Abstract
Twelve bacterial strains isolated from shrimp farming ponds were screened for their growth activity on chitin as the sole carbon source. The highly chitinolytic bacterial strain was detected by qualitative cup plate assay and tentatively identified to be Cohnella sp. A01 based on 16S rDNA sequencing and by matching the key morphological, physiological, and biochemical characteristics. The cultivation of Cohnella sp. A01 in the suitable liquid medium resulted in the production of high levels of enzyme. The colloidal chitin, peptone, and K2HPO4 represented the best carbon, nitrogen, and phosphorus sources, respectively. Enzyme production by Cohnella sp. A01 was optimized by the Taguchi method. Our results demonstrated that inoculation amount and temperature of incubation were the most significant factors influencing chitinase production. From the tested values, the best pH/temperature was obtained at pH 5 and 70 °C, with Km and Vmax values of chitinase to be 5.6 mg/mL and 0.87 μmol/min, respectively. Ag+, Co2+, iodoacetamide, and iodoacetic acid inhibited the enzyme activity, whereas Mn2+, Cu2+, Tweens (20 and 80), Triton X-100, and EDTA increased the same. In addition, the study of the morphological alteration of chitin treated by enzyme by SEM revealed cracks and pores on the chitin surface, indicating a potential application of this enzyme in several industries.
Keywords: Biochemical characterization, Chitinase, Cohnella sp. A01, Isolation, Thermostable
Introduction
Chitin, a β-1,4 polymer of N-acetyl-d-glucosamine (GlcNAc), which is widely distributed among fungi, crustaceans, molluscs, coelenterates, protozoan, and green algae, is the second-most abundant biopolymer found in nature after cellulose.1, 2 Several million tons of chitin is synthesized and degraded each year in the biosphere.3 This natural resource is relatively easily accessible, e.g., from sources such as shrimp, crab, and krill, which are considered as waste; chitin accounts for 20–58% of the dry weight of these wastes.4 Chitinous wastes are also produced in large amounts in industries such as seafood processing industry, which produces prawn waste (containing 23% chitin).5 These wastes may pose as an environmental threat on their accumulation and due to extremely slow decomposition.6 Therefore, organisms that produce chitin-degrading enzymes can be useful in bioremediation and waste management as well as help release nutrients and maintain the carbon, nitrogen, and other biogeochemical cycles in the environment.5, 7, 8
Chitinases (EC 3.2.1.14) are present in a wide range of organisms, including viruses, bacteria, fungi, insects, higher plants, and animals; these enzymes are capable of catalyzing the hydrolysis of chitin.9 Chitinase participates in a variety of functions, including defense, nutrient digestion, morphogenesis, and pathogenesis.3 Most chitin-degrading prokaryotes are the gliding bacteria, pseudomonad, vibrio, enterobacteria, actinomycete, bacilli, and clostridia.10 Bacterial chitinases have a size range of 20–60 kDa.11, 12 Chitinases have potential applications in various areas of biotechnology, biomedicine, agriculture, and nutrition.13, 14
Microorganisms adapt to the condition in which they have to live and survive. Thermophiles synthesize proteins that are thermostable and resist denaturation and proteolysis. Due to their ecological role and growing interests of their application in biotechnology, a large number of chitin-degrading bacteria have been isolated and their respective genes have been cloned and characterized. However, only few thermostable chitinases have been reported in microorganisms.15, 16 The thermostable chitinolytic enzymes can hydrolyze their substrates at high temperatures and represent important advantages against their mesophilic counterparts, for example, chemical and thermal stability, decreased viscosity, increased solubility, and significantly reduced contamination risk.16 Therefore, researches have been focused on microorganisms capable of producing such enzymes that can tolerate extreme environmental conditions.
Several articles have been published on the classical method of medium optimization by changing one independent parameter while fixing the others fixed.9 This process can be extremely time consuming, expensive, and unmanageable when involving a large number of variables as well as it cannot describe the combined effect of all the factors involved. Several factors have been reported to influence enzyme production by bacteria.17 Optimizing all of these affecting factors by statistical experimental designs can address these limitations. The methods of Taguchi have been used extensively in experiment designing.18 However, the application of Taguchi method in biological science is scarce.17
The genus Paenibacillus was originally defined in 1993 by Ash et al. after an extensive comparative analysis of 16S rRNA gene sequences of approximately 50 species of the genus Bacillus.19, 20, 21 They were reported to possess inhibitory effect on bacteria or fungi owing to their cell wall-degrading enzymes.22 Cohnella is a member of the Paenibacillaceae family.23 Earlier, we had reported the Taguchi method of chitinase production optimization from Serratia marcescens B4A9 and polygalacturonase production from Macrophomina phaseolina.24 In this study, we attempted to isolate and characterize the thermostable chitinase from the novel thermophilic strain, Cohnella sp. A01. Moreover, with the objective of obtaining accurate data and economizing the use of time and materials, we decided to use the Taguchi method for the optimization of culture medium instead of using traditional method. Only limited studies have reported statistical optimization for the production of chitinase.25 The present report is an attempt to formulate a suitable production medium by using statistical optimization that can substantially enhance chitinase production by Cohnella sp. A01.
Materials and methods
Materials
Flake crab shell chitin, 3,5-dinitrosalicylic acid (DNS), N-acetyl-d-glucosamine, and bovine serum albumin (BSA) were obtained from Sigma (St. Louis, Mo. USA). Colloidal chitin was prepared by the modified method of Roberts and Selitrennikoff.26 Taq DNA polymerase, 1-kb DNA ladder, standard proteins for molecular weight determination, T4 DNA ligase, IPTG, and X-Gal were purchased from Fermentas (Burlington, Canada). DNA extraction kit was purchased from Metabion (Martinsried, Germany). The High-Pure PCR Purification Kit was sourced from Roche (Indianapolis, USA). All other chemicals were purchased from Merck (Darmstadt, Germany) and were of the highest analytical grade available.
Isolation of microorganisms
Samples collected from shrimp farming wastewater located in Choebdeh-Abadan (southwestern of Iran) and used for isolation studies in our laboratory. The climate in Abadan is arid. Summers are dry and hot with temperatures of >45 °C (average 55 °C); the soaring temperatures may advance to >65 °C.
