tarboush F. Thymoquinone decreases F-actin polymerization and the proliferation of human multiple myeloma cells by suppressing STAT3 phosphorylation and Bcl2/Bcl-XL expression. Lipids Health Dis. 2011;10:236. doi: 10.1186/1476-511X-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dergarabetian E.M., Ghattass K.I., El-Sitt S.B., Al-Mismar R.M., El-Baba C.O., Itani W.S. Thymoquinone induces apoptosis in malignant T-cells via generation of ROS. Front Biosci Elite Ed. 2013;5:706–719. doi: 10.2741/e651. [DOI] [PubMed] [Google Scholar]
- 96.Attoub S., Sperandio O., Raza H., Arafat K., Al-Salam S., Al Sultan M.A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharm. 2013;27(5):557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 97.Alhazmi M.I., Hasan T.N., Shafi G., Al-Assaf A.H., Alfawaz M.A., Alshatwi A.A. Roles of p53 and caspases in induction of apoptosis in MCF- 7 breast cancer cells treated with a methanolic extract of Nigella sativa seeds. Asian Pac J Cancer Prev. 2014;15(22):9655–9660. doi: 10.7314/apjcp.2014.15.22.9655. [DOI] [PubMed] [Google Scholar]
- 98.Ichwan S.J., Al-Ani I.M., Bilal H.G., Suriyah W.H., Taher M., Ikeda M.A. Apoptotic activities of thymoquinone, an active ingredient of black seed (Nigella sativa), in cervical cancer cell lines. Chin J Physiol. 2014;57(5):249–255. doi: 10.4077/CJP.2014.BAB190. [DOI] [PubMed] [Google Scholar]
- 99.El-Baba C., Mahadevan V., Fahlbusch F.B., SM S., Rau T.T., Gali-Muhtasib H. Thymoquinone-induced conformational changes of PAK1 interrupt prosurvival MEK-ERK signaling in colorectal cancer. Mol Cancer. 2014;13(1):201. doi: 10.1186/1476-4598-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salim L.Z., Othman R., Abdulla M.A., Al-Jashamy K., Ali H.M., Hassandarvish P. Thymoquinone inhibits murine leukemia WEHI-3 cells in vivo and in vitro. PLoS One. 2014;9(12):e115340. doi: 10.1371/journal.pone.0115340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shafi G., Munshi A., Hasan T.N., Alshatwi A.A., Jyothy A., Lei D.K. Induction of apoptosis in HeLa cells by chloroform fraction of seed extracts of Nigella sativa. Cancer Cell Int. 2009;9:29. doi: 10.1186/1475-2867-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumara S.S., Huat B.T. Extraction, isolation and characterisation of antitumor principle, alpha-hederin, from the seeds of Nigella sativa. Planta Med. 2001;67(1):29–32. doi: 10.1055/s-2001-10628. [DOI] [PubMed] [Google Scholar]
- 103.Villani P., Orsière T., Sari-Minodier I., Bouvenot G., Botta A. In vitro study of the antimutagenic activity of alphahederin. Ann Biol Clin Paris. 2001;59(3):285–289. [PubMed] [Google Scholar]
- 104.Rooney S., Ryan M.F. Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005;25(3B):2199–2204. [PubMed] [Google Scholar]
- 105.Rooney S., Ryan M.F. Modes of action of alpha-hederin and thymoquinone, active constituents of Nigella sativa, against HEp-2 cancer cells. Anticancer Res. 2005;25(6B):4255–4259. [PubMed] [Google Scholar]
- 106.Bun S.S., Elias R., Baghdikian B., Ciccolini J., Ollivier E., Balansard G. Alpha-hederin potentiates 5-FU antitumor activity in human colon adenocarcinoma cells. Phytother Res. 2008;22(10):1299–1302. doi: 10.1002/ptr.2483. [DOI] [PubMed] [Google Scholar]
- 107.Cheng L., Xia T.S., Wang Y.F., Zhou W., Liang X.Q., Xue J.Q. The anticancer effect and mechanism of α-hederin on breast cancer cells. Int J Oncol. 2014;45(2):757–763. doi: 10.3892/ijo.2014.2449. [DOI] [PubMed] [Google Scholar]
- 108.Worthen D.R., Ghosheh O.A., Crooks P.A. The in vitro antitumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18:1527–1532. [PubMed] [Google Scholar]
- 109.Kruk I., Michalska T., Lichszteld K., Kładna A., Aboul-Enein H.Y. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere. 2000;41(7):1059–1064. doi: 10.1016/s0045-6535(99)00454-3. [DOI] [PubMed] [Google Scholar]
- 110.Marsik P., Kokoska L., Landa P., Nepovim A., Soudek P., Vanek T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005;71(8):739–742. doi: 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- 111.Ivankovic S., Stojkovic R., Jukic M., Milos M., Milos M., Jurin M. The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp Oncol. 2006;28:220–224. [PubMed] [Google Scholar]
- 112.Tesarova H., Svobodova B., Kokoska L., Marsik P., Pribylova M., Landa P. Determination of oxygen radical absorbance capacity of black cumin (Nigella sativa) seed quinone compounds. Nat Prod Commun. 2011;6(2):213–216. [PubMed] [Google Scholar]
- 113.Archana P.R., Nageshwar Rao B., Satish Rao B.S. Modulation of gamma ray-induced genotoxic effect by thymol, a monoterpene phenol derivative of cymene. Integr Cancer Ther. 2011;10(4):374–383. doi: 10.1177/1534735410387421. [DOI] [PubMed] [Google Scholar]
- 114.Deb D.D., Parimala G., Saravana Devi S., Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact. 2011;193(1):97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Satooka H., Kubo I. Effects of thymol on B16-F10 melanoma cells. J Agric Food Chem. 2012;60(10):2746–2752. doi: 10.1021/jf204525b. [DOI] [PubMed] [Google Scholar]
- 116.Liang W.Z., Lu C.H. Carvacrol-induced [Ca2+]i rise and apoptosis in human glioblastoma cells. Life Sci. 2012;90(17–18):703–711. doi: 10.1016/j.lfs.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 117.Horvathova E., Navarova J., Galova E., Sevcovicova A., Chodakova L., Snahnicanova Z. Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. J Agric Food Chem. 2014;62(28):6632–6639. doi: 10.1021/jf501006y. [DOI] [PubMed] [Google Scholar]