Abstract
Porcine circovirus type 2 (PCV2) is the primary causative agent of porcine circovirus disease, a complex multisystem syndrome in domestic pigs. Despite the significant economic losses caused by porcine circovirus disease, the mechanisms of pathogenesis underlying the clinical findings remain largely unclear. As various reports have highlighted the potential key role of vascular lesions in the pathogenesis of porcine circovirus disease, the aim of this work was to investigate effects of PCV2 infection on vascular endothelial cells, focusing on cell viability and expression of adhesion/junction molecules. PCV2 infection reduced endothelial cell viability, while viral infection did not affected the viability of several other classical cell lines. Also, PCV2 infection in endothelial cells displayed a dual/biphasic effect: initially, infection increased ICAM-1 expression, which can favor leukocyte recruitment and emigration to tissues and possibly inducing characteristic porcine circovirus disease inflammatory lesions; then, secondarily, infection caused an increase in zonula occludens 1 tight junction protein (ZO-1) expression, which in turn can result in difficulties for cell traffic across the endothelium and a potential impairment the immune response in peripheral tissues. These virus-induced endothelial changes could directly impact the inflammatory process of porcine circovirus disease and associated vascular/immune system disturbances. Data suggest that, among the wide range of effects induced by PCV2 on the host, endothelial modulation can be a pivotal process which can help to explain PCV2 pathogenesis in some porcine circovirus disease presentations.
Keywords: Swine, Infectious diseases, Pathogenesis, Viral infection, Cell adhesion
Introduction
Porcine circovirus type 2 (PCV2) is a small non-enveloped virus, with a single-stranded circular DNA genome1 and is the primary causative agent of a complex syndrome in domestic pigs called porcine circovirus disease (PCVD).2, 3 Several clinical presentation forms of PCVD have been reported worldwide, all exhibiting multisystem clinical manifestations, such as reduced weight gain in piglets as well as respiratory, enteric, renal, vascular and dermatological disorders.3, 4 The pathogenic mechanisms underlying PCV2 infection and the clinical findings for PCVD remain unclear.
In addition to the effects of PCV2 on the immune system, endothelial cell alterations and vascular system disturbances could be (at least partially) implicated in the pathogenesis of PCVD,5 particularly in cases of pneumonia, dermatitis and necrotizing lymphadenitis. This hypothesis is supported by several previous pathological findings6, 7, 8, 9 that proved the importance and high frequency of vasculitis cases in swine with PCVD. Recently, some seminal reports highlight the involvement of vascular lesions/alterations in the pathogenesis of some PCVD presentations.10, 11, 12
It is important to consider that even a non-cytopathic virus such as PCV2 can disrupt several organ systems and cause severe lesions as a result of slight alterations (caused by virus-induced cell changes) to the finely-balanced physiological processes. This becomes more important when the virus-altered endothelial cells and play an essential role in several key physiological processes (e.g. immune migration and cell nutrition).13, 14, 15
It has been previously demonstrated that PCV2-infected endothelial cells display an activated and prothrombotic phenotype, leading to vascular leakage, leukocyte migration, tissue inflammation and necrosis associated with PCVD clinical findings.5 These processes are finely regulated through several endothelial cell signaling systems and mediators such surface molecules, as adhesins and junction proteins, which in turn modulate cell-to-cell signaling, and consequently cell migration, fluid leakage and chemotaxys.14, 16, 17 In this sense, studies that address the phenomena induced by PCV2 infection of endothelial cells should improve the understanding of viral pathogenesis. Therefore, the aim of this study was to investigate the effects of PCV2 infection on endothelial cells, focusing on cell viability and the expression of adhesion/junction molecules.
Material and methods
Virus strain
The PCV2b strain used in this work was isolated in 2006 from kidneys of naturally infected piglets from Rio Grande do Sul state (Southern Brazil) before the introduction of PCV2 vaccines in Brazil.5 This strain was isolated from animals showing the classical triad of clinical findings indicative of PCVD18: clinical signs – wasting, reduced weight gain, diarrhea, dermatitis; characteristic lesions – lymph node atrophy with lymphoid depletion and histiocytic replacement of follicles in lymphoid tissues, dermatitis and vasculitis, and pale kidney with diffuse cortical white foci; PCV2 presence confirmed by immunohistochemistry and PCR. Additionally, the isolated viral inoculums were negative for other pathogens, such as pestivirus, swine parvovirus, influenza A virus, Torque-Teno virus, porcine reproductive and respiratory syndrome virus (PRRSV) and Mycoplasma spp.5
Molecular analysis
Total DNA was extracted from viral inoculum and cells using size-fractionated silica particles according to a previously described protocol.19 TaqMan™ qPCR was conducted to confirm and quantify PCV2 infection according to a protocol previously described,20 using the ABI Prism 7300 detection system and sequence detection software (Applied Biosystems, Forster City, CA, USA). The quantification was expressed as virus copies.
Cell cultures
Primary cell cultures of swine testicle (ST) cells were generated as previously described.21 Briefly, testicles from healthy piglets were aseptically removed and further processed in a laminar flow hood where the testicles were minced into small pieces, washed and digested with 0.25% trypsin. The resulting cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal calf serum (FCS), penicillin (200 U/mL) and streptomycin (200 mg/L), and maintained at 37 °C and 5% CO2.
EAhy926 cell line (derived from human endothelial cells) was used as a model to study the effects of PCV2 on endothelial cells (as previously reported).5 The cell lines PK-15 (porcine kidney cells), Vero (African green monkey kidney cells) and HEK293 (human embryonic kidney cells) were also used in this work. Cells were cultivated in DMEM containing 10% FCS, penicillin (200 U/mL) and streptomycin (200 mg/L) at 37 °C in a 5% CO2 atmosphere. Medium for EAhy926 cells was supplemented with 100 mM hypoxanthine, 0.4 mM aminopterin and 10 mM thymidine (HAT) as previously described.22 All cells used in this work were free of PCV1 contamination, as confirmed by routine molecular analysis.
PCV2 infection in cultured cells
Cells were seeded into 25-cm2 cell culture flasks and after a 24 h incubation period at 37 °C in 5% CO2 atmosphere, the culture medium was discarded and the cells (≈80% confluence) were inoculated with PCV2 (2 log10 virus copies, as determined by qPCR) or an equal volume of DMEM (control cultures). After 1 h, the supernatant was discarded and DMEM containing 3% FCS was added.
PCV2 infection and replication in cultured cells was confirmed and quantified by qPCR analysis. At 72 h after infection of EAhy926 cells, PCV2 viral load (DNA copies) was more than 15 times higher than initial viral load (1 h after infection) in all set of experiments used in this work, which confirmed PCV2 replication in endothelial cells. As a rule, for other cell lines and ST cells, PCV2 viral load also increase at least three times at 72 h after post-infection (compared to initial viral load, 1 h after infection) in all set of replicates used in this work.
Cells used for flow cytometry analysis were prepared as follows. After incubation time (36 or 72 h), medium was discarded, cells washed with sterile phosphate-buffered saline (PBS) solution and detached from the flask by trypsin (for 2 min at 37 °C). DMEM containing 10% FCS was added to cell flasks, the cell suspension centrifuged at 900 × g for 10 min, the supernatant was discarded and cell pellet resuspended in sterile PBS at 80 cells/μL. Immediately after this procedure, cells were analyzed by flow cytometry.
Cell viability assay
Cell viability was evaluated by flow cytometry using the Guava ViaCount Reagent (EMD Millipore Corporation, Billerica, MA, USA) and flow cytometry analysis of cell viability was performed using a Guava® EasyCyte™ Plus Flow Cytometer (Guava Technologies, Hayward, CA, USA). Results were expressed as the percentage of viable cells from the analyzed cells/events (minimum 20,000 events/technical replicate) and were the mean of twelve replicates.
Expression of cell adhesion and cell junction molecules
Immunophenotyping by flow cytometry was used to evaluate the expression of cell adhesion/junction molecules for EAhy926 endothelial cells using the following antibodies (antibody diluted according to the manufacturer instructions): phycoerythrin (PE)-conjugated monoclonal anti-CD54 (ICAM-1) antibody (source rat, class IgG2bκ, assay concentration 0.1 μg of antibody per 106 cells), PE-conjugated monoclonal anti-CD106 (VCAM-1) antibody (source rat, class IgG1κ, assay concentration 0.2 μg of antibody per 106 cells), PE-conjugated monoclonal anti-CD62P (P-selectin) antibody (source mouse, class IgG1κ, assay concentration 1 μg of antibody per 106 cells), fluorescein (FITC)-conjugated monoclonal anti-ZO-1 (tight junction protein zonula occludens 1) antibody (source mouse, class IgG1, assay concentration 2 μg of antibody per 106 cells), and FITC-conjugated monoclonal anti-occludin antibody (source mouse, class IgG1κ, assay concentration 2 μg of antibody per 106 cells). All antibodies were purchased from Thermo Fisher Scientific Inc. Antibodies were kept protected from light at 4 °C and were diluted some minutes before flow cytometry assays.
Cells were incubated with antibodies for 20 min at 4 °C in the dark and flow cytometry analysis of protein expression was performed using a Guava® EasyCyte™ Plus Flow Cytometer (Guava Technologies, Hayward, CA, USA). Results were expressed as percent positive cells of analyzed cells/events (minimum 30,000 events/technical replicate) or as mean cell fluorescence intensity comparing the control with the infected cells (from nine replicates).
Data analysis
Data from flow cytometry was analyzed with the CytoSoft™ software version 5.0 (Guava Technologies, Hayward, CA, USA) and results expressed as mean ± SEM of n replicates. Statistical significance of results was analyzed using the Students t-test for unpaired samples or one-way analysis of variance (ANOVA) with a Tukey post hoc test. The statistical analyses were conducted using GraphPad Prism 3.0 software (GraphPad Software Inc., San Diego, CA, USA) and p ≤ 0.05 was considered statistically different.
Results
Cell viability assay
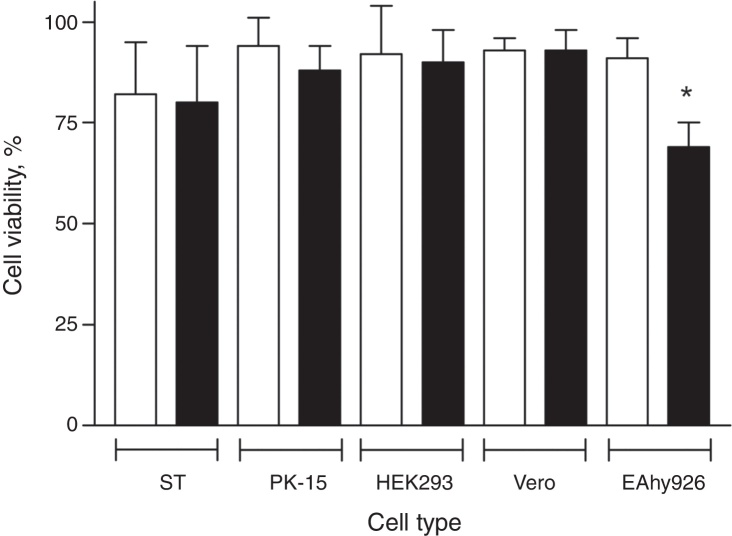
Preliminary experiments suggested PCV2 infection resulted in a slight reduction in the viability of EAhy926 cells, although no cytopathic effect (CPE) could be observed. Though it was uncommon for other cell lines to be infected with PCV2, we infected other classical cell lines and measured their cell viability in comparison with EAhy926 cells. EAhy926, PK-15, Vero, HEK293 and primary swine testicle (ST) cell cultures were inoculated with PCV2 and 36 and 72 h post-infection PCR analysis confirmed that PCV2 infected all cell types (data not shown). Cell viability was measured by flow cytometry at 72 h post-infection. Viral infection did not alter viability of PK-15, Vero, HEK293 and ST cells, while PCV2 infection reduced the viability of EAhy926 endothelial cells by ∼25% compared to uninfected EAhy926 cells (control) (p = 0.012). Results are summarized in Fig. 1.
Fig. 1.
Viability of PCV2-infected cells assessed by flow cytometry. EAhy926, PK-15, Vero and HEK293 cell lines and primary cell culture of swine kidney (ST) were infected with 2 log10 virus copies and cell viability assessed 72 h post-infection. Results are expressed as mean ± SEM of twelve biological replicates of uninfected control cells (open bars) and PCV2-infected cells (black bars). Statistical analysis was performed using Student's t-test for unpaired samples, comparing infected and non-infected cells (*p < 0.05).
Expression of cell adhesion and cell junction molecules
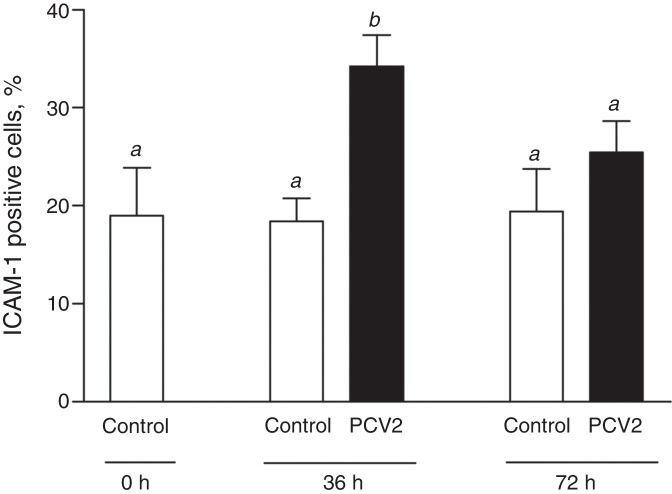
Expression of endothelial cell adhesion molecules (ICAM-1, VCAM-1 and P-selectin) was evaluated in EAhy926 endothelial cells after PCV2 infection. Viral infection did not alter VCAM-1 (CD106) or P-selectin (CD62P) expression on endothelial cells either at 36 and 72 h post-infection (p.i.) (data not shown). Number of endothelial cells expressing ICAM-1 (CD54) was significantly increased by ∼80% at 36 h p.i. in PCV2-infected EAhy926 cells (p < 0.01) (Fig. 2). At 72 h p.i., ICAM-1 positive cells were slightly increased though the difference was not statistically different from uninfected cells (control) (Fig. 2).
Fig. 2.
ICAM-1 expression in EAhy926 endothelial cells. ICAM-1 (CD54) expression was assessed by flow cytometry after infection of EAhy926 cells with 2 log10 virus copies. Results are expressed as mean ± SEM of nine biological replicates. Statistical analysis was performed using the one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Different letters indicate statistically significant differences (p < 0.05).
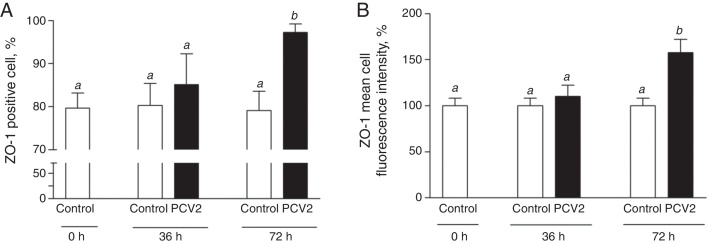
Expression of cell junction molecules was also investigated in PCV2-infected EAhy926 endothelial cells. At 72 h p.i., PCV2 infected endothelial cells showed a ∼57% increase in ZO-1 expression (mean cell fluorescence intensity) and a ∼23% increase in the number of cells expressing ZO-1 (Fig. 3A and B). There were no changes in occludin expression in PCV2-infected endothelial cells at either 36 or 72 h p.i. (data not shown).
Fig. 3.
ZO-1 expression in EAhy926 endothelial cells. ZO-1 expression was assessed by flow cytometry after EAhy926 cells infection with 2 log10 virus copies. Panel A, number of cells expressing ZO-1. Panel B, expression of ZO-1 molecules/cell by mean cell fluorescence intensity. Results are expressed as mean ± SEM of nine biological replicates. Statistical analysis was performed using the one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Different letters indicate statistically significant differences (p < 0.05).
Discussion and conclusions
Endothelial cells, as wells as the vascular system, play a key-role in several viral infections.13, 15 A recent comprehensive review focusing on porcine circovirus 2 (PCV2)-associated lesions indicates that vascular changes are found (to varying degrees) in nearly all organ systems, and concludes that infection of endothelial cell by PCV2 and the associated vascular changes appear to be very significant for the pathogenesis of porcine circovirus disease (PCVD).12 This observation was consistent with the study by Szeredi and co-workers11 which indicates that detection of PCV2 positively correlates with the presence of the vascular lesions in swine lungs. Also noteworthy are the increasing reports of acute pulmonary edema (APE), a novel PCVD presentation in North America, which shows fibrinoid necrosis of the blood vessel wall.23
Results of this study confirmed that PCV2 did not induce cell death in classical cell lineages used for virological studies, which was consistent with previous studies where PCV2 infection usually did not interfere with cell viability.24, 25 However, PCV2 infection significantly reduced cell viability of the endothelial EAhy926 cell line. Although it was an in vitro result, it is reasonable to hypothesize that it could occur during natural infection in swine, resulting in a considerable impact on PCV2 pathogenesis and leading to findings previously associated with PCVD, such as activation of endothelium, degeneration of endothelial cells, perivascular and intramural edema, necrosis of endothelium, necrotizing lymphohistiocytic and plasmacytic vascular lesions, vasculitis, perivasculitis, arteritis, widespread petechiae, thrombosis, massive fibrin deposition, microvascular thrombosis in kidneys and mesentery, obliterated blood vessels, alveolar hemorrhage, hypertrophy and hyperplasia of venules, and endothelial hypertrophy.5, 12 Since endothelial integrity is necessary for proper functioning of all organs and body systems, disruption of the endothelium could be linked to several typical multisystemic PCVD lesions, suggesting a key role for endothelial cells in the pathogenesis of PCV2.
A vital role of the endothelium in the regulation of leukocyte infiltration into tissues depends on the expression of adhesion and junction proteins. Two major types of proteins regulate cell migration process across endothelium, adhesins (which participate in leukocyte attachment, rolling, arrest, spread and finally cell emigration) and junction proteins (which regulate the intercellular space traffic). The primary adhesins involved in leukocyte extravasation are part of the immunoglobulin superfamily (ICAM and VCAM) and the selectin family, while junction proteins include tight junction protein zonula occludens 1 (ZO-1) and occludin. As a general rule, an increase in adhesin expression and a reduction in junction protein expression favor cell extravasation, while the opposite impairs extravasation.26, 27, 28 As leukocyte migration into tissues is a key process involved in viral infection, it can influence viral pathogenesis and the clinical outcome.29 Indeed, modulation of adhesion/junction molecules expression and leukocyte migration during virus infection is demonstrated for several other viral infections.30, 31
This study showed that 36 h after PCV2 infection, there were an increased number of endothelial cells expressing ICAM-1, one of the major molecules involved in leukocyte migration and tissue inflammation. Apparently, this result is paradoxical to the increased ZO-1 expression at 72 h post-infection. In light of these data, PCV2 infection of endothelial cells was hypothesized to have a dual/biphasic effect. Initially, leukocyte recruitment/extravasation to tissues was favored and induced the characteristic PCVD inflammatory lesions followed by a phase where viral infection caused an increase in the number of cell-cell junctions, negatively affecting leukocyte emigration and the development of an adequate immune response in peripheral tissues. It is important to note that this does not mean that these phenomena occurred sequentially during PCV2 infection, rather as dynamic processes which occurred concurrently in different swine tissues, since PCV2 infection of endothelial cells was presumed to be an ongoing process in infected swine.
This hypothesis of cell adhesion and leukocyte migration modulation during PCV2 infection is supported by a microarray analysis study which demonstrates that the main genes regulated by infection are those involved in cell adhesion and migration.32 Additionally, positive/negative modulation of endothelial adhesion molecules and leukocyte migration is not an uncommon finding during viral infections, and has been demonstrated to occur during other viral infections such as measles,30 West Nile virus,31, 33 Dengue,34 Japanese encephalitis virus,35 human immunodeficiency virus,36 equine herpesvirus137 and classical swine fever virus.38 Though this dual/biphasic effect hypothesis needs further in vivo evidence from swine with PCVD, it may help to explain, when associated with lymphoid depletion, the complex effects of PCV2 on the swine immune system.
These data, taken in conjunction with previously published work, demonstrated significant endothelial changes during the course of PCV2 infection seen as endothelial cell activation and thrombin formation,5 decreased cell viability and the expression of adhesion/junction molecules. These endothelial changes resulting from viral infection may directly impact the inflammatory process of PCVD and disturbances of the vascular and immune systems. It is reasonable to conclude that among the wide range of effects induced by PCV2, endothelial modulation can be a pivotal process which could explain (at least partially) PCV2 pathogenesis in some PCVD presentations.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Estadual de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support of this work. Special thanks to I. Vaz (UFRGS) for help in flow cytometry analysis and to Simbios Biotecnologia (Canoas, RS) for the use of real-time PCR facility.
Associate Editor: João Pessoa Araújo Junior
References
- 1.Tischer I., Gelderblom H., Vettermann W., Koch M.A. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- 2.Segalés J., Rosell C., Domingo M. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet Microbiol. 2004;98:137–149. doi: 10.1016/j.vetmic.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Opriessnig T., Meng X.J., Halbur P.G. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19:591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 4.Segalés J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. 2012;164(1–2):10–19. doi: 10.1016/j.virusres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Marks F.S., Reck J., Jr., Almeida L.L. Porcine circovirus 2 (PCV2) induces a procoagulant state in naturally infected swine and in cultured endothelial cells. Vet Microbiol. 2010;141:22–30. doi: 10.1016/j.vetmic.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Quezada M., Ramírez E., Muñoz D., Ruiz A. Porcine dermatitis and nephropathy syndrome: one of the principal cause of glomerulonephritis in pigs. Agro-Ciencia. 2004;20:119–130. [Google Scholar]
- 7.Corrêa A.M., Zlotowski P., Barcellos D.E.S.N., Cruz C.E., Driemeier D. Brain lesions in pigs affected with postweaning multisystemic wasting syndrome. J Vet Diagn Invest. 2007;19:109–112. doi: 10.1177/104063870701900120. [DOI] [PubMed] [Google Scholar]
- 8.Seeliger F.A., Brügmann M.L., Krüger L. Porcine circovirus type 2-associated cerebellar vasculitis in postweaning multisystemic wasting syndrome (PMWS)-affected pigs. Vet Pathol. 2007;44:621–634. doi: 10.1354/vp.44-5-621. [DOI] [PubMed] [Google Scholar]
- 9.Szeredi L., Szentirmai C. Proliferative and necrotising pneumonia and severe vascular lesions in pigs naturally infected with porcine circovirus type 2. Acta Vet Hung. 2008;56:101–109. doi: 10.1556/AVet.56.2008.1.10. [DOI] [PubMed] [Google Scholar]
- 10.Galindo-Cardiel I., Grau-Roma L., Pérez-Maíllo M., Segalés J. Characterization of necrotizing lymphadenitis associated with porcine circovirus type 2 infection. J Comp Pathol. 2011;144(1):63–69. doi: 10.1016/j.jcpa.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Szeredi L., Dán A., Solymosi N., Cságola A., Tuboly T. Association of porcine circovirus type 2 with vascular lesions in porcine pneumonia. Vet Pathol. 2012;49(2):264–270. doi: 10.1177/0300985811406888. [DOI] [PubMed] [Google Scholar]
- 12.Opriessnig T., Langohr I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet Pathol. 2013;50(1):23–38. doi: 10.1177/0300985812450726. [DOI] [PubMed] [Google Scholar]
- 13.Hajjar D.P., Nicholson A.C. Viral activation of coagulation: implications for thrombosis and atherosclerosis. Ann N Y Acad Sci. 1997;15:155–165. doi: 10.1111/j.1749-6632.1997.tb51998.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons G.H. The vascular response to injury. In: Loscalzo J., Schafer A.I., editors. Thrombosis and Hemorrhage. 2nd ed. Williams and Wilkins; Baltimore: 1998. pp. 307–320. [Google Scholar]
- 15.González X., Notario M. Alteraciones de la hemostasia en las enfermedades virales. Rev Cubana Hematol Immunol Hemoter. 1999;15:21–24. [Google Scholar]
- 16.Ebnet K., Suzuki A., Ohno S., Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117(Pt 1):19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 17.Reglero-Real N., Marcos-Ramiro B., Millán J. Endothelial membrane reorganization during leukocyte extravasation. Cell Mol Life Sci. 2012;69(18):3079–3099. doi: 10.1007/s00018-012-0987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorden S.D. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS) Swine Health Prod. 2000;8:133–136. [Google Scholar]
- 19.Boom R., Sol C.J., Salimans M.M., Jansen C.L., Wertheim-van Dillen P.M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olvera A., Sibila M., Calsamiglia M., Segalés J., Domingo M. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in post weaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J Virol Methods. 2004;117:75–80. doi: 10.1016/j.jviromet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Freshney R.I. Wiley-Liss; New York: 2000. Culture of Animal Cells: A Manual of Basic Techniques. [Google Scholar]
- 22.Edgell C.J., McDonald C.C., Graham J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cino-Ozuna A.G., Henry S., Hesse R. Characterization of a new disease syndrome associated with porcine circovirus type 2 in previously vaccinated herds. J Clin Microbiol. 2011;49(5):2012–2016. doi: 10.1128/JCM.02543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent I.E., Carrasco C.P., Herrmann B. Dendritic cells harbor infectious porcine circovirus type 2 in the absence of apparent cell modulation or replication of the virus. J Virol. 2003;77(24):13288–13300. doi: 10.1128/JVI.77.24.13288-13300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang H.W., Jeng C.R., Liu J.J. Reduction of porcine reproductive and respiratory syndrome virus (PRRSV) infection in swine alveolar macrophages by porcine circovirus 2 (PCV2)-induced interferon-alpha. Vet Microbiol. 2005;108(3–4):167–177. doi: 10.1016/j.vetmic.2005.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolton S.J., Anthony D.C., Perry V.H. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood–brain barrier break down in vivo. Neuroscience. 1998;86(4):1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 27.McEver R.P. Interactions of leukocytes with the vessel wall. In: Loscalzo J., Schafer A.I., editors. Thrombosis and Hemorrhage. 2nd ed. Williams and Wilkins; Baltimore: 1998. pp. 321–336. [Google Scholar]
- 28.Luscinskas F.W., Ma S., Nusrat A., Parkos C.A., Shaw S.K. Leukocyte transendothelial migration: a junctional affair. Semin Immunol. 2002;14(2):105–113. doi: 10.1006/smim.2001.0347. [DOI] [PubMed] [Google Scholar]
- 29.Friedl P., Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 30.Dittmar S., Harms H., Runkler N., Maisner A., Kim K.S., Schneider-Schaulies J. Measles virus-induced block of transendothelial migration of T lymphocytes and infection-mediated virus spread across endothelial cell barriers. J Virol. 2008;82(22):11273–11282. doi: 10.1128/JVI.00775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z., Waeckerlin R., Urbanowski M.D., van Marle G., Hobman T.C. West Nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins. PLoS ONE. 2012;7(5):e37886. doi: 10.1371/journal.pone.0037886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomás A., Fernandes L.T., Sánchez A., Segalés J. Time course differential gene expression in response to porcine circovirus type 2 subclinical infection. Vet Res. 2010;41(1):12. doi: 10.1051/vetres/2009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma S., Lo Y., Chapagain M. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood–brain barrier. Virology. 2009;385:425–433. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh T.M., Liu S.H., Lin K.C. Dengue virus enhances thrombomodulin and ICAM-1 expression through the macrophage migration inhibitory factor induction of the MAPK and PI3K signaling pathways. PLOS ONE. 2013;8(1):e55018. doi: 10.1371/journal.pone.0055018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai C.Y., Ou Y.C., Chang C.Y. Endothelial Japanese encephalitis virus infection enhances migration and adhesion of leukocytes to brain microvascular endothelia via MEK-dependent expression of ICAM1 and the CINC and RANTES chemokines. J Neurochem. 2012;123(2):250–261. doi: 10.1111/j.1471-4159.2012.07889.x. [DOI] [PubMed] [Google Scholar]
- 36.Duan M., Yao H., Hu G., Chen X., Lund A.K., Buch S. HIV Tat induces expression of ICAM-1 in HUVECs: implications for miR-221/-222 in HIV-associated cardiomyopathy. PLOS ONE. 2013;8(3):e60170. doi: 10.1371/journal.pone.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith D.J., Hamblin A.S., Edington N. Infection of endothelial cells with equine herpesvirus-1 (EHV-1) occurs where there is activation of putative adhesion molecules: a mechanism for transfer of virus. Equine Vet J. 2001;33(2):138–142. doi: 10.1111/j.2042-3306.2001.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 38.Li S., Qu H., Hao J. Proteomic analysis of primary porcine endothelial cells after infection by classical swine fever virus. Biochim Biophys Acta. 2010;1804(9):1882–1888. doi: 10.1016/j.bbapap.2010.05.011. [DOI] [PubMed] [Google Scholar]