Screening of thermophilic chitinase-producing microorganism
For the direct screening of chitinase activity from bacterial colonies, clear zone production was monitored at 60 °C.27 The isolated microorganisms were cultured on agar plates containing 0.5% colloidal chitin, 0.07% K2HPO4, 0.03% KH2PO4, 0.05% MgSO4·7H2O, 2% agar, 0.2% NH4NO3, 0.1% NaCl (w/v), and 0.1% trace elements (pH 7.8). The cultures were incubated for 3 days at 60 °C. Only one chitinolytic bacterial strain (detected by a colony producing a halo around itself) was transferred into fresh chitin containing nutrient broth medium and incubated at 60 °C, following which, the strain was preserved as cell suspensions in 10% glycerol at −80 °C.
Culture and growth conditions
For chitinase production, the strain selected from the primary screening was cultured in a preculture medium (trace element 0.1%, tryptone 1%, yeast extract 0.5%, NaCl 0.5%, agar 0.2%, peptone 0.03%, K2HPO4 0.07%, KH2PO4 0.03%, CaCl2·2H2O 0.013%, NH4NO3 0.1%, glucose 0.2%, colloid chitin 0.5%, MgSO4·7H2O 0.05%) for 24 h at 60 °C on a shaker incubator (180 rpm). Then, 4 mL of the preculture (3.5 × 108 cells/mL) was added to 100 mL of the production medium (preculture medium without glucose). The resultant inoculated medium was cultured at 60 °C for 6 days on a rotary shaker (180 rpm). The sample (4 mL) of the culture medium was removed every 24 h and centrifuged at 12,000 × g for 15 min at 4 °C. The resulting supernatant was then collected and used for subsequent chitinase activity assays.
PCR amplification and study of 16S rRNA gene
A partial DNA sequence for the 16S rRNA gene (ca. 1.4-kbp fragment) was amplified by PCR with the primers f16s: AGAAAGGAGGTGATCCAGCCGC and r16s: TCTTTTGGAGAGTTTGATCCTG. Isolation of genomic DNA and PCR amplification were conducted as suggested by.28 The amplification was performed by using the Techne® TC-512 Gradient Thermal Cycler (UK) with the following cycling parameters: 94 °C for 1 min, followed by 35 cycles of 30 s at 94 °C, 1 min at 51 °C, and 1.5 min at 72 °C, with a final extension of 5 min. The PCR fragment was purified by using the High Pure PCR Purification Kit (Roche, Germany). The purified PCR fragment was then ligated into the pTZ75R (Fermentas, Canada) by the T/A cloning procedure, and the construct was transformed into Escherichia coli DH5α. The amplified construct harboring the 16S rDNA was then extracted, purified, and sequenced by Seqlab (Germany) with appropriate primers. The nucleotide sequences were analyzed by using the DNASIS program (version 3.2; Hitachi Software Engineering Co. Ltd.); the obtained sequences were compiled and compared with the sequences in the databases (http://www.ncbi.nlm.nih.gov) by using the BLAST program. Other morphological and physiological characteristics for the selected strain were analyzed according to the Bergey's Manual of Systematic Bacteriology.
Selection of carbon, nitrogen, and phosphorus sources
For the selection of the best source of carbon, nitrogen, and phosphorus for chitinase production, several carbon, nitrogen, and phosphorus sources were used in the production liquid medium. To select the carbon sources in the production medium without chitin, 1% (w/v) glucose, galactose, mannose, fructose, arabinose (as monosaccharide); lactose, sucrose, maltose (as disaccharide); and starch, polygalacturonic acid, cellulose, chitin plus glucose, and chitin powder (as polysaccharide) were added to each basic medium separately. To select the nitrogen sources, 1% (w/v) peptone, NH4HCO3, (NH4)2SO4, NH4NO3, NH4Cl, Ca(NO3)2·4H2O, C4H11NO3, (NH4)2HPO4, and triptone NH4H2PO4 were added to the basic medium with optimal carbon source. Similarly, 1% (w/v) Na2HPO4, NaH2PO4, K2HPO4, KH2PO4, and NH4H2PO4 were added to the medium to determine the optimum phosphorus sources. The carbon:nitrogen (C:N) ratio are the same in all the media.
Experimental design and analysis
We first determined the various factors to be optimized in the culture medium that can have critical effect on the chitinase yield. Factors and their related levels were selected based on the consensus among the design engineers, scientists, and technicians with relevant experience involved in this experiment. Based on the obtained experimental data, eight factors were considered to significantly influence the chitinase yield.29 Table 1 displays the eight control factors and their levels employed in the Taguchi's robust experimental design. A standard orthogonal array L2730 was used to examine this eight-factor (six chemical parameters, two physical parameters) system. A run involved the corresponding combination of levels to which the factors in the experiment were set. Thus, the Taguchi's method can calculate the condition of least variability from the SN ratios and the condition of the best reaction performed by maximizing the overall desirability.31
Table 1.
Variables and their levels employed in the Taguchi's robust design method for optimal chitinase production by Cohnella sp. A01.
| Serial number | Factors | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| 1 | Temperature (°C) | 55 | 60 | 65 |
| 2 | pH | 6.5 | 7.5 | 8.5 |
| 3 | NaCl % (w/v) | 0.17 | 0.5 | 1.5 |
| 4 | K2HPO4% (w/v) | 0.035 | 0.07 | 0.14 |
| 5 | NH4NO3% (w/v) | 0.5 | 1.0 | 2.0 |
| 6 | Chitin % (w/v) | 0.17 | 0.5 | 1.5 |
| 7 | Trace element % (v/v) | 0.05 | 0.1 | 0.2 |
| 8 | Inoculation % (v/v) | 2 | 4 | 8 |
Extraction and partial purification of chitinase
All experimental steps were conducted at 4 °C in a cold room. The resulting supernatant (100 mL) of the production medium was fractionated by 20–80% solid ammonium sulfate precipitation and then centrifuged at 12,000 × g for 15 min at 4 °C; the precipitated proteins were suspended in 20 mM phosphate buffer (pH 7.8) and dialyzed against the same buffer for 24 h at 4 °C.
Enzymatic activity
The chitinase activity was determined by measuring the reducing end-group N-acetylglucosamine (GlcNAc) (as a final product) produced from colloidal chitin as a substrate. Colloidal chitin was prepared from chitin, as described previously.9 The standard reaction mixture containing 0.1 mL of 1% (w/v) colloidal chitin and 0.1 mL of the partially purified enzyme was incubated at 60 °C for 60 min. The reaction was stopped by the addition of 0.2 mL of DNS, followed by heating at 100 °C for 5 min. Then, the samples were cooled to room temperature and centrifuged at 6000 × g for 15 min. The concentration of the reducing sugar was determined by the modified DNS method.32 The absorption of the test sample was measured at 540 nm by a UV spectrophotometer (Beckman DU530, USA) along with reaction blanks. One unit (U) of the chitinase activity was defined as the amount of enzyme required to liberate 1 mmol of the reducing sugar per minute under the described conditions.
Effect of temperature and pH on the chitinase activity and stability
The best pH value of partially purified chitinase was determined by using 50-mM mixed buffer (glycine, NaHCO3, NaH2PO4) (pHs 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0) by performing the standard assay. The best temperature value of the chitinase activity was tested at 10, 20, 30, 40, 50, 60, 70, 80, and 90 °C in 50-mM phosphate buffer (pH 7.8). After that, the residual activity of the chitinase was measured by using the standard assay.
To determine the temperature stability, chitinase was initially preincubated at different temperatures (10–90 °C). Every 10 and 20 min (up to 90 min), the samples were placed on an ice bath for 30 min and then the colloidal chitin was added and the standard enzyme assay was performed at 60 °C. To obtain the pH stability, the enzyme was added to buffers with different pHs (3–12) for 90 min at 25 °C, followed by the standard enzyme assay at the best pH value.
Effect of metal ions and detergents on chitinase activity
To study the detrimental effects of metal ions and detergents, the chitinase activity was determined by the standard assay method in the presence of Ag+, Al3+, Ba2+, Ca2+, Co2+, Cu2+, Fe2+, Fe3+, K+, Ni2+, Mg2+, Mn+2 (1, 5, 10, and 15 mM) and ethylenediaminetetra acetic acid (EDTA) iodoacetamide, iodoacetic acid, Tween 20, Tween 80, Triton X-100, and SDS at 0.1% (w/v). The relative activity was calculated with respect to the control, where the reaction was performed in the absence of any additive.
Michaelis and rate constant determination
The Michaelis constant (Km) and the maximum velocity (Vmax) of the enzyme (U/mL) were evaluated by using a substrate concentration of 0.0–10 mg/mL. The reaction mixture was incubated at 60 °C for 1 h. Analysis of the Michaelis–Menten curve was conducted by using the GraphPad Prism 6 software to determine the enzyme's Km and Vmax.
Scanning electron microscopy (SEM)
In order to observe the morphological features of the microorganisms and the effect of chitinase on the morphological changes of chitin, the bacteria and chitin in the absence and presence of enzyme were provided and photographed under an SEM (LEO, 1455 VP; Germany) as described in previous articles.9, 33, 34
Statistical analyses
All data are presented as means ± standard deviation (SD) of at least three independent experiments. Statistical analysis was performed by using the Student's t-test. p < 0.05 was considered as statistically significant.
Software
Qualitek-4 software (Nutek Inc., MI) was used for automatic designing of experiments by using the Taguchi approach. Qualitek-4 software is provided to use L-4 to L-64 arrays along with a selection of 2–63 factors with two, three, and four levels to each factor. The automatic layout option permits Qualitek-4 to select the array used and to assign factors to suitable columns.
GraphPad Prism 6 (San Diego, CA, USA) was used to determine the enzyme's Michaelis–Menten kinetic parameters, Km (substrate concentration that yields a half-maximal velocity), and Vmax (maximum velocity) values by nonlinear regression analysis.
Results
Isolation and identification of chitinolytic microorganisms
We isolated 12 strains from the water and waste water of Abadan shrimp farming ponds in southwestern Iran. In the initial screening, qualitative cup plate assay was performed for chitinase production, indicating one isolate as the most active one.9 Biochemical and microbiological analyses were performed to characterize the screened strain (Table 2). In accordance with the Bergey's Manual of Systematic Bacteriology, the A01 strain was classified as a bacteria belonging to the genus Cohnella. Subsequent sequence analysis of the 16 S rDNA gene confirmed the isolate as being Cohnella sp.
Table 2.
Morphological, physiological and biochemical characteristic of Cohnella sp. A01.
| Characters | Results |
|---|---|
| Form | Rod |
| Gram stain | Positive |
| Spore | Spore central and oval |
| Motility | Positive |
| Catalase | Positive |
| Oxidase | Negative |
| Utilization of | |
| Glucose, maltose | Positive |
| Arabinose, xylose, mannitol, lactose, fructose | Negative |
| Hydrolysis of starch | Positive |
| Hydrolysis of gelatin | Negative |
| Indol formation | Negative |
| Growth on NaCl | Positive |
| Growth temperature | 40–60 °C |
Nucleotide sequence accession number
The nucleotide sequence of 16S rDNA from Cohnella sp. A01 would be made available in the GenBank nucleotide sequence databases (accession number: JN208862).
Effects of incubation time, carbon, nitrogen, and phosphorus sources on chitinase production
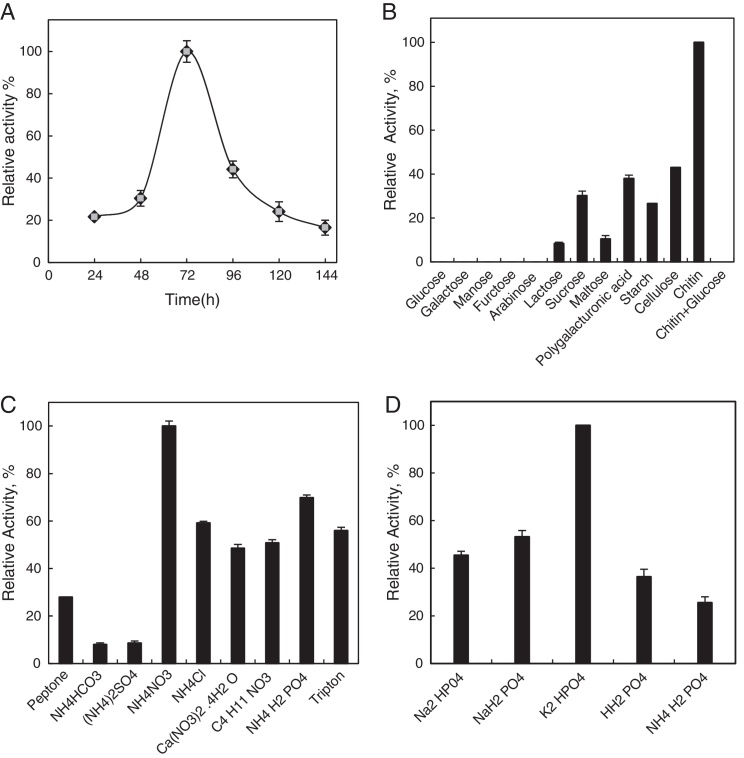
Chitinase was produced in the media containing 0.5% of colloidal chitin. During the early period of the incubation, minimal enzyme activity was detected in the culture; however, after 72 h of incubation, the enzyme activity was significantly increased (Fig. 1A).
Fig. 1.
Effects of incubation time (A), carbon (B), nitrogen (C), and phosphorus (D) sources on chitinase production. All experiments were carried out in triplicate, and each point illustrates the average of three independent experiments. For more details, please refer to the “Materials and methods” section.
The growth of Cohnella sp. and the production of enzyme by this bacterium were studied by using different carbon sources. Chitinase production was induced by chitin and inhibited in the presence of easily metabolized monosaccharides, such as glucose, galactose, mannose, arabinose, and fructose (Fig. 1B). These compounds inhibited enzyme biosynthesis and presented with low chitinase activities when these monosaccharides were used as carbon sources. Lactose, sucrose, and maltose detracted chitinase production (approximately 70–80%). Polygalacturonic acid, starch, and cellulose decreased enzyme production by approximately 50–60%; nevertheless, chitin gave the highest enzyme activity (100%). The medium containing NH4NO3 (Fig. 1C) and K2HPO4 (Fig. 1D) showed the highest enzyme activity as compared to the others.
Results of Taguchi design analysis
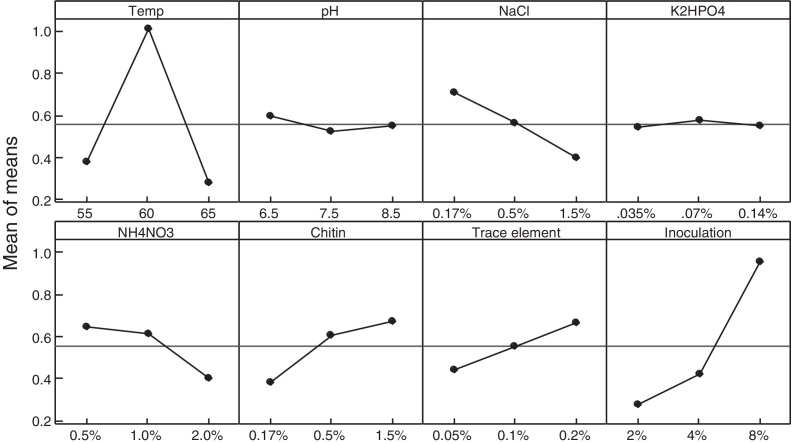
We used different media with different inducers and under different conditions to analyze the possible fractions of chitinases synthesized by Cohnella sp. The main effects of the eight control factors at their three levels on crude enzyme biosynthesis are shown in Fig. 2. The contribution of each factor is presented in Table 3. According to these data, inoculation amount and temperature were the most significant factors influencing the production of chitinase in addition to trace elements and NaCl constitution.
Fig. 2.
The main effect plot (data means) for means on chitinase production. For more details, please refer to the “Materials and methods” section.
Table 3.
The analysis of variance (ANOVA) table.
| Serial number | Factors | Degree of freedom (DOF) | Sum of squares | Variance | F-ratio | p-Value |
|---|---|---|---|---|---|---|
| 1 | Temperature | 2 | 7.677 | 3.839 | 70.763 | 0.000 |
| 2 | pH | 2 | 0.101 | 5.056E−02 | 0.932 | 0.0399 |
| 3 | NaCl | 2 | 1.533 | 0.767 | 14.131 | 0.000 |
| 4 | K2HPO4 | 2 | 0.117 | 5.836E−02 | 1.076 | 0.347 |
| 5 | NH4NO3 | 2 | 7.127E–02 | 3.563E−02 | 0.657 | 0.522 |
| 6 | Chitin | 2 | 0.599 | 3.678E−02 | 0.678 | 0.511 |
| 7 | Trace element | 2 | 7.642 | 0.299 | 5.517 | 0.006 |
| 8 | Inoculation | 2 | 3.472 | 3.821 | 70.443 | 0.006 |
Effect of temperature and pH on enzyme activity and stability
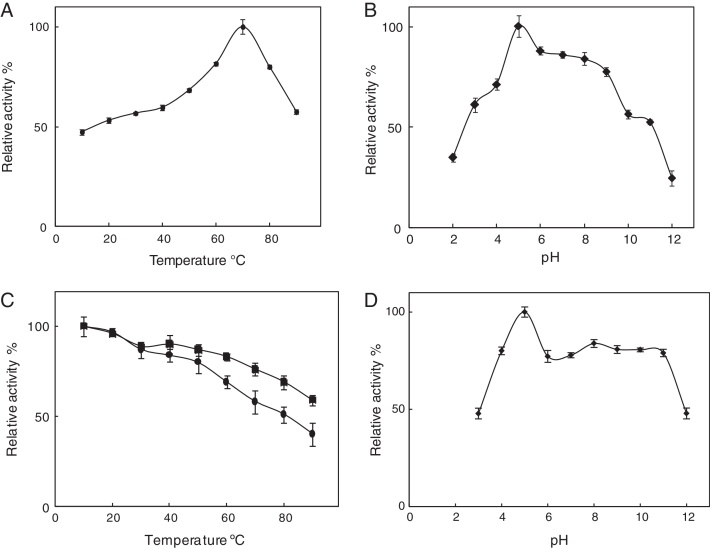
The effect of temperature on the crude enzyme activity was studied at several temperatures. The best temperature value was obtained at 70 °C (Fig. 3A) and the best pH value at 5 (Fig. 3B). The temperature stability in 10- and 20-min incubation of enzyme in different temperature conditions showed that the enzyme possessed >50% activity until at 80 °C temperature (Fig. 3C). The enzyme displayed activity over a relatively wide range of pH (4.0–11.0), i.e., >75% (Fig. 3D).
Fig. 3.
Temperature (A) and pH (B) profiles and temperature (10 “■” and 20 “●” minute incubation) (C) and pH (D) stability of partially purified chitinase. All experiments were carried out in triplicate, and each point illustrates the average of three independent experiments. For more details, please refer to the “Materials and methods” section.
Effect of metal ions, chelators, and some chemicals on chitinase activity
The crude chitinase activity was measured at the optimum pH and temperature in the presence of various metal ions and chemical compounds. Metal ions such as Ag2+ and Co2+ inhibited the enzyme activity up to 40% at 15 mM concentration. On the other hand, Mn2+ and Cu2+ increased the enzyme activity up to 25% at 5 mM concentration (Table 4). Iodoacetamide and idoacetic acid (1% w/v) inhibited the enzyme activity by approximately 55%. Tweens (20 and 80) and Triton X-100 slightly increased the enzyme activity (110, 115, and 108%, respectively), while EDTA stimulated the activity up to 120% (Table 5).
Table 4.
Effect of some metal ions on the enzyme activity.
| Residual activity at 15 mM | Residual activity at 10 mM | Residual activity at 5 mM | Residual activity at 1 mM | Metal ions |
|---|---|---|---|---|
| 89.2 | 91.3 | 86.9 | 85.9 | LiCl |
| 126.1 | 124.6 | 127.1 | 113 | CuSO4 |
| 99.1 | 98.9 | 101.2 | 100 | AlCl3 |
| 94.9 | 96.9 | 100 | 100 | K2Cr2O7 |
| 93.6 | 92.6 | 93.3 | 88 | KCl |
| 95 | 94.4 | 88.8 | 92.7 | MgSO4 |
| 92.4 | 91.2 | 100 | 96.5 | FeCl3 |
| 59.1 | 63.6 | 68.4 | 69.9 | AgNO3 |
| 95 | 94.4 | 100 | 95.9 | BaCl2 |
| 125.8 | 125 | 126.2 | 110 | MnSO4 |
| 55.2 | 67.1 | 81.2 | 97.4 | Co(NH2)2 |
| 93 | 98.8 | 99.2 | 100 | ZnSO4 |
Table 5.
Effect of some chemical compounds and chelators on the enzyme activity.
| Chemical compounds and chelators (1%) | Enzyme activity (%) |
|---|---|
| Control | 100 |
| Citric acid | 96.9 |
| EDTA | 120 |
| SDS | 87.4 |
| PMSF | 100 |
| Iodoacetamide | 45.7 |
| Iodoacetic acid | 45.5 |
| Tween 20 | 110 |
| Tween 80 | 115 |
| Triton X-100 | 108 |
Determination of Michaelis and rate constant
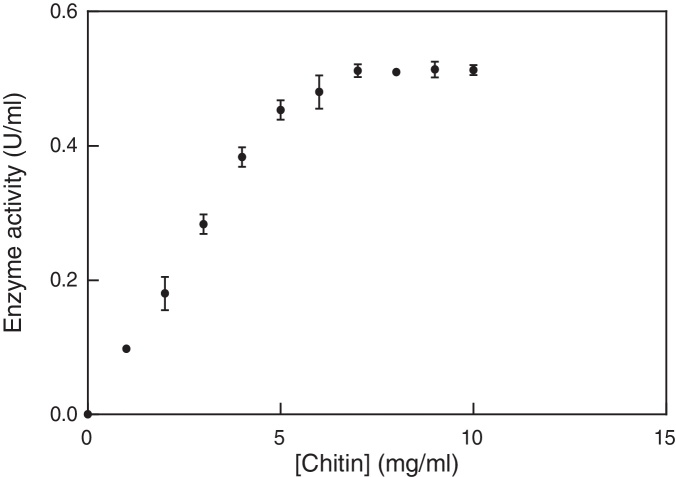
The Michaelis constant (Km) and the maximum rate (Vmax) of the enzyme (U/mL) were assayed at chitin concentration of 0.0–10 mg/mL. Analysis of the Michaelis–Menten curve was accomplished with GraphPad Prism 6. In enzyme kinetics, Vmax illustrated the maximum velocity received by the system at saturating substrate concentrations. The substrate concentration at which the reaction velocity was half of Vmax value represented the Michaelis–Menten constant (Km). The Km and Vmax values of the enzyme were 5.7 mg mL−1 and 0.87 units (μmol min−1) in the presence of colloidal chitin, respectively (Fig. 4).
Fig. 4.
Michaelis–Menten curve of chitinase activity versus different concentrations of chitin incubated at the best temperature and pH values. The Km and Vmax values of the enzyme were 5.7 mg mL−1 and 0.87 units (μmol min−1). For more details, please refer to the “Materials and methods” section.
SEM
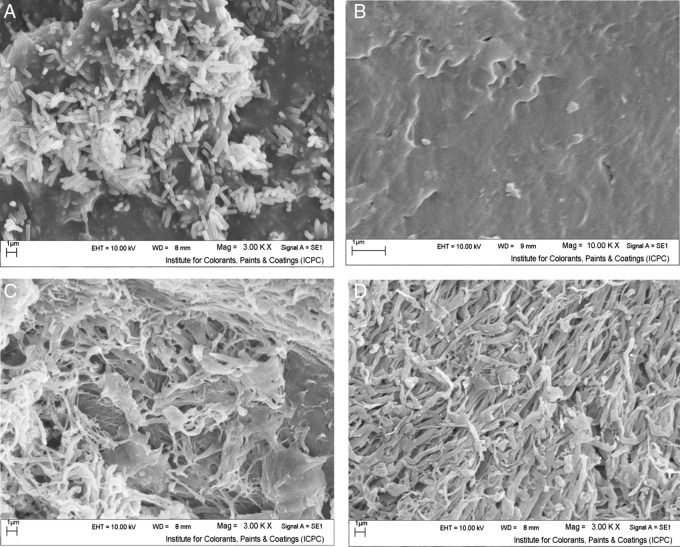
The SEM image illustrated in Fig. 5A shows Cohnella sp. A01 cells as long, straight, rod-shaped bacilli. To investigate the morphological changes in chitin after chitinase treatment, we performed SEM trials. In the absence of enzyme, chitin illustrated more or less a smooth outside layer (Fig. 5B). After chitinase treatment (Fig. 5C and D), the SEM image revealed cracks and several small holes on the chitin surface, with the amount of pores remarkably increasing with the period of exposure (incubation).
Fig. 5.
Scanning electronic microscopy photographs of Cohnella A01 (A); untreated chitin (B); treated chitin after 1 h (C); and 2 h (D). For more details, please refer to the “Materials and methods” section.
Discussion
The increase of enzyme products often depends on screening a large number of microorganisms for an enzyme activity.9, 35, 36 In this study, a chitinolytic strain was isolated from the shrimp farming wastewater located in Choebdeh-Abadan and confirmed to be Cohnella sp. A01 based on its morphology, physiological and biochemical tests, and the 16S rRNA sequence analysis reports. The 16S rRNA gene sequences are largely used for the identification of prokaryotes.37
This paper present product optimization of chitinase by Cohnella A01 for the first time. Other studies have reported similar studies on Pseudomonas sp. TKU015,38 Aeromonas schubertii,39 and Bacillus sp. 13.26.40 In the A. hydrophila HS4, maximum chitinase activity was detected to occur after 24 h of incubation, and remained invariant up to 48 h, while A. punctata HS6 produced maximum enzyme after 48 h of incubation.41 The primary reasons for the reduction in chitinase production may be due to the lack of nutrients in the culture medium or the production of toxic chemicals in the medium, eventuating in the inactivation of secretary machinery of the enzymes or breakup of enzyme by proteases.42
In the first stage of the medium ingredients optimization for maximum enzyme production by Cohnella sp. A01, the optimum carbon, nitrogen, and phosphorus sources were selected by the one factor-at-a-time procedure. The study on the effect of carbon sources showed that monosaccharides inhibited chitinase biosynthesis and its synthesis in small levels with low activity. Inhibition of the enzyme efficiency in the presence of simple sugars may be due to catabolite repression.43 These outcomes are in agreement with that for chitinase production by Streptomyces viridificans44 and Trichoderma harzianum,45 respectively. The results of the present survey demonstrates that the addition of monosaccharides to the culture medium reduces the chitinase activity of Cohnella sp. A01, which was similar to chitinase production by Streptomyces sp.46 and Serratia marcescens B4A.9
The efficacy of nitrogen sources on enzyme production demonstrated that NH4NO3 presumably is the most optimal nitrogen source for enzyme production. It may be due to ammonium (in ammonium nitrate) is the inorganic nitrogenous form of simpler assimilation and also because of lower energetic costs of metabolization.47
K2HPO4 was recognized as the best phosphorus source for the enzyme production by Cohnella sp. A01, in concordance with that reported for chitinase biosynthesis by Paenibacillus sp. D148 and Vibrio alginolyticus JN863235.49
Cultural conditions are significant factors that affect the product efficiency and cell growth. In this research, the effects of various parameters, including incubation temperature, initial pH, NaCl, K2HPO4, NH4NO3, chitin levels, trace elements, and inoculation percentage on the efficiency of chitinase production were evaluated and optimized by the Taguchi L27 array method. The data obtained from this method suggested that the temperature and NaCl as well as inoculation amount were more effective than others on the enzyme production. The temperature of incubation and inoculation percentage were the most important factors influencing the change in the production level (Fig. 2).
The temperature and pH of enzyme are usually distinctive parameters that determine whether an enzyme is appropriate for biotechnological applications. The chitinase of Cohnella sp. A01 was active at 80 °C after 20 min of temperature treatment and showed the best activity at 70 °C (Fig. 3A and C). Therefore, Cohnella sp. A01 chitinase can be called as a thermostable enzyme. A comparable thermostability has been reported for alkaline chitinase by Bacillus thuringiensis subsp. Kurstaki strain HBK-51,50 Bacillus thuringiensis Mexican isolates,51 Brevibacillus formosus BISR-1,52 and Rhodothermus marinus.15 The highest enzyme activity was found at pH 5 (Fig. 3B), although activity was detected over a wide pH range of 4–11 (Fig. 3D). A comparable pH optimum has been reported for Bbchit1 of Beauveria bassiana NCIM 1216.10 This enzyme has it is the best pH in the acidic range and can possibly be used for the control of plant fungal pathogens.
The studied enzyme did not show requirement of any specific metal ions for its activity, although Mn2+ and Cu2+ had a stimulatory effect on chitinase activity. Idoacetic acid and iodoacetamide inhibited the enzyme activity at 1 mM concentration by approximately 55%, indicating that several cysteine residues form a part of the catalytic site of chitinase. Tweens 20, 80, and Triton X-100 stimulated the chitinase activity by up to 15%. As the surface-active reagents may have enhanced the turnover number of enzyme due to the increased contact frequency between the substrate and active site of the enzyme with disrupting surface tension of the aqueous medium.33, 43
In the test of substrate concentration against chitinase activity, the activity of enzyme was found to increase with increase in the substrate concentration to about 7 mg/mL of colloidal chitin, but no effect with further increase. The enzyme showed a Km value of 5.6 mg/mL against colloidal chitin, which is relatively lower than some other reported Km values, such as 12 mg/mL for chitinase from Bacillus sp. BG-1153 and 8.3 mg/mL for chitinase from Serratia marcescens B4A33; the chitinase of Cohnella sp. A01 revealed a high affinity for colloidal chitin and possibly greater potential in the industry.
Study of the morphological alterations of chitin treated by enzyme via SEM experiments exhibited cracks and pores in the substrate surface. The same morphological changes have been reported for Serratia marcescens B4A chitinase33 and chitinase from penicillium sp. LYG0704.54 This direct evidence implies that the isolated chitinase was quite effective on chitin degradation.
Conclusions
The isolation study revealed that shrimp farming ponds are an appropriate environment for screening of chitinolytic enzyme-producing bacteria. Our study affirmed the efficiency of the Taguchi experimental design for specifying the most appropriate medium components to obtain maximum production of enzyme by Cohnella sp. A01. This work revealed that the production of chitinase is feasible from a newly isolated Cohnella sp. A01. Chitinase produced from Cohnella sp. A01 is active over a wide range of temperature and pH, with good tolerance to high temperatures. The growth of enzyme products mostly relies on screening of extensive number of organisms for their enzyme activity with a specific set of biochemical characteristics that suits the targeted population. Thus, merging enzyme screening with protein engineering, directed evolution, and metagenome studies, novel enzymes with improved efficiency under specific applications and conditions can be built.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by International Foundation for Science (IFS), COMSTECH (Committee on Scientific and Technological Cooperation) Grant no. F/4900-2, and National Institute of Genetic Engineering and Biotechnology (NIGEB) (Grant No: 463). We would like to thank them for financial support of this project.
Associate Editor: Lucy Seldin
References
- 1.Matroodi S., Zamani M., Haghbeen K., Motallebi M., Aminzadeh S. Physicochemical study of a novel chimeric chitinase with enhanced binding ability. Acta Biochim Biophys Sin (Shanghai) 2013;45:845–856. doi: 10.1093/abbs/gmt089. [DOI] [PubMed] [Google Scholar]
- 2.Hao Z., Cai Y., Liao X., Zhang X., Fang Z., Zhang D. Optimization of nutrition factors on chitinase production from a newly isolated Chitiolyticbacter meiyuanensis SYBC-H1. Braz J Microbiol. 2012;43:177–186. doi: 10.1590/S1517-838220120001000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S.Y., Zhou J.J., Shao B., Lu Y.J., Rao P.F. A thermostable chitinase with chitin-binding activity from Phaseolus limensis. J Food Sci. 2008;73:C452–C457. doi: 10.1111/j.1750-3841.2008.00800.x. [DOI] [PubMed] [Google Scholar]
- 4.Dahiya N., Tewari R., Hoondal G.S. Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol. 2006;71:773–782. doi: 10.1007/s00253-005-0183-7. [DOI] [PubMed] [Google Scholar]
- 5.Waghmare S.R., Ghosh J.S. Chitobiose production by using a novel thermostable chitinase from Bacillus licheniformis strain JS isolated from a mushroom bed. Carbohydr Res. 2010;345:2630–2635. doi: 10.1016/j.carres.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Shahidi F., Arachchi J.K.V., Jeon Y. Food applications of chitin and chitosans. Trends Food Sci Technol. 1999;10:37–51. [Google Scholar]
- 7.Lang S., Hüners M., Lurtz V. Springer; Berlin: 2005. Marine Biotechnology II. [Google Scholar]
- 8.Lang S., Huners M., Verena L. Bioprocess engineering data on the cultivation of marine prokaryotes and fungi. Adv Biochem Eng Biotechnol. 2005;97:29–62. doi: 10.1007/b135822. [DOI] [PubMed] [Google Scholar]
- 9.Zarei M., Aminzadeh S., Zolgharnein H. Serratia marcescens B4A chitinase product optimization using Taguchi approach. Iran J Biotechnol. 2010;8:252–262. [Google Scholar]
- 10.Pinnamaneni R., Kalidas P., Sambasiva Rao K.R. Studies on the cloning and expression of Bbchit1 gene of Beauveria bassiana NCIM 1216. Indian J Microbiol. 2011;51:396–402. doi: 10.1007/s12088-011-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya D., Nagpure A., Gupta R.K. Bacterial chitinases: properties and potential. Crit Rev Biotechnol. 2007;27:21–28. doi: 10.1080/07388550601168223. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y.S., Park I.H., Yoo J.S. Cloning, purification, and characterization of chitinase from Bacillus sp. DAU101. Bioresour Technol. 2007;98:2734–2741. doi: 10.1016/j.biortech.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 13.Babashpour S., Aminzadeh S., Farrokhi N., Karkhane A., Haghbeen K. Characterization of a chitinase (Chit62) from Serratia marcescens B4A and its efficacy as a bioshield against plant fungal pathogens. Biochem Genet. 2012;50:722–735. doi: 10.1007/s10528-012-9515-3. [DOI] [PubMed] [Google Scholar]
- 14.Narayana K.J., Vijayalakshmi M. Chitinase production by Streptomyces sp. ANU 6277. Braz J Microbiol. 2009;40:725–733. doi: 10.1590/S1517-83822009000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobel C.F., Hreggvidsson G.O., Marteinsson V.T., Bahrani-Mougeot F., Einarsson J.M., Kristjansson J.K. Cloning, expression, and characterization of a highly thermostable family 18 chitinase from Rhodothermus marinus. Extremophiles. 2005;9:53–64. doi: 10.1007/s00792-004-0422-3. [DOI] [PubMed] [Google Scholar]
- 16.Andronopoulou E., Vorgias C.E. Isolation, cloning, and overexpression of a chitinase gene fragment from the hyperthermophilic archaeon Thermococcus chitonophagus: semi-denaturing purification of the recombinant peptide and investigation of its relation with other chitinases. Protein Expr Purif. 2004;35:264–271. doi: 10.1016/j.pep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Gohel V., Chaudhary T., Vyas P., Chhatpar H.S. Statistical screenings of medium components for the production of chitinase by the marine isolate Pantoea dispersa. Biochem Eng J. 2006;28:50–56. [Google Scholar]
- 18.Oku T., Ishikawa K. Analysis of the hyperthermophilic chitinase from Pyrococcus furiosus: activity toward crystalline chitin. Biosci Biotechnol Biochem. 2006;70:1696–1701. doi: 10.1271/bbb.60031. [DOI] [PubMed] [Google Scholar]
- 19.Ash C., Priest F.G., Collins M.D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek. 1993;64:253–260. doi: 10.1007/BF00873085. [DOI] [PubMed] [Google Scholar]
- 20.Ash C., Farrow J.A.E., Wallbanks S., Collins M.D. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett Appl Microbiol. 1991;13:202–206. [Google Scholar]
- 21.Yoon M.H., Ten L.N., Im W.T. Cohnella panacarvi sp. nov., a xylanolytic bacterium isolated from ginseng cultivating soil. J Microbiol Biotechnol. 2007;17:913–918. [PubMed] [Google Scholar]
- 22.Singh A.K., Ghodke I., Chhatpar H.S. Pesticide tolerance of Paenibacillus sp. D1 and its chitinase. J Environ Manag. 2009;91:358–362. doi: 10.1016/j.jenvman.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Kampfer P., Rossello-Mora R., Falsen E., Busse H.J., Tindall B.J. Cohnella thermotolerans gen. nov., sp. nov., and classification of ‘Paenibacillus hongkongensis’ as Cohnella hongkongensis sp. nov. Int J Syst Evol Microbiol. 2006;56(Pt 4):781–786. doi: 10.1099/ijs.0.63985-0. [DOI] [PubMed] [Google Scholar]
- 24.Aminzadeh S., Farrokhi N. Product optimization, purification and characterization of a novel polygalacturonase produced by Macrophomina phaseolina. Biol J Microorganism. 2013;1:21–34. [Google Scholar]
- 25.Abiri N., Aminzadeh S., Bihamta M.R. Partial purification and assessment of antifungal activity of an extracellular chitinase from an Iranian thermophile strain of Cohnella sp. A01. Genet Eng Biosaf J. 2012;1:15–22. [Google Scholar]
- 26.Takiguchi Y. 1991. Physical Properties of Chitinous Materials: Advanced in Chitin Science. [Google Scholar]
- 27.Hau S.C., Lockwood J.L. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol. 1975;29:422–426. doi: 10.1128/am.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbour Laboratory; New York: 1986. Molecular Cloning. [Google Scholar]
- 29.Prasad K.K., Mohan S.V., Bhaskar Y.V. Laccase production using Pleurotus ostreatus 1804 immobilized on PUF cubes in batch and packed bed reactors: influence of culture conditions. J Microbiol. 2005;43:301–307. [PubMed] [Google Scholar]
- 30.Taguchi G. Kraus International; White Plains, NY: 1987. System of Experimental Design: Engineering Methods to Optimize Quality and Minimize Costs. [Google Scholar]
- 31.Dasu V.V., Panda T., Chidambaram M. Determination of significant parameters for improved griseofulvin production in a batch bioreactor by Taguchi's method. Process Biochem. 2003;38:877–880. [Google Scholar]
- 32.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 33.Zarei M., Aminzadeh S., Zolgharnein H. Characterization of a chitinase with antifungal activity from a native Serratia marcescens B4A. Braz J Microbiol. 2011;42:1017–1029. doi: 10.1590/S1517-838220110003000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adibzadeh N., Aminzadeh S., Jamili S., Karkhane A.A., Farrokhi N. Purification and characterization of pepsin-solubilized collagen from skin of sea cucumber Holothuria parva. Appl Biochem Biotechnol. 2014;173:143–154. doi: 10.1007/s12010-014-0823-4. [DOI] [PubMed] [Google Scholar]
- 35.Aminzadeh S., Naderi-Manesh H., Khajeh K., Naderi-Manesh M. Purification, characterization, kinetic properties, and thermal behavior of extracellular polygalacturonase produced by filamentous fungus Tetracoccosporium sp. Appl Biochem Biotechnol. 2006;135:193–208. doi: 10.1385/abab:135:3:193. [DOI] [PubMed] [Google Scholar]
- 36.Aminzadeh S., Naderi-Manesh H., Khajeh K., Ranjbar B., Farrokhi N. Characterization of acid-induced partially folded conformation resembling a molten globule state of polygalacturonase from a filamentous fungus Tetracoccosporium sp. Appl Biochem Biotechnol. 2010;160:1921–1932. doi: 10.1007/s12010-009-8723-8. [DOI] [PubMed] [Google Scholar]
- 37.Chun J., Lee J.H., Jung Y. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57(Pt 10):2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- 38.Wang S.L., Chen S.J., Wang C.L. Purification and characterization of chitinases and chitosanases from a new species strain Pseudomonas sp. TKU015 using shrimp shells as a substrate. Carbohydr Res. 2008;343:1171–1179. doi: 10.1016/j.carres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Guo S., Chen J., Lee W. Purification and characterization of extracellular chitinase from Aeromonas schubertii. Enzyme Microb Technol. 2004;35:550–556. [Google Scholar]
- 40.Yuli P.E., Suhartono M.T., Rukayadi Y., Hwang J.K., Pyun Y.R. Characteristics of thermostable chitinase enzymes from the Indonesian Bacillus sp. 13.26. Enzyme Microb Technol. 2004;35:147–153. [Google Scholar]
- 41.Kuddus S.M., Ahmad R.I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Gen Eng Biotechnol. 2013;11:39–46. [Google Scholar]
- 42.Binod P., Sandhya C., Suma P., Szakacs G., Pandey A. Fungal biosynthesis of endochitinase and chitobiase in solid state fermentation and their application for the production of N-acetyl-d-glucosamine from colloidal chitin. Bioresour Technol. 2007;98:2742–2748. doi: 10.1016/j.biortech.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Aminzadeh S., Naderi-Manesh H., Khajeh K., Soudi M.R. Isolation and characterization of polygalacturonase produced by Tetracoccosporium sp. Iran J Chem Chem Eng. 2007;26:47–54. [Google Scholar]
- 44.Gupta R., Saxena R.K., Chaturvedi P., Virdi J.S. Chitinase production by Streptomyces viridificans: its potential in fungal cell wall lysis. J Appl Bacteriol. 1995;78:378–383. doi: 10.1111/j.1365-2672.1995.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 45.Ulhoa C.J., Peberdy J.F. Regulation of chitinase synthesis in Trichoderma harzianum. J Gen Microbiol. 1991;137:2163–2169. doi: 10.1099/00221287-137-9-2163. [DOI] [PubMed] [Google Scholar]
- 46.Nawani N.N., Kapadnis B.P. Optimization of chitinase production using statistic based experimental designs. Process Biochem. 2005;40:651–660. [Google Scholar]
- 47.Von Rückert G., Giani A. Effect of nitrate and ammonium on the growth and protein concentration of Microcystis viridis Lemmermann (Cyanobacteria) Braz J Bot. 2004;27:325–331. [Google Scholar]
- 48.Singh A.K. Optimization of culture conditions for thermostable chitinase production by Paenibacillus sp. D1. Afr J Microbiol Res. 2010;40:2291–2298. [Google Scholar]
- 49.Ravikumar V., Meignanalakshmi S. Screening of marine Vibrio sp isolated from the Bay of Bengal, India for chitinase enzyme production. Int J Pharm Bio Sci. 2013;4:238–248. [Google Scholar]
- 50.Kuzu S.B., Guvenmez H.K., Denizci A.A. Production of a thermostable and alkaline chitinase by Bacillus thuringiensis subsp. kurstaki strain HBK-51. Biotechnol Res Int. 2012;2012:135498. doi: 10.1155/2012/135498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosas-Garcia N.M., Fortuna-Gonzalez J.M., Barboza-Corona J.E. Characterization of the chitinase gene in Bacillus thuringiensis Mexican isolates. Folia Microbiol (Praha) 2013;58:483–490. doi: 10.1007/s12223-013-0233-y. [DOI] [PubMed] [Google Scholar]
- 52.Meena S., Gothwal R.K., Krishna Mohan M., Ghosh P. Production and purification of a hyperthermostable chitinase from Brevibacillus formosus BISR-1 isolated from the Great Indian Desert soils. Extremophiles. 2014;18:451–462. doi: 10.1007/s00792-014-0630-4. [DOI] [PubMed] [Google Scholar]
- 53.Bhushan B. Production and characterization of a thermostable chitinase from a new alkalophilic Bacillus sp. BG-11. J Appl Microbiol. 2000;88:800–808. doi: 10.1046/j.1365-2672.2000.01016.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee Y.G., Chung K.C., Wi S.G., Lee J.C., Bae H.J. Purification and properties of a chitinase from Penicillium sp. LYG0704. Protein Expr Purif. 2009;65:244–250. doi: 10.1016/j.pep.2008.12.004. [DOI] [PubMed] [Google Scholar]