Abstract
Background
Root extracts of Withania somnifera (Ashwagandha) are known to possess analgesic, anti-inflammatory and chondroprotective effects. An aqueous extract of roots plus leaves of this plant has shown to yield higher percentages of withanolide glycosides and, accordingly, may possess better analgesic, anti-inflammatory and chondroprotective effects than root alone extracts.
Objectives
To evaluate efficacy and tolerability of a standardized aqueous extract of roots plus leaves of W. somnifera in patients with knee joint pain and discomfort.
Material and methods
Sixty patients with knee joint pain and discomfort were randomized in a double-blind manner to W. somnifera 250 mg, W. somnifera 125 mg and placebo, all given twice daily. Assessment was done by Modified WOMAC, Knee Swelling Index (KSI), Visual Analogue Scale (VAS) at baseline and at the end of 4, 8, 12 weeks. Tolerability was assessed by incidence of adverse effects in treatment groups. Student's ‘t’ test and ANOVA were used to compare mean change from baseline within and between the study groups. A p < 0.05 was considered significant.
Results
At the end of 12 weeks, compared to baseline and placebo, significant reductions were observed in mean mWOMAC and KSI in W. somnifera 250 mg (p < 0.001), W. somnifera 125 mg (p < 0.05) groups. VAS scores for pain, stiffness and disability were significantly reduced in W. somnifera 250 mg (p < 0.001), W. somnifera 125 mg (p < 0.01) groups. W. somnifera 250 mg group showed earliest efficacy (at 4 weeks). All treatments were well tolerated.
Conclusions
Both the doses of an aqueous extract of W. somnifera produced significant reduction in outcome variables, with the 250 mg group showing significantly better response. In addition, the therapeutic response appears to be dose-dependent and free of any significant GI disturbances.
Keywords: Withania somnifera, Ashwagandha, Knee joint, WOMAC
Highlights
-
•
Aqueous extract of Withania somnifera roots plus leaves demonstrated dose – dependent therapeutic response.
-
•
Onset of therapeutic activity is seen as early as 4 weeks with the W. somnifera 250 mg group.
-
•
Aqueous extract of W. somnifera roots plus leaves has good safety profile and is well tolerated.
1. Introduction
1.1. Background
Knee joint pain and discomfort are the most prevalent of the chronic rheumatic symptoms and is a leading cause of disability in most countries worldwide [1].The prevalence of joint pain and discomfort due to osteoarthritis (OA) increases with age and more so with female gender, though males are also affected. OA contributes to a higher disease burden in men below the age of 50 and in women over the age of 50 [2]. Most of the disability arising due to OA is due to involvement of hip and knee joints [3]. Knee OA is likely to become the fourth most important global cause of disability in women and eighth most important in men [4].
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly used drugs for the symptomatic treatment of pain in OA. However, NSAIDs are associated with serious gastrointestinal adverse effects which limit their use in many patients [5], [6]. Other drugs like opioids and non-opioid analgesics and intra-articular steroids may not be effective in all patients [5], [6]. Hence, there is a specific need for effective and safe drugs in the treatment of OA.
Herbal medicines have been explored for their usefulness in OA for a long time. Withania somnifera (Ashwagandha), a plant belonging to the family Solanaceae, is widely used in Ayurvedic medicine for this purpose. It is an ingredient in many formulations prescribed for a variety of musculoskeletal conditions (e.g., arthritis, rheumatism), and as a general tonic to improve overall health [7]. Roots of the plant reportedly exhibit anti-inflammatory, anti-tumour, anti-stress, antioxidant, immunomodulatory, haematopoietic and rejuvenating properties [8]. There is evidence of effectiveness of W. somnifera in various rheumatologic conditions due to its anti-inflammatory properties [9]. In a randomized, double-blind, placebo-controlled, cross-over study in patients with OA, treatment with roots of W. somnifera produced a significant drop in severity of pain and disability score. It also acts as an analgesic that soothes nervous system from pain response [10]. Chemical composition of W. somnifera extracts vary widely depending on which part of the plant is used as well as the extraction solvent and procedure, and thus different extracts are expected to elicit different clinical response. Sensoril® is an aqueous extract of W. somnifera roots plus leaves and contains withanolide glycosides, Withaferin-A and oligosaccharides as the major components. There are very few human studies evaluating the effects of W. somnifera root extracts, in combination with other herbal products, in patients with symptoms of knee joint pain and disability and there are no human studies reported with an aqueous extract of roots plus leaves of W. somnifera.
2. Objectives
To evaluate the efficacy and tolerability of a standardized aqueous extract of roots plus leaves of W. somnifera using Modified WOMAC index score, pain relief as assessed by Visual Analogue Scale (VAS) and changes in Knee Swelling Index (KSI) in patients with pain and discomfort of knee joint.
3. Methods
3.1. Study design
The study was a prospective, randomized, double-blind, placebo-controlled trial with 1:1:1 allocation ratio of the participants in to the 3 study groups. The study was approved by the local Institutional Ethics Committee.
3.2. Study participants
3.2.1. Eligibility criteria
The patients were screened for their eligibility to participate in the study during the screening visit (Visit 1).
-
a.
Inclusion Criteria
Patients with knee joint pain and discomfort of either gender aged between 40 and 70 years, for at least 6 months duration and meeting the American Rheumatology Association (ARA) functional class I to III and who recorded baseline pain scores of at least 40 mm on the VAS monitored at baseline visit were enrolled. Patients who discontinued all current analgesic therapy, including NSAIDs, over the counter pain medications and topical analgesics for 7–10 days prior to the start of the study were randomized into the study.
-
b.
Exclusion Criteria
Patients with severe OA (ARA functional class IV), on alternative system of medicine, any psychiatric disorder or who have been using systemic/intra-articular steroids within 12 weeks of study and hyaluronic acid in the last 9 months, or potential candidates for imminent joint replacement and patients with uncontrolled hypertension or diabetes, hepatic or renal impairment, pregnant or lactating females, or with a recent trauma of the involved knee were excluded from the study.
3.3. Study interventions
The study medications included capsules of W. somnifera in the strengths of 125 mg and 250 mg and identical placebo capsules, supplied by Natreon, Inc, New Jersey, USA.
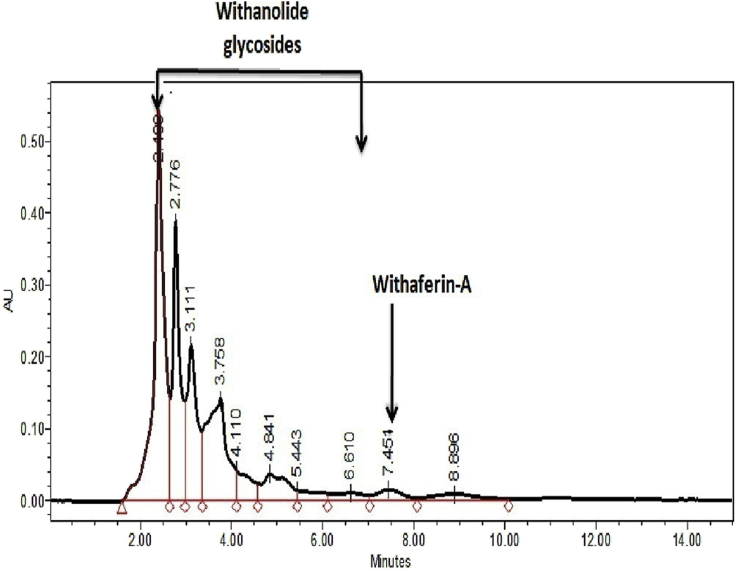
A W. somnifera capsule consists of standardized aqueous extract of roots and leaves of W. somnifera (Sensoril®) containing not less than 10% Withanolide glycosides, not less than 32% oligosaccharides and not more than 0.5% of Withaferin- A and is standardized by HPLC (Fig. 1). The excipients used in these capsules include microcrystalline cellulose, croscarmellose sodium, silicon dioxide, magnesium stearate and gelatin from the capsule shell.
Fig. 1.
HPLC Chromatogram of Sensoril®.
3.4. Study procedure
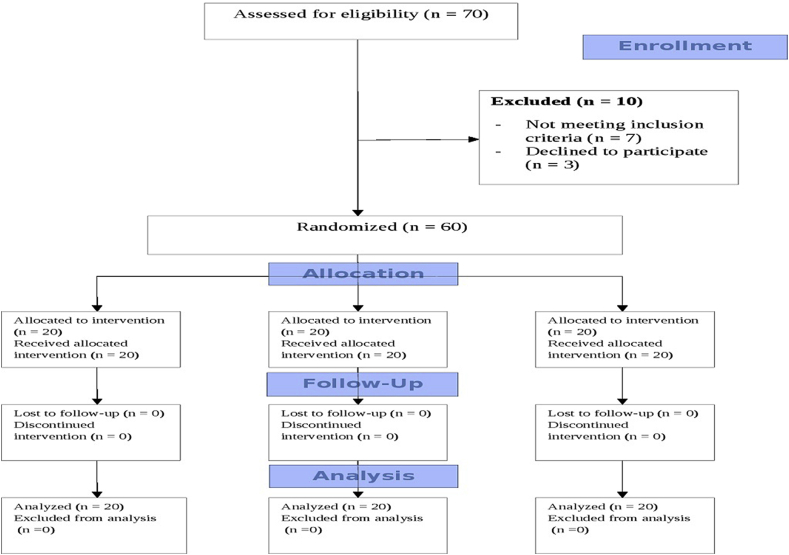
The study was conducted in the Department of Clinical Pharmacology and Therapeutics. The patients were randomized by the principal investigator using a computer generated simple randomization sequence with a block size of 20 patients per group. Case record numbers and sequentially numbered containers were used for random allocation sequence. The study was performed in a double-blinded manner, with both the study patients and the investigator blinded to study interventions. The participant flow chart is shown in Fig. 2.
Fig. 2.
Participant flow chart.
A run-in period was allowed between screening visit (Visit 1) and randomization visit (Visit 2) to ensure that the were weaned off all medications 7–10 days prior to randomization. At the baseline/randomization visit (Visit 2, Day 0), all eligible were randomized to receive either W. somnifera 250 mg or W. somnifera 125 mg or identical placebo capsules for 4 weeks, with one capsule of the study medication to be taken twice daily after food with a glass of water. Paracetamol 650 mg tablets were used as and when required as rescue medication. The subsequent visits were scheduled at 4 weeks interval (Visit 3-after 4 weeks of treatment initiation, Visit 4 -after 8 weeks of treatment initiation and Visit 5- after 12 weeks of treatment initiation). The study and rescue medications were dispensed at visits 2, 3 and 4 and compliance to study medications was checked by pill count method during the subsequent visits. The total duration for which the patients received study medications was 12 weeks.
All the efficacy variables, pill count, use of rescue medication and Physician's Global Assessment were evaluated in the subsequent visits. Adverse reactions/serious adverse effect (ADR/SAE) monitoring was done throughout the course of study. Safety lab were done before and after treatment and as and when required.
3.5. Outcomes
The primary outcome measure was percentage change in the Modified Western Ontario and McMaster University Osteoarthritis Index (mWOMAC, Ref. www.copcord.org/images/WOMAC.pdf) score at the end of 12 weeks from baseline.
The secondary outcome measures were percentage changes in mWOMAC score at the end of 4 and 8 weeks, Knee Swelling Index (KSI) as measured by joint circumference (mm) and VAS for pain, disability and stiffness at the end of 4, 8 and 12 weeks, extent of use of rescue medication in treatment groups, Physician Global Assessment scale and tolerability. The Physician Global Assessment was used to classify the patients based on symptoms. Accordingly, the 5 categories were Excellent (complete relief of symptoms), Good (partial relief of symptoms), Fair (minimal relief of symptoms), Poor (no relief of symptoms) and Very poor (worsening of symptoms). Tolerability was assessed by 3 categories, viz., Good (no side effects), Fair (mild to moderate side effects) and Poor (severe side effects and withdrawal of therapy).
3.6. Sample size
The sample size calculation was based on the assumption that there will be a decrease of 10% in total mWOMAC score from baseline to end of treatment. A sample size of 60 evaluable cases would provide an 80% power to estimate the reduction of total mWOMAC score at 5% level of significance at the end of the study. Anticipating 15% dropouts, we enrolled 70 to get 60 evaluable cases for the study.
3.7. Statistical analysis
Primary and secondary end points were analyzed as average change in the response from baseline. Student's ‘t’ test and ANOVA were used to compare the mean change from baseline to post-treatment period within and between study medications and placebo groups, respectively, at 80% power. A p < 0.05 was used to test the significance. All statistical analysis was performed using the GraphPad Prism Software 4 (GraphPad Software Inc., San Diego, California, USA).
4. Results
A total of seventy patients were recruited and screened over a period of 10 months (4/7/2014 to 16/5/2015), out of which 60 eligible patients (43 males, 17 females) with a mean age of 57.78 ± 4.49 years were enrolled into the study. Patients were divided into three groups of 20 each and were randomized to receive W. somnifera 250 mg, W. somnifera 125 mg or identical placebo capsules, one capsule twice daily for 12 weeks. All patients completed 12 weeks of treatment.
The demographic characteristics of all the three study groups are shown in Table 1. There were no significant differences between the treatment groups in baseline characteristics including age, weight and body mass index, indicating a homogenous population.
Table 1.
Demographic data.
| W. somnifera 250 mg (A) | W. somnifera 125 mg (B) | Placebo (C) | |
|---|---|---|---|
| No. of Subjects | 20 | 20 | 20 |
| Gender (M/F) | 13/7 | 14/6 | 16/4 |
| Age (Yrs) | 58.92 ± 6.07 | 55.42 ± 3.69 | 58.95 ± 3.73 |
| BMI(Kg/m2) | 23.79 ± 2.99 | 23.82 ± 1.86 | 24.15 ± 2.17 |
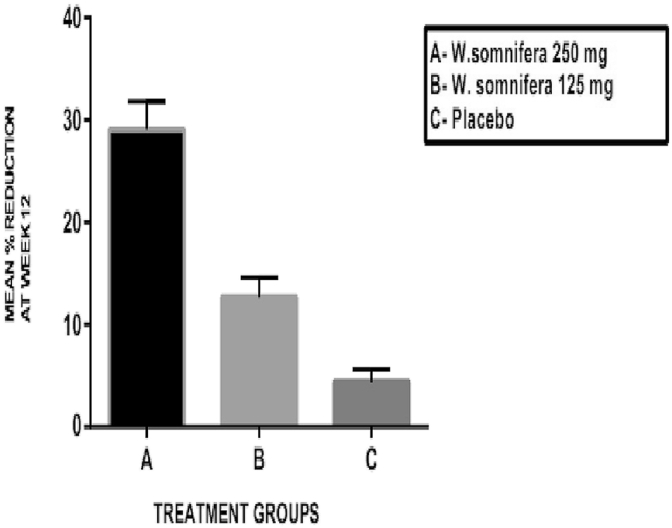
The mWOMAC scores at the end of 12 weeks are shown in Table 2(a) and the mean percentage reduction in mWOMAC scores are shown in Fig. 3. The baseline values of the mWOMAC score were comparable in all the three groups without any statistically significant difference between them. There was a significant reduction in the mWOMAC score at the end of 12 weeks from baseline in W. somnifera 250 mg group (A) (p < 0.001) and W. somnifera 125 mg group (B) (p < 0.05). The mean percentage reduction in the mWOMAC score at the end of 12 weeks showed significant differences between W. somnifera 250 mg (A) and W. somnifera 125 mg groups (B) (p < 0.001), W. somnifera 250 mg (A) and placebo groups (C) (p < 0.001) and W. somnifera 125 mg (B) and placebo groups (C) (p < 0.01).
Table 2a.
Summary of results − I.
| mWOMAC score |
Knee swelling index |
|||||
|---|---|---|---|---|---|---|
| W. somnifera 250 mg (A) | W. somnifera 125 mg (B) | Placebo (C) | W. somnifera 250 mg (A) | W. somnifera 125 mg (B) | Placebo (C) | |
| Baseline | 53.1 ± 2.90 | 51.15 ± 2.39 | 50.45 ± 2.11 | 423.25 ± 9.63 | 415.5 ± 20.76 | 408.5 ± 15.05 |
| End of 4 weeks | 48.6 ± 2.41# | 49.95 ± 2.11 | 49.97 ± 2.04 | 415.25 ± 9.66# | 413.75 ± 19.65 | 404.6 ± 15.30 |
| End of 8 weeks | 43.4 ± 2.47* | 47.4 ± 1.91 | 49.31 ± 2.11 | 404.75 ± 10.44* | 409.25 ± 19.61 | 401 ± 15.61 |
| End of 12 weeks | 37.65 ± 2.41* | 44.6 ± 1.42@ | 48.24 ± 2.31 | 396.25 ± 10.98* | 405.75 ± 19.21@ | 399.5 ± 15.55 |
| Absolute change at end of 12 weeks | 15.45 ± 1.73* | 6.5 ± 1.23# | 2.15 ± 0.75 | 27 ± 6.76* | 9.75 ± 3.43NS | 9 ± 2.62 |
| Mean percentage change at end of 12 weeks | 29.07 ± 2.73* | 12.7 ± 1.96# | 4.27 ± 1.46 | 6.37 ± 1.58* | 2.34 ± 0.78NS | 2.2 ± 0.65 |
@ p value < 0.05; #p value < 0.01; *p value < 0.001 compared to baseline; NS- Non-significant.
Absolute change in mWOMAC score: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
Mean percentage change in mWOMAC score:* A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
Absolute change in KSI: * A vs B, A vs C (p < 0.001),NS B vs C.
Mean percentage change in KSI: * A vs B, A vs C (p < 0.001), NS B vs C.
Fig. 3.
Mean percentage change in modified WOMAC score at the end of 12 weeks.
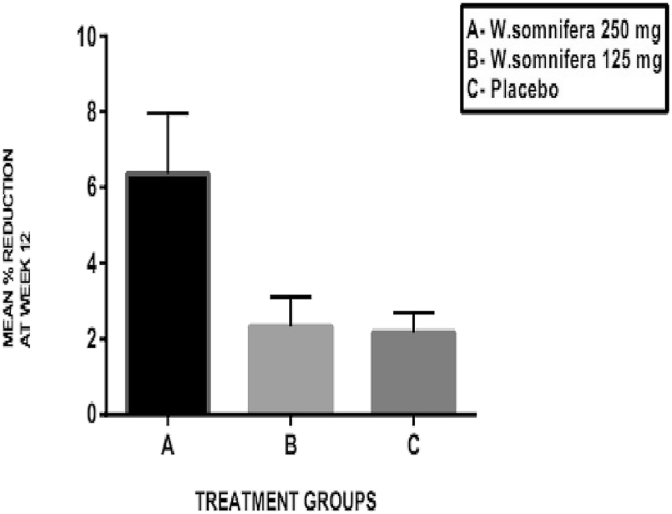
The KSI scores at the end of 12 weeks are shown in Table 2(a) and mean percentage change in KSI scores are shown in Fig. 4. The baseline values of the KSI scores were comparable in all the three groups without any statistically significant difference between them. There was a significant reduction in the KSI score at the end of 12 weeks from baseline in W. somnifera 250 mg group (A) (p < 0.001) and W. somnifera 125 mg group (B) (p < 0.05). The mean percentage reduction in the KSI scores in all the three groups at the end of 12 weeks showed a significant difference between W. somnifera 250 mg (A) and W. somnifera 125 mg (B) groups (p < 0.001) and W. somnifera 250 mg (A) and placebo (C) groups (p < 0.001).
Fig. 4.
Mean percentage change in knee swelling index score at the end of 12 weeks.
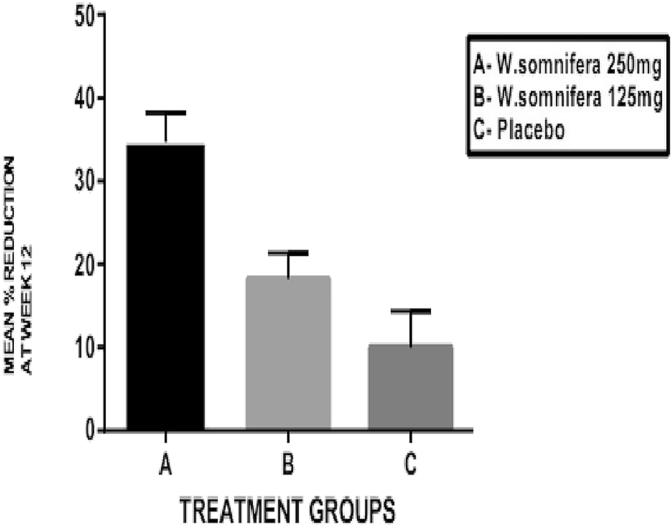
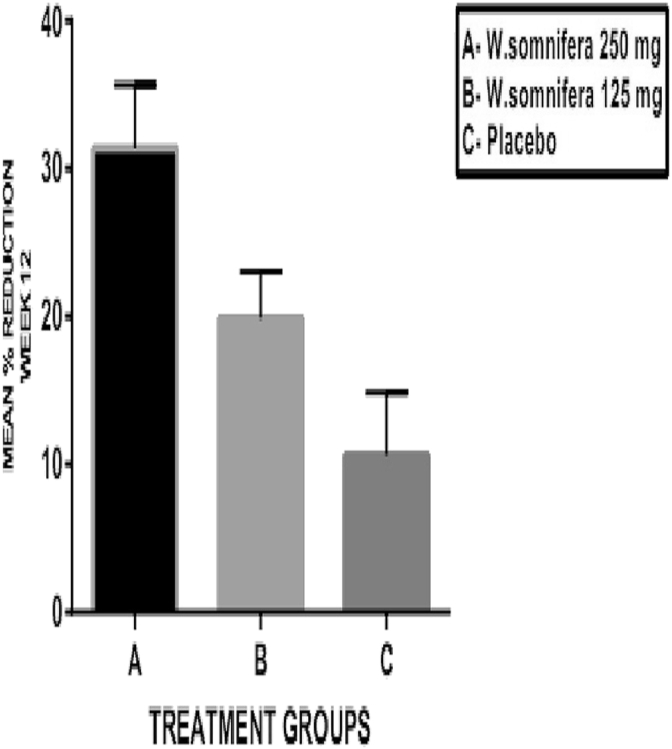
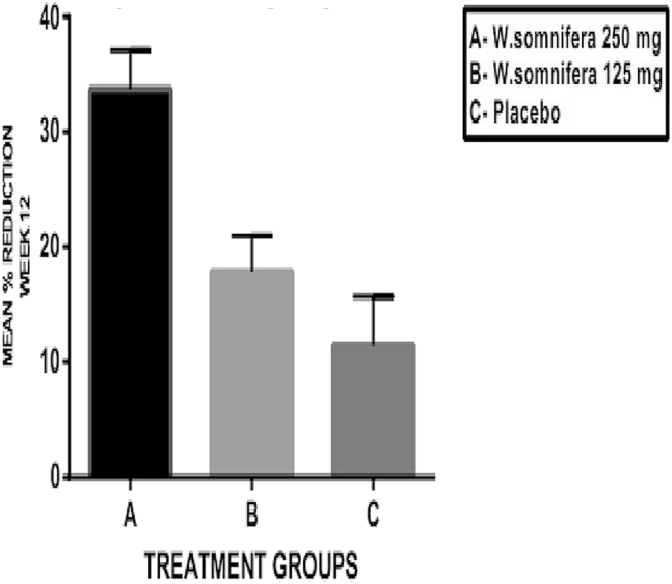
The VAS score for pain, stiffness and disability are shown in Table 2(b) and the mean percentage changes in these scores are shown in Fig. 5, Fig. 6, Fig. 7, respectively. The baseline values for pain, stiffness and disability measured by VAS were comparable in all the three groups without any statistically significant differences between them. In W. somnifera 250 mg group (A), there was a significant reduction in pain, stiffness and disability (p < 0.001) at the end of 12 weeks from baseline. In W. somnifera 125 mg group (B), there was a significant reduction in pain (p < 0.01), stiffness (p < 0.01) and disability (p < 0.05). The mean percentage reduction in the pain, stiffness and disability in all the three groups at the end of 12 weeks showed a significant difference between W. somnifera 250 mg (A) and W. somnifera 125 mg (B) groups (p < 0.001) and W. somnifera 250 mg (A) and placebo (C) groups (p < 0.001) and W. somnifera 125 mg (B) and placebo (C) groups (p < 0.01).
Table 2b.
Summary of results − II.
| VAS, pain |
VAS, stiffness |
VAS, disability |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| W. somnifera 250 mg (A) | W. somnifera 125 mg (B) | Placebo (C) | W. somnifera 250 mg (A) | W. somnifera 125 mg (B) | Placebo (C) | W. somnifera 250 mg (A) | W. somnifera 125 mg (B) | Placebo (C) | |
| Baseline | 67.25 ± 4.48 | 65.6 ± 4.56 | 63.8 ± 2.78 | 60.25 ± 4.11 | 62.55 ± 4.43 | 61.8 ± 2.71 | 50.45 ± 4.35 | 63.95 ± 4.71 | 58.6 ± 4.30 |
| End of 4 weeks | 60.95 ± 4.22# | 63.05 ± 4.67 | 60.5 ± 3.24 | 55.8 ± 3.83# | 59.45 ± 4.13 | 58.6 ± 2.85 | 46 ± 3.98# | 61 ± 5.03 | 55.35 ± 4.06 |
| End of 8 weeks | 52.9 ± 3.37* | 59.5 ± 4.07 | 58.65 ± 3.56 | 49.4 ± 3.28* | 56.3 ± 4.05 @ | 56.7 ± 2.96 | 40.35 ± 3.64* | 57.75 ± 5.03 | 53.5 ± 4.42 |
| End of 12 weeks | 43.9 ± 3.30* | 53.3 ± 4.02# | 57.4 ± 3.68 | 41.3 ± 3.54* | 50.1 ± 4.12# | 55.2 ± 2.98 | 33.55 ± 3.79* | 52.6 ± 5.37@ | 51.87 ± 4.48 |
| Absolute change at end of 12 weeks | 23.35 ± 3.04* | 12 ± 2.31# | 6.4 ± 1.57 | 18.95 ± 3.08* | 12.45 ± 2.08# | 6.6 ± 1.82 | 17.4 ± 3.08* | 11.35 ± 1.56# | 6.5 ± 1.79 |
| Mean percentage change at end of 12 weeks | 34.73 ± 3.49* | 18.29 ± 3.13# | 10.08 ± 2.71 | 31.4 ± 4.26* | 19.9 ± 3.15# | 10.67 ± 2.93 | 33.7 ± 3.37* | 17.74 ± 3.13# | 11.15 ± 3.18 |
@ p value < 0.05; #p value < 0.01; *p value < 0.001 compared to baseline.
Absolute change in VAS scores:
a) Pain: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
b) Stiffness: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
c) Disability: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
Mean percentage change in VAS scores:
a) Pain: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
b) Stiffness: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
c) Disability: * A vs B, A vs C (p < 0.001); #B vs C (p < 0.01).
Fig. 5.
Mean percentage change in VAS – pain score at the end of 12 weeks.
Fig. 6.
Mean percentage change in VAS – stiffness score at the end of 12 weeks.
Fig. 7.
Mean percentage change in VAS – disability score at the end of 12 weeks.
5. Rescue medication usage
The mean number of rescue medication tablets (Paracetamol 650 mg) was 10, 13 and 17 in W. somnifera 250 mg (A), W. somnifera 125 mg (B) and placebo (C) groups, respectively.
6. Physician Global Assessment
The Physician Global Assessment was done at the end of 12 weeks. In W. somnifera 250 mg group, 15 patients were assessed to be excellent and 5 were assessed as good by the Physician Global Assessment scale.
In W. somnifera 125 mg group, 17 patients were assessed to be good and 3 were assessed as fair and in the placebo group, 1 patient was assessed to be fair and 19 patients were assessed as poor.
7. Results at 4 and 8 weeks
At the end of 4 weeks, patients treated with W. somnifera 250 mg twice daily showed statistically significant reductions in the mWOMAC score (p < 0.01), KSI (p < 0.01), pain (p < 0.01), stiffness (p < 0.01) and disability (p < 0.01) as measured by VAS when compared to baseline. However, patients treated with W. somnifera 125 mg twice daily did not show any significant changes at the end of 4 weeks compared to baseline.
At the end of 8 weeks, patients treated with W. somnifera 250 mg showed statistically significant reductions in the mWOMAC score (p < 0.001), KSI (p < 0.001), pain (p < 0.001), stiffness (p < 0.001) and disability (p < 0.001) as measured by VAS, compared to baseline. W. somnifera 125 mg (B) showed significant change from baseline only in VAS stiffness at the end of 8 weeks (p < 0.05).
There were no significant changes in the efficacy variables in the placebo group at the end of 4 and 8 weeks. The results at the end of 4 and 8 weeks are summarized in Table 2a, Table 2b(a), 2(b).
8. Safety and tolerability
All safety hematological and biochemical variables were within normal limits in all the three treatment groups at the baseline recording. Both W. somnifera 250 mg and W. somnifera 125 mg were well tolerated. In W. somnifera 250 mg group, 4 patients complained of nausea and 1 patient developed mild gastritis. In W. somnifera 125 mg group, 2 patients complained of nausea and mild headache. None of the patients in placebo group had any adverse effects. No patients in any of the groups discontinued the study.
9. Discussion
W. somnifera has multiple bioactive components which contribute to it's biological activity as an analgesic, anti-inflammatory, anti-arthritic and chondroprotective [11]. The chemical analysis of W. somnifera shows its main constituents to be alkaloids and steroidal lactones. The biologically active alkaloids include withanine, somniferine, somnine, isopelletierine and anferine, and the steroidal lactones include withanolides and withaferins [12], [13]. The roots and leaves of W. somnifera have been shown to be rich in withanolides, which resemble steroids in their action and are considered to account for the biological activities of W. somnifera. Other components include saponins like sitoindoside VII and VIII and iron. Much of W. somnifera's pharmacological activity has been attributed to Withaferin A and Withanolide D [13]. However, chemical composition of W. somnifera extracts vary widely depending on which part of the plant is used as well as the extraction solvent and procedure, and thus are expected to elicit different clinical response. Sensoril® is an aqueous extract of W. somnifera roots plus leaves and contains withanolide glycosides, Withaferin-A and oligosaccharides as the major components.
In the present study, the mWOMAC score was reduced significantly at the end of 12 weeks from baseline in both W. somnifera 250 mg and W. somnifera 125 mg groups, with the reduction being greater in W. somnifera 250 mg group. This reduction in mWOMAC scores by W. somnifera may be attributed to its analgesic and chondroprotective effects. The analgesic activity of W. somnifera is mainly mediated by Withaferin A which has been shown to block the cyclo-oxygenase (COX) pathway involved in the production of prostaglandins, the endogenous pain mediators [14]. The analgesic activity is also due to its action of soothing the nervous system from pain responses [15]. Also it is suggested that serotonin may be involved in the analgesic effects of W. somnifera [16]. An experimental study has demonstrated the chondroprotective activity of W. somnifera in human cartilage tissue [17].
Another important biological effect of W. somnifera is its anti-inflammatory activity which can be determined by its effect on the KSI. The KSI scores were significantly reduced at the end of 12 weeks from baseline in both W. somnifera 250 mg and W. somnifera 125 mg groups. However, greater reduction was seen in W. somnifera 250 mg group. This anti-inflammatory effect of W. somnifera has also been attributed to Withaferin A [18]. The possible role of anti-oxidant activity of W. somnifera on its anti-inflammatory properties has also been proposed. An experimental study in rats has shown that the anti-inflammatory activity of W. somnifera may be due to the inhibition of biological changes like increase in levels of lipid peroxides and glycoproteins and decreased antioxidant status and bone collagen in the affected joint [19]. In the affected joint, reactive oxygen species are known to activate a number of intracellular signaling pathways such as NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells), which further activate the transcription of various pro-inflammatory cytokines (interleukins and TNFα), cell adhesion molecules and COX 2 [20]. Some of the constituents of W. somnifera like flavonoids and phenolic acid such as gallic acid, rutein, vanillic acid, quercetin and kaempferol block the distinct signal transduction events necessary for NF-kB activation and thus inhibit transcription factors such as NF-kB, activating protein-1 (AP-1) and nuclear factor-erythroid 2-related factor 2 (Nrf2) [21].
VAS scores for pain, stiffness and disability were used to assess the physical function of the knee joint. W. somnifera, in both 250 mg and 125 mg twice daily doses, significantly reduced the pain, stiffness and disability at the end of 12 weeks. However, the extent of reduction of these variables was higher with W. somnifera 250 mg twice daily dose. Also, the mean percentage reduction in all the three variables at the end of 12 weeks was significant with W. somnifera 250 mg, when compared to W. somnifera 125 mg and placebo groups.
The clinical assessment of patients with knee joint pain and discomfort was made by the Physician Global Assessment scale. Accordingly, patients in W. somnifera 250 mg group were assessed to have performed better in terms of clinical improvement compared to the other two groups.
Rescue medication in the form of Paracetamol 650 mg was allowed as and when required during the course of the study. We evaluated the usage of rescue medication as one of the outcomes measures of efficacy of W. somnifera treatment. The usage of rescue medication was the least in W. somnifera 250 mg group and the highest in the placebo group, indicating effectiveness of W. somnifera in reducing the symptoms of knee joint pain and discomfort.
The efficacy was also evaluated at 4 weeks and 8 weeks to determine the earliest onset of action. At the end of 4 and 8 weeks, patients treated with W. somnifera 250 mg twice daily showed statistically significant reductions in all efficacy variables, compared to baseline. However, patients treated with W. somnifera 125 mg twice daily did not show any significant changes at the end of 4 weeks compared to baseline and showed only significant change in VAS stiffness (p < 0.05) at the end of 8 weeks, compared to baseline. This suggests that treatment with W. somnifera 250 mg twice daily produces earlier and better symptomatic relief thus increasing patient compliance and satisfaction. In addition, the response with W. somnifera seems to be linearly dose-dependent.
The safety of W. somnifera was evaluated by monitoring the occurrence of any adverse effects. Both W. somnifera 250 mg and W. somnifera 125 mg were well tolerated and our study did not report any serious adverse effects with either of the doses of W. somnifera. This is consistent with the available literature on safety of W. somnifera [22]. Gastritis, nausea and headache that were reported were treated symptomatically with standard care of treatment.
10. Conclusions
Treatment with W. somnifera 250 mg and W. somnifera 125 mg, both taken twice daily in patients with knee joint pain and discomfort for a period of 12 weeks showed significant reduction in the outcome variables of efficacy and safety, when compared to baseline and placebo. On further analysis, W. somnifera 250 mg produced better reduction in outcomes when statistically compared to W. somnifera 125 mg. Also, this effect was seen earlier (in 4 weeks) in W. somnifera 250 mg group than in W. somnifera 125 mg group. The need for rescue medication (Paracetamol 650 mg tablets) was the least with W. somnifera 250 mg group suggesting its analgesic and anti-inflammatory effects. All the study medications were well tolerated and mild gastrointestinal adverse effects like nausea and gastritis were observed in few patients. None of the patients discontinued the study suggesting the favorable safety profile of W. somnifera. Further studies with W. somnifera, hence, are needed to confirm its therapeutic potential in patients with knee joint pain and discomfort and other painful rheumatologic conditions.
Registration of the trial
The trial was registered in the Clinical Trial Registry-India (CTRI) with the reference number REF/2014/08/007385.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.World Health Organization . WHO; Geneva: 2002. World health report 2002. Reducing risks, Promoting Healthy Life. [Google Scholar]
- 2.Saloni Tanna, Osteoarthritis: Opportunities to Address Pharmaceutical Gaps; 7 October 2004; Priority Medicines for Europe and the World; “A Public Health Approach to Innovation” – Background Paper available on http://archives.who.int/prioritymeds/report/background/osteoarthritis.doc (accessed on 31st August 2016).
- 3.Australian Orthopaedic Association . 2009. Hip and knee arthroplasty. Natl Jt Replace Regist Annu Rep 2009. [Google Scholar]
- 4.Jordan K.M., Arden N.K., Doherty M., Bannwarth B. EULAR Recommendations 2003: An evidence based approach to the Management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tramer M.R., Moore R.A., Reynolds D.J., McQuay H.J. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000;85:169–182. doi: 10.1016/s0304-3959(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan W. Implications of NSAID therapy in elderly in-subjects. J Rheumatol. 1990;4:29–32. [PubMed] [Google Scholar]
- 7.Chatterjee A., Pakrashi S.C. vol. 4. 1995. pp. 208–212. (The treatise on Indian medicinal plants). [Google Scholar]
- 8.Mishra L.C., Singh B.B., Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med. 2000;5:334–346. [PubMed] [Google Scholar]
- 9.Anbalagan K., Sadique J. Influence of an Indian medicine (Ashwagandha) on acute-phase reactants in inflammation. Indian J ExpBiol. 1981;19:245–249. [PubMed] [Google Scholar]
- 10.Kulkarni R.R., Patki P.S., Jog V.P., Gandage S.G., Patwardhan Bhushan. Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, cross-over study. J Ethnopharmacol. May–June 1991;33(1–2):91–95. doi: 10.1016/0378-8741(91)90167-c. [DOI] [PubMed] [Google Scholar]
- 11.Bhutani KK, Gupta DK, Kapil RS. A process for the isolation of bioactive peptide fraction from the plant W somnifera. 1994; Indian Patent No.1195/DEL/94 DT.23.9.94.
- 12.Davis L., Kuttan G. Suppressive effect of cyclophosphamide-induced toxicity by Withania somnifera extract in mice. J Ethno Pharmacol. 1998;62:209–214. doi: 10.1016/s0378-8741(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 13.Singh G., Sharma P.K., Dudhe R., Singh S. Biological activities of Withania somnifera. Ann Biol Res. 2010;1(3):56–63. [Google Scholar]
- 14.Sabina Evan Prince, Chandel Sonal, Rasool Mahaboob Khan. Evaluation of analgesic, antipyretic and ulcerogenic effect of Withaferin A. Int J Integr Biol. 2009;6(2):52–56. [Google Scholar]
- 15.Twaij H.A.A., Elisha E.E., Khalid R.M. Analgesic studies on some Iraqi medicinal plants. Int J Crude Drug Res. 1989;27:109–112. [Google Scholar]
- 16.Singh Narendra, Bhalla Mohit, de Jager Prashanti, Gilca Marilena. An overview on Ashwagandha: a Rasayana (Rejuvenator) of ayurveda. Afr J Tradit Complement Altern Med. 2011;8(S):208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumantran Venil N., Kulkarni Asavari, Boddul Sanjay, Chinchwade Trushna, Koppikar Soumya J., Harsulkar Abhay. Chondroprotective potential of root extracts of Withania somnifera in osteoarthritis. J Biosci. 2007;32(2):299–307. doi: 10.1007/s12038-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 18.Khare C.P. Springer (India) Pvt. Ltd.; New Delhi (: 2007. Indian medicinal plants–an illustrated dictionary. First Indian reprint; pp. 717–718. [Google Scholar]
- 19.Rasool M., Varalakshmi P. Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundam Clin Pharmacol. 2007 Apr;21(2):157–164. doi: 10.1111/j.1472-8206.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 20.Pavlick K.P., Laroux F.S., Fuseler J., Wolf R.E., Gray L., Hoffman J. Free Radic Biol Med. 2002;33:311–322. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 21.Serafini M., Peluso I., Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. Aug 2010;69:273–278. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 22.Umadevi M., Rajeswari R., SharmilaRahale C., Selvavenkadesh S., Pushpa R., Sampath Kumar K.P. Traditional and medicinal uses of Withania somnifera. Pharma Innov. 2012;1(9):106. [Google Scholar